Abstract
One of the most common malformations of the central nervous system is related to embryonic neural tube alterations. We hypothesized that anencephaly affects the development of the uterus during the human second trimester of pregnancy. The objective of this study was to study the biometric parameters of the uterus in fetuses with anencephaly and compare them with normocephalic fetuses at that important. In our study, 34 female fetuses were analyzed, 22 normal and 12 anencephalic, aged between 12 and 22 weeks post-conception (WPC). After dissection of the pelvis and individualization of the genital tract, we evaluated the length and width of the uterus using the Image J software. We compared the means statistically using the Wilcoxon-Mann–Whitney test and performed linear regression. We identify significant differences between the uterus length (mm)/weight (g) × 100 (p = 0.0046) and uterus width (mm)/weight (g) × 100 (p = 0.0013) when we compared the control with the anencephalic group. The linear regression analysis indicated that 80% significance was found in the correlations in normocephalic fetuses (12.9 to 22.6 WPC) and 40% significance in anencephalic fetuses (12.3 to 18.6 WPC). The measurements of the uterus were greater in anencephalic group but there are no difference in the uterine width and length growth curves during the period studied. Further studies are required to support the hypothesis suggesting that anencephaly may affect uterine development during the human fetal period.
Similar content being viewed by others
Introduction
Neural tube defects (NTDs) are one of the most common congenital malformations of the central nervous system, with an average prevalence at birth of 1 in 10001. Anencephaly is the most severe NTD, resulting from failure of the neural tube in the third to fourth week (days 26 to 28) after conception2. The possibility of secondary effects of anencephaly on other organs and systems, have motivated studies to better understand that condition.
Recently, the urogenital tract of anencephalic fetuses has been studied, including the kidneys and collecting system2,3, ureters4, bladder5, prostate6,7, penis8, testicles9, female external genitalia10 and vagina11. Some authors have indicated a different response of male and female anencephalic fetuses to hormonal stimulation12. One of the reasons for this difference was explained in articles describing the underdevelopment of the adrenal cortex in anencephalic fetuses13,14,15, since that segment of adrenal tissue is also responsible for the production of virilizing hormones. In the absence of this signaling, an XY fetus would not achieve full growth of its sexual characteristics. Thus, in theory anencephaly would not negatively influence the development of gynecological structures, including the uterus from a neuroendocrine perspective. But from another point of view, NTD can affect the development of the nerve plexuses that surround the uterus.
The uterus is derived from the paramesonephric (or Müllerian) ducts. By the eighth week, the paramesonephric ducts merge in the cranio-caudal direction and at the end of the first-trimester, development of the uterus and the other Müllerian structures is complete16,17. Research of the effect of anencephaly on uterine morphogenesis is still scarce.
We hypothesized that anencephaly impacts the uterine development during the human fetal period. The confirmation of these alterations could be important in future studies about the impact of NTDs on female genital development. The objective of this study was to compare the uterus diameters in fetuses with anencephaly and compare them with the biometric parameters of normocephalic fetuses at different gestational ages.
Material and methods
The fetuses used in this study (both Controls and with AWDs all with informed consent of the parents) were obtained from the Department of Pathology of the Fernandes Figueira Institute, Oswaldo Cruz Foundation, Ministry of Health, in partnership with our University, via an official Cooperation Term.
The study was approved by the Ethical Committee on Human Research—University Hospital of the State University of Rio de Janeiro (CEP / HUPE), with the number (IRB: 2.475.334, CAAE: 78881317.4.0000.5259).
The study has also been registered in the Brazil Plataform, Ministry of Health, National Health Council, National Research Ethics Commission (CONEP) for studies with human beings. We confirm that all methods used in this paper were carried out in accordance with relevant guidelines and regulation.
Thirty-four female fetuses (22 without apparent anomalies and 12 anencephalic) were studied, aged 12 to 22 weeks post-conception (WPC), which had been aborted because of hypoxia. All of them were macroscopically well preserved and were donated by the hospital’s obstetrics department.
The gestational age was determined in WPC according to the foot-length criterion. This criterion is currently considered the most acceptable parameter to estimate gestational age18,19,20. The fetuses were also evaluated regarding, crown-rump length (CRL) and body weight immediately before dissection. All measurements were carried out by the same observer.
After compiling anthropometric data, specimens were thoroughly dissected through bilateral subcostal incision laparotomy, allowing visualization of abdominal organs and extraction of fetal pelvis “en bloc”.
