Abstract
For millennia, Aspilia africana has been used across Africa to treat various diseases including malaria, wounds, and diabetes. In this study, temperature influenced the in vitro germination of A. africana with highest final germination percentage (FGP) and germination index (GI) of 65.0 ± 7.64% and 2.26 ± 0.223, respectively, at 19.8 °C. Priming seeds with H2O, KNO3, and GA3 (gibberellic acid 3) improved both in vitro germination and ex vitro emergence of A. africana seeds. Seed priming with \(1.44 \times 10^{ - 3 }\) M GA3 produced overall highest in vitro FGP (from 90.0 ± 4.08% to 100 ± 0.00%) and GI (from 2.97 ± 0.385 to 3.80 ± 0.239) across all priming durations. Seeds primed with KNO3 had better germination parameters for 6 and 12 h compared to 18 and 24 h. Furthermore, the highest in vitro FGP (100 ± 0.00%) was observed in seeds primed for 12 h with \(1.44 \times 10^{ - 3 }\) M GA3. Ex vitro A. africana seed emergence was significantly enhanced by GA3 priming. Priming A. africana seeds with H2O, KNO3, and GA3 improved their growth after 3 months, with the overall best growth for seeds primed with \(2.89 \times 10^{ - 4 }\) M GA3. Seed priming of A. africana is a feasible approach for improving germination and seed emergence, and enhancing plant growth.
Similar content being viewed by others
Introduction
Aspilia africana (Pers.) C. D. Adams, also known as wild sunflower, hemorrhage plant, or African iodine plant, has been used for millennia to treat several diseases across many countries in Africa1,2. Diseases and health conditions treated using A. africana include malaria, osteoporosis, tuberculosis, febrile headaches, diabetes, stomach ache, cough, rheumatic pains, measles, diarrhea, ear infections, wounds, sores, gastric ulcers, gonorrhea, and stings from bees, wasps and scorpions3,4,5. In a recent study, Niyonizigiye, et al.6 demonstrated the plant’s anti-cancer activity. The biological activity of the plant is attributed to its richness in secondary metabolites such as phenolic compounds (including chlorogenic acid and gallic acid), flavonoids (e.g., quercetin), tannins, saponins, and terpenes (such as caryophyllene, phytol, and pinene)1,5. A. africana, although indigenous to Eastern African counties, inhabits forest zones of tropical Africa and the savanna5,7.
Apart from soil moisture, the most vital abiotic factor that greatly influences seed germination is temperature8. The effects of temperature on germination vary across species or even among the seeds of a species from different provenances8,9. Temperature not only influences germination but also greatly regulates growth and development in plants10,11. The temperature at which germination percentage is highest is termed as the optimum temperature and this varies from one species to another8,10. Understanding the emergence and germination responses of plants to temperature is critical as it not only provides a basis for temperature tolerance identification but also provides an understanding of optimal climatic conditions for germination and successful establishment of plants10 in addition to assisting in model construction to predict developmental processes12.
Seed priming is a widely used low-cost pre-sowing strategy for improving imbibition and inducing DNA repair processes and antioxidant responses linked to pre-germinative metabolism without radicle protrusion13,14,15. After the seeds are primed, they are dehydrated, stored, or commercialized13. Different priming techniques, such as osmopriming, hydropriming, chemical priming, hormonal priming, and nutrient priming have been employed to improve seed germination and crop yield16. Seed priming enhances germination and results in fast and uniform emergence of plants13. Furthermore, priming increases tolerance of plants to abiotic and biotic stresses, greatly improving plant population density and performance13.
A. africana is not only a plant of great cosmetic and pharmaceutical interests5,7 but is also highly browsed by domestic animals such as cattle, goats, rabbits, and sheep17. A recent study by Okello, et al.18 on the effects of different commercial soils on the germination of A. africana indicated a very low plant germination rate. In this study, we investigated the effects of temperature and seed priming conditions on the germination of A. africana. This contributes to improving seed germination of this important medicinal plant and its domestication. To the best of our knowledge, this is the first study on the effects of temperature and seed priming on the germination, emergence, and growth of A. africana.