The pelvis blocks were then reserved in an formaldehyde prefilled container until the moment of microdissection, performed in our laboratory with aid of stereoscopic magnification lenses (Zeiss Discovery V8 microscope 16/25×). All fetuses were dissected under identical conditions by the same researcher, who has practical experience in microsurgery.
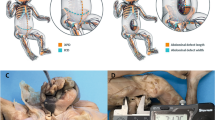
The pelvis was opened to expose and identify the urogenital organs and separate the genital and urinary tracts. After complete dissection of the uterus, photographs were taken by the camera attached to the microscope (Zeiss Axiocam 506 Color, 6 megapixels), and images were stored in a TIFF file. The biometric parameters were recorded, with measurements performed by the same observer using the Image J software, version 1.46r, because of the high intra observer precision compared to interobserver analysis21. Uterine dimensions were measured assuming the length from the cervix to the fundus and its width was equal to the distance between the utero-tubal junctions (Fig. 1). The data were expressed in millimeters. All data were collected from July 2019 to December 2021.
Measurements of uterus in human fetuses. (A) Schematic drawing of female organs showing the metric standards used to measure the uterus width (1) and uterus length (2) and (B) genital organs of a female fetus with 18 weeks post conception, after the dissection with the aid of the microscope (with ×16/25 magnification). The measurement of uterus width (1) and uterus length (2) was done using the Image J software, version 1.46r.
Statistical analysis
All parameters were statistically processed and graphically described. The Shapiro–Wilk test was used to verify the normality of the data. After that, the Wilcoxon-Mann–Whitney test was used for comparison of quantitative data of normocephalic fetuses vs. fetuses with anencephaly (p < 0.05) and the level of significance was adjusted by the correction of Bonferroni.
Simple linear correlations (r2 values less than 0.4 reflect very weak correlation, while r2 between 0.4 and 0.7 reflect moderate correlation and r2 greater than 0.7 indicates strong correlation) were calculated for uterine measurements, according to fetal age. The statistical analysis was performed with the GraphPad Prism software (Version 9.2.0).
Compliance with ethical standards
This study was supported by the National Council for Scientific and Technological Development (CNPQ—Brazil) (Grant number: 301522/2017) and The Rio de Janeiro State Research Foundation (FAPERJ) (Grant number: E26/202.873/2017).
Ethical approval
This study was carried out in accordance with the ethical standards of the hospital’s institutional committee on human experimentation. (IRB: 2.475.334, CAAE: 78881317.4.0000.5259).
Statement
We confirm that all data generated or analysed during this study are included in this published article submitted to Scientific Reports.
Results
All biometric data of the 34 fetuses of the control and anencephalic group are reported in Table 1. The statistical analysis of all fetal biometric parameters and uterine measurements is reported in Table 2.
The gestational age of fetuses ranged from 12 to 22 weeks post-conception (WPC). The normocephalic group’s average gestational age was 17 WPC while for the anencephalic group it was 15 WPC. This difference was not statistically significant (p-value 0.0819).
The fetuses’ weight ranged from 22 to 248 g (g). The normocephalic group’s average weight was 170.64 g while for the anencephalic group it was 79.917 g. This difference was statistically significant (p-value 0.0017).
The measurement of the crown-rump length (CRL) of the fetuses in the whole sample ranged from 7 to 20 cm (cm). The normocephalic group’s average CRL was 13.95 cm, while for the anencephalic group it was 9.75 cm, a difference that was statistically significant (p-value 0.0015).
We did not identify statistical significance between the groups for the measurements of uterus length (Control: 2.02–6.36 mm/mean = 3.59 mm/SD+ −1.43 vs. Anencephalic: 1.71–5.44 mm/mean = 2.97 mm/SD + −1.21, p = 0.1070) and uterus width (Control: 1.99–7.91 mm/mean = 3.84 mm/SD+ −1.27 vs. Anencephalic: 2.08–6.48 mm/mean = 3.65 mm/SD+ −1.23; p = 0.3360).
When the uterine length and weight data were normalized to the fetal weight in each case, and then compared we calculate two new variables: uterine length/fetal weight × 100 and uterine width/fetal weight × 100. We identify significant differences between the uterus length (mm)/weight (g) × 100 (p = 0.0046) and uterus width (mm)/weight (g) × 100 (p = 0.0013) when we compare the control with the anencephalic group. The summary of the findings regarding the correlations studied between the uterus length (mm)/weight (g) × 100 (p = 0.0046) and uterus width (mm)/weight (g) × 100 (p = 0.0013) in the normal and anencephalic groups is reported in Table 2.
The summary of the findings regarding the correlations studied in the normal and anencephalic groups is reported in Table 3. The linear regression analysis indicated that 80% significance was found in the correlations in normocephalic fetuses (12.9 to 22.6 WPC) and 40% significance in anencephalic fetuses (12.3 to 18.6 WPC) during the period studied.
The gestational age (WPC) was correlated with the length of the uterus (mm) in the control group (12.9–22.6 WPC) (y = 32.44x − 2.015) and anencephalic group (12.3–18.6 WPC) (y = 39.71x – 3.190). The results showed that gestational age was significantly and positively correlated with the uterine length of the normal (control) and anencephalic fetuses. The gestational age (WPC) was correlated with the width of the uterus (mm) in the control group (12.9–22.6 WPC) (y = 19.31x + 50.89) and anencephalic group (12.3–18.6 WPC) (y = 24.211x – 11.25). The results showed that the correlation of gestational age with the uterine width of the normal (control) and anencephalic fetuses was not statistically significant (Fig. 2 and Table 3).
The figure shows the linear regression analysis comparing the biometric data of the uterus with fetal age (WPC), weight (g) and crown-rump length (cm). (A) Uterus width × fetal age: the linear regression analysis shows non-significant correlation between fetal age and uterine width in normal and anencephalic groups; (B) uterus length × fetal age: the linear regression analysis shows a significant and positive correlation between fetal age and uterine length in normal and anencephalic groups; (C) uterine width × fetal age: the linear regression analysis shows a significant and positive correlation between fetal weight and uterine width in the normal and anencephalic groups; (D) uterine length × fetal weight: the linear regression analysis shows a significant and positive correlation between fetal weight and uterine length in the normal group, but not significant in the anencephalic group; (E) uterine width × crown-rump length: the linear regression analysis shows a significant and positive correlation between fetal weight and uterine length in the anencephalic group, but not significant in the normal group and (F) uterine length × crown-rump length: the linear regression analysis shows a significant and positive correlation between fetal weight and uterine length in the normal group, but not significant in the anencephalic group.
The fetal weight was correlated with the length of the uterus (mm) in the control group (12.9–22.6 WPC) (y = 0.00864x + 2.113) and anencephalic group (12.3–18.6 WPC) (y = 0.004112x + 2.646). The results showed that fetal weight was significantly and positively correlated with the uterine length of the normal group, but not for the anencephalic group. The fetal weight was correlated with the width of the uterus (mm) in the control group (12.9–22.6 WPC) (y = 0.006672x + 2.706) and anencephalic group (12.3–18.6 WPC) (y = 0.009322x + 2.901). The results showed that fetal weight was significantly and positively correlated with the uterine length of the normal group and the anencephalic group (Fig. 2 and Table 3).
The CRL (cm) was correlated with the length of the uterus (mm) in the normal fetuses (12.9–22.6 WPC) (y = 0.2142x + 0.5989) and anencephalic fetuses (12.3–18.6 WPC) (y = 0.06239x + 2.366). The results showed that CRL was significantly and positively correlated with the uterine length of the normal group, but not of the anencephalic group. The CRL was correlated with the width of the uterus (mm) in normal fetuses (12.9–22.6 WPC) (y = 0.1436x + 1.841) and anencephalic fetuses (12.3–18.6 WPC) (y = 0.2579x + 1.132). CRL was significantly and positively correlated with the uterine width of the anencephalic group, but not of the normal group (Fig. 2 and Table 3).
Discussion
In normal fetuses, GnRH-containing neurons are present in the human brain at the end of the first month of pregnancy22. Hypophysis is able to synthesize gonadotropins from at least 9 weeks of fetal life23,24, so the release of gonadotropins into the fetal circulation can be demonstrated by the beginning of the second trimester. Circulating luteinizing hormone (LH) and follicle stimulating hormone (FSH) levels were significantly higher in female than in male fetuses at mid-gestation25.
Differentiation of the genital tract depends on metabolic pathways initially orchestrated by the presence/absence of the SRY gene and by the action of anti-Müllerian hormones (AMH) and testosterone. Thus, it is expected that a female embryo, genetically XX, will not susceptible to the direct action of anti-Müllerian hormones (AMH) and testosterone.