Materials and methods
Seed material and sterilization
Mature, dry, and ripe A. africana seeds randomly collected with permission from the local authority from at least 50 healthy plants in the wild from Pece, located in Gulu, Uganda, East Africa, and were provided by the Natural Chemotherapeutics Research Institute (NCRI). The seeds were transported to the Korea Institute of Oriental Medicine, Republic of Korea, and stored in a dry room maintained at 25 ± 1 °C until the start of the experiment. A voucher specimen (number KYM-KIOM-2021-1) was deposited at the Korean Herbarium of Standard Herbal Resources (Index Herbarium code: KIOM) at the Korea Institute of Oriental Medicine (KIOM), Herbal Medicine Resources Research Center, Republic of South Korea by Dr. Sungyu Yang. The seeds used in this experimental work were obtained from A. africana var. africana plants. To limit the tendencies of contamination, the seeds were sterilized as follows: the seeds were first washed under running tap water for 2 min, quickly transferred to a laminar flow cabinet, re-washed with distilled autoclaved water, surface sterilized in 70% (v/v) ethanol for 1 min, followed by 2% (v/v) sodium hypochlorite for 3 min, and then rinsed three times with distilled autoclaved water. Sterilized A. africana seeds were used in all in vitro experimental setups. A summary of the sterilization process, in vitro seed arrangement and illustration of the in vitro seed arrangement are shown in Fig. 1a, a1 and a2, respectively. All methods were carried out in accordance with relevant guidelines and regulations.
(a) Summary of sterilization process of A. africana seeds. (a1) Arrangement of A. africana seeds in a Petri dish (a2) Illustration of arrangement of A. africana seeds in a Petri dish (b) Seeds of A. africana in Petri dishes incubated in a thermogradient germinator at different temperatures (1–17.6 °C, 2–18.7 °C, 3–19.8 °C, 4–20.9 °C, 5–22.0 °C, 6–23.1 °C, 7–24.2 °C, 8–25.3 °C, 9–26.4 and 10–27.5 °C.
Experiment 1: effect of temperature on in vitro germination of A. africana seeds
A. africana seeds were placed using sterile forceps in crystal-grade polystyrene Petri dishes (100 × 20 mm) containing filter papers moistened with 5 ml distilled water. Each Petri dish contained 10 seeds with six replicates for each temperature, ranging from 17.6 to 27.5 °C with an increment of 1.1 °C, and dishes were placed on a thermogradient germinator chamber under dark conditions (Fig. 1b).
The seeds were monitored for germination every 24 h for 15 d. The Petri dishes with seeds were only exposed to light for a short time during the counting of the germinated seeds. Seeds with a minimum of 2 mm radicle length were counted as germinated. The germination parameters considered for determining the effects of temperature on seed germination of A. africana were final germination percentage (FGP), germination index (GI), mean germination rate (MGR), and time required for 50% germination (T50). The same parameters were used to investigate the effects of priming treatments and durations on the in vitro and ex vitro germination of A. africana seeds. The following formulae were used to calculate the germination parameters: FGP = \(\frac{N}{Nt}\) × 100 (N is the number of germinated seeds at the final count; Nt is the total number of seeds in the Petri dish); GI = ∑ (Gt/Dt) (Gt is the number of germinated seeds on day t, and Dt is time corresponding to Gt in days); and MGR = \( \frac{(\sum n)}{{\sum (nt)}}\) (n is the number of newly germinated seeds at time t, and t is the number of days from planting). T50 was calculated according to the formula modified from Farooq, et al.19, T50 = ti + \(\frac{{\left[ {\left( {\frac{N}{2} - n_{i} } \right)\left( {t_{j} - t_{i} } \right)} \right]}}{{n_{j} - n_{i} }}\) (N is the final number of germinated seeds, ni and nj are the cumulative number of germinated seeds counted at time ti and tj, respectively, when ni < \(\frac{N}{2}\) < nj).