Fetal ovaries are unable to secrete AMH, but their ovarian expression can be detected by immunohistochemistry at the time of the third trimester, suggesting that AMH plays a role in ovarian development as early as the fetal period16,26. Circulating FSH concentrations in female fetuses are high at mid-gestation, then decrease to low levels at birth, but transiently increase again during postnatal pituitary activation27. In premature girls, extremely high postnatal levels of FSH have been described, indicating an alteration in pituitary-ovarian function in infancy27,28.
In this sense, since the exposure of the central nervous system to amniotic fluid causes irreversible damage to the encephalic tissue, we could assume that the primary hormonal axis would be compromised in anencephalic fetuses, which could reflect the abnormal development of the reproductive organs1,9.
This correlation would be more assertive for male fetuses since the fetal ovaries are not thought to play any part in female sex differentiation, because they secrete little estrogen, even though follicles begin to develop at about 16 weeks and primordial follicles containing granulosa cells are present by 20 weeks16. It has been described that female external genitalia development is not subject to fetal gonadal hormones as in male fetuses29,30.
In male fetuses, the comparative study of the development of the genital tract of normal and anencephalic fetuses has been conducted over the years. Zondek & Zondek, during the 1960s, observed that the prostate showed marked, and in some instances extreme, metaplastic changes, sometimes even surpassing the appearance in normal controls7. In another paper, those researchers noted that the volume of the testis was smaller than that of controls with similar periods of development12.
In our department, Pires et al. observed that the testicular volume of anencephalic fetuses did not increase with fetal age, and developed more slowly than in normal fetuses. On the other hand, the same research team did not observe significant differences in development of the prostate in fetuses with anencephaly6,9. In the same sense, Carvalho and colleagues, using histological and immunolabeling techniques, concluded that anencephaly does not cause structural alterations in the fetal penis8.
As for the female fetuses, Baker and Scrimgeour demonstrated that gonadal development was almost identical in the ovaries of anencephalic and control fetuses31. Zondek and Zondek reported a different result, suggesting that the volume of the ovary in anencephalics was larger than that of the controls up to 36 weeks of gestation and somewhat smaller in the last month of pregnancy; with no marked degree of hypoplasia12.
Previous data from our department showed no differences in vaginal morphology, but the vaginal length and width were smaller in the anencephalic group during the second trimester of pregnancy11. Changes were perceived in relation to the external genitalia, with anencephalic fetuses tending to have more rudimentary external genitalia, with a reduction in anatomical distances from some reference points: length and width of the clitoris, length and width of the vaginal introitus, and distance between the labia majora and the clitoris-anus distance. Despite these findings, there was no significant change in the distance between the vaginal opening and the anus of these fetuses10.
Comparative studies of the uterus between normal and anencephalic fetuses are scarce in the literature. Zondek and Zondek evaluated genital structures of female anencephalic fetuses, stillborn in the third trimester. Their histological investigation demonstrated normal uterine development, with vascular, stromal and glandular proliferation, glandular secretion present in varying degrees, and full development during the last month of pregnancy32.
The present paper reports the first normative parameters of uterine development during the second gestational trimester in human fetuses. The statistical analysis of our measurements indicates that the uterine diameters were different between the evaluated groups. The measurements of the uterus were significantly greater in anencephalic group and the linear regression analysis indicated that 80% significance was found in the correlations in control group and 40% significance in anencephalic fetuses but without statistical difference in the uterine width and length growth curves during the 2nd gestational trimester. We can speculate that NTDs could have impact on uterine development during the human gestational period probably because of commitment of the primary hormonal axis in anencephalic fetuses and the consequent alteration in the growth of organs of the genital system in fetuses as evidenced in previous studies5,9,10. However we need more studies including ultrastructural and sophisticated histological analysis, providing better assessment of these changes in fetuses of the 3rd gestational trimester to support this hypothesis.
Uterine development could be influenced by other factors independently of anencephaly. It is assumed that the Vangl2 gene is vital for neural tube closure (mainly a recessive effect) and independently for female reproductive tract development (a dominant effect). In a previous experimental study, heterozygotes for the Vangl2-Lp mutation develop mild spinal neural tube defects but also show a high frequency of imperforate vagina33. Moreover, it is important to make clear that the case of Vangl2 mutants in mice is just an example, where the primary NTD effect appears separate from the effect on the female genital tract. There may well be other genes or environmental factors that also have a similar effect.
Some limitations of our study should be mentioned: (a) the unequal WPC of the anencephalic fetuses and the control group; (b) the lack of 3rd trimester fetuses to confirm the uterine growth pattern in the two groups studied; (c) the lack of uterine histopathological analysis in our sample; (d) the small sample size (however, anencephalic fetuses are rare, so the observations of a small sample are still relevant); and (e) measurement of uterine biometric parameters by a single observer, potentially leading to measurement bias.
Conclusions
The comparative study of uterine dimensions between normal and anencephalic fetuses indicated differences between the two groups. The measurements of the uterus were greater in anencephalic group but there are no difference in the uterine width and length growth curves during the period studied. Further studies are required to support the hypothesis suggesting that anencephaly may affect uterine development during the human fetal period. We reiterate that the translational aspects of the anencephalic model are promising in the fields of embryology, fetal medicine, neonatology and pediatric urology.
Abbreviations
- NTDs:
-
Neural tube defects
- WPC:
-
Weeks post-conception
- CRL:
-
Crown-rump length
- LH:
-
Luteinizing hormone
- FSH:
-
Follicle stimulating hormone
- AMH:
-
Anti-Müllerian hormones
- CAAE:
-
Certificate of Ethical Appreciation Presentation
- CNPQ:
-
National Council for Scientific and Technological Development
- FAPERJ:
-
Rio de Janeiro State Research Fundation
- IRB:
-
Institutional Review Board
- mm:
-
Millimeters
- mm2 :
-
Square millimeters
- p:
-
P-value
- r2 :
-
Pearson correlation coefficient
- SD:
-
Standard deviation
- Vs.:
-
Versus
References
Blatter, B. M., van der Star, M. & Roeleveld, N. Review of neural tube defects: Risk factors in parental occupation and the environment. Environ. Health Perspect. 102, 140–145 (1994).
Diniz, A., Rodrigues, N., Sampaio, F. & Favorito, L. A. Study of the renal Parenchymal volume during the human fetal period. Int. Braz. J. 45, 150–160 (2019).
Diniz, A., Sampaio, F. & Favorito, L. A. Anencephaly alters renal parenchymal volume in human fetuses?. Int. Braz. J. Urol. 46, 1075–1081 (2020).
Costa, S. et al. Study of the ureter structure in anencephalic fetuses. Int. Braz. J. Urol. 39, 853–860 (2013).
Pazos, H. M. et al. Do neural tube defects lead to structural alterations in the human bladder?. Histol. Histopathol. 26, 581–588 (2011).
Favorito, L. A., Pires, R. S., Gallo, C. M. & Sampaio, F. Study of prostate growth in prune belly syndrome and anencephalic fetuses. J. Pediatr. Surg. 55, 2221–2225 (2020).
Zondek, L. H. & Zondek, T. The human prostate in anencephaly. Acta Endocrinol. 64, 548–556 (1970).
de Carvalho, J. P., Costa, W. S., Sampaio, F. J. & Favorito, L. A. Anencephaly does not cause structural alterations in the fetal penis. J. Sex. Med. 9, 735–742 (2012).
Pires, R. S., Gallo, C. M., Sampaio, F. J. & Favorito, L. A. Do prune-belly syndrome and neural tube defects change testicular growth? A study on human fetuses. J. Pediatr. Urol. 15(557), e1-557.e8 (2019).
Vieiralves, R. R., Ribeiro, G. S. Jr., Alves, E. F., Sampaio, F. J. & Favorito, L. A. Are anogenital distance and external female genitalia development changed in neural tube defects? Study in human fetuses. J. Pediatr. Urol. 16(654), e1-654.e8 (2020).
Ribeiro-Julio, G. S., Vieiralves, R. R., Sampaio, F. J., Gallo, C. M. & Favorito, L. A. Vaginal development during 2nd gestational trimester: Translational study in human female fetuses with disorders of the neural tube. Arch. Gynecol. Obstet. https://doi.org/10.1007/s00404-021-06357-4 (2022).
Zondek, L. H. & Zondek, T. Ovarian hilar cells and testicular Leydig cells in anencephaly. Biol. Neonate 43, 211–219 (1983).
Tuchmann-duplessis, H. Etude des glandes endocrines des anencéphales; déduction sur les corrélations hypophyso-nerveuses du foetus humain [Study of the endocrine glands in anencephalus; data on pituitary-nervous system correlations of the human fetus]. Biol. Neonatorum Neonatal Stud. 1, 8–32 (1959).
Gray, E. S. & Abramovich, D. R. Morphologic features of the anencephalic adrenal gland in early pregnancy. Am. J. Obstet. Gynecol. 137, 491–495 (1980).
Osamura R.Y. Functional prenatal development of anencephalic and normal anterior pituitary glands. In human and experimental animals studied by peroxidase-labeled antibody method. Acta Pathol. Japonica 27, 495–509 (1977).
Warne, G. L. & Kanumakala, S. Molecular endocrinology of sex differentiation. Semin. Reprod. Med. 20, 169–180 (2002).
Robbins, J. B., Broadwell, C., Chow, L. C., Parry, J. P. & Sadowski, E. A. Müllerian duct anomalies: Embryological development, classification, and MRI assessment. J. Magnet. Resonan. Imaging (JMRI) 41, 1–12 (2015).
Hern, W. M. Correlation of fetal age and measurements between 10 and 26 weeks of gestation. Obstet. Gynecol. 63, 26–32 (1984).
Mercer, B. M., Sklar, S., Shariatmadar, A., Gillieson, M. S. & D’Alton, M. E. Fetal foot length as a predictor of gestational age. Am. J. Obstet. Gynecol. 156, 350–355 (1987).
Platt, L. D. et al. Fetal foot length: relationship to menstrual age and fetal measurements in the second trimester. Obstet. Gynecol. 71, 526–531 (1988).
Tello, C., Liebmann, J., Potash, S. D., Cohen, H. & Ritch, R. Measurement of ultrasound biomicroscopy images: intraobserver and interobserver reliability. Invest. Ophthalmol. Vis. Sci. 35, 3549–3552 (1994).
Winters, A. J., Eskay, R. L. & Porter, J. C. Concentration and distribution of TRH and LRH in the human fetal brain. J. Clin. Endocrinol. Metab. 39, 960–963 (1974).
Hagen, C. & McNeilly, A. S. The gonadotrophins and their subunits in foetal pituitary glands and circulation. J. Steroid Biochem. 8, 537–544 (1977).
Levina, S. E. Endocrine features in development of human hypothalamus, hypophysis, and placenta. Gen. Comp. Endocrinol. 11, 151–159 (1968).
Beck-Peccoz, P. et al. Maturation of hypothalamic-pituitary-gonadal function in normal human fetuses: Circulating levels of gonadotropins, their common alpha-subunit and free testosterone, and discrepancy between immunological and biological activities of circulating follicle-stimulating hormone. J. Clin. Endocrinol. Metab. 73, 525–532 (1991).
Kuiri-Hänninen, T. et al. Postnatal developmental changes in the pituitary-ovarian axis in preterm and term infant girls. J. Clin. Endocrinol. Metab. 96, 3432–3439 (2011).
Tapanainen, J., Koivisto, M., Vihko, R. & Huhtaniemi, I. Enhanced activity of the pituitary–gonadal axis in premature human infants. J. Clin. Endocrinol. Metab. 52, 235–238 (1981).
Greaves, R. F., Hunt, R. W., Chiriano, A. S. & Zacharin, M. R. Luteinizing hormone and follicle-stimulating hormone levels in extreme prematurity: Development of reference intervals. Pediatrics 121, e574–e580 (2008).
Flück, C. E. et al. Why boys will be boys: two pathways of fetal testicular androgen biosynthesis are needed for male sexual differentiation. Am. J. Hum. Genet. 89(2), 201–218 (2011).
Makiyan, Z. Studies of gonadal sex differentiation. Organogenesis 12, 42–51 (2016).
Baker, T. G. & Scrimgeour, J. B. Development of the gonad in normal and anencephalic human fetuses. J. Reprod. Fertil. 60, 193–199 (1980).
Zondek, L. H. & Zondek, T. Reproductive organs in anencephaly with special reference to the uterus. Biol. Neonate 51, 346–351 (1987).
Guyot, M.C., Bosoi, C.M., Kharfallah, F., Reynolds, A., Drapeau, P., Justice, M., Gros, P., & Kibar, Z. A novel hypomorphic looptail allele at the planar cell polarity Vangl2 gene. Dev. Dyn. 240, 839–849 (2011).
Author information
Authors and Affiliations
Contributions
A.L.D.: Project development, Data Collection, Manuscript writing. R.R.V.: Project development, Data Collection, Manuscript writing. C.M.G.: Statistics, Manuscript writing, Data Collection. L.A.F.: Project development, Data Collection, Manuscript writing. F.J.B.S.: Project development, Data Collection, Manuscript writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Diniz, A.L.L., Vieiralves, R.R., Sampaio, F.J.B. et al. Neural tube defects and uterus development in human fetuses. Sci Rep 12, 14051 (2022). https://doi.org/10.1038/s41598-022-18431-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-18431-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.