Experiment 2: effect of priming duration and priming treatments on in vitro germination of A. africana seeds
Sterilized A. africana seeds were primed at varying times of 6 h, 12 h, 18 h, and 24 h in different priming solutions, such as distilled water (hydro-priming) and potassium nitrate (halo-priming) at different concentrations (0.1, 0.5, and 1 M) and gibberellic acid-3 (GA3; hormo-priming) at concentrations of \(2.89 \times 10^{ - 5 }\), \(2.89 \times 10^{ - 4 }\), and \(1.44 \times 10^{ - 3 }\) M. For priming treatments, 60 seeds were placed in 5 ml of each priming solution in crystal-grade polystyrene Petri dishes (100 × 40 mm). Upon completion of the treatment, the primed seeds were rinsed three times with sterile water, blotted, and dried back to their near initial weight at ambient temperature. The seeds were then placed in crystal-grade polystyrene Petri dishes (100 × 20 mm) containing filter paper saturated with autoclaved distilled water. Each Petri dish (100 × 20 mm) contained 10 seeds with five replicates for each treatment. The Petri dishes containing the seeds were kept in a thermogradient germinator chamber maintained at 19.8 °C (determined to be ideal from experiment 1) in darkness. Non-primed A. africana seeds were used as a control. Seeds showing signs of fungal contamination were removed from the Petri dishes. The seeds were monitored for germination every 24 h for 15 d and were only exposed to light for a short time during the counting of germinated seeds. Seeds with a minimum of 2 mm radicle length were counted as germinated. The same parameters and formulae used to assess germination in Experiment 1 were used in Experiments 2 and 3.
Experiment 3: effect of different priming treatments on ex vitro emergence and early growth of A. africana plants
To examine the effect of various priming treatments on the ex vitro emergence of A. africana seeds, the priming procedure used in Experiment 2 was repeated, except that seeds were treated with the priming solutions for a uniform priming duration of 12 h. The primed seeds were then planted in an autoclaved mixture of horticulture soil (consisting of about 40% mineral soil, 10% organic matter and 50% pore space filled by water and air) containing perlite (Kyungdong ONE Co. Ltd, Republic of Korea), one of the natural volcanic aluminosilicate glasses20 and peat pellet soil (Jiffy-7, 33 mm from Jiffy Products International AS, Norway) in a 1:1 ratio (determined as an ideal composition for the growth of A. africana18) in a plastic planting tray (30 × 25 × 10 cm). Twenty seeds of A. africana were planted in each tray at a depth of 1 cm and a distance of 5 cm from each other. Each treatment was replicated three times. The seeds in the soil were watered and the trays were kept in growth chambers maintained at 19.8 ± 1 °C for a 16 h photoperiod. Light intensity was maintained at 33.73 µmol/m2/s using cool white fluorescent tubes. The relative humidity in the growth chamber was maintained at 70%. The seeds in the tray were watered every two days until the end of the experiment. The number of seeds germinated every 24 h was counted and recorded for each treatment until no further emergence occurred. Seeds were counted as emerged when the hypocotyl length was at least 3 mm. The FGP, MGR, T50, and GI were calculated. To determine the effects of different priming treatments on the early growth of A. africana, seedlings from the differently primed seeds were uniformly re-spaced and allowed to continue growing in the growth chambers, and the growth rates were determined after three months. Each planting tray was carefully immersed in water to soak the soil, enabling easy uprooting of the plants. The roots of the uprooted plants were carefully and thoroughly washed to remove soil particles and debris, and then blotted dry with paper towels. The lengths of roots and shoots of each A. africana plant from the different treatments were measured using a meter ruler. The number of leaves and roots of each plant were counted. Fresh weights of the A. africana plants from the different treatments were obtained. Thereafter, the plants were oven dried at 60 °C for 48 h and their dry weights were recorded.
Results
Effect of temperature on in vitro germination of A. africana seeds
With respect to FPG, the germination response of A. africana seeds across all temperatures was better at lower temperatures than at higher temperatures (Fig. 2a). The highest FGP and GI of 65.0 ± 7.64% and 2.26 ± 0.223, respectively, were attained at 19.8 °C, although these did not vary significantly from the values recorded at other temperatures (Fig. 2a, b). The lowest FGP (38.3 ± 6.01%) and GI (1.48 ± 0.150) values were obtained at 27.5 °C and 17.6 °C, respectively (Fig. 2a, b). A. africana seed germination was faster at higher temperatures than at lower temperatures with increasing MGR (highest (0.385 ± 0.050) at 27.5 °C) and decreasing T50 (longest (2.79 ± 0.121 days) at 17.6 °C) (Fig. 2c, d). Similar to FGP and GI, the MGR and T50 values across all temperatures investigated did not differ significantly (Fig. 2a–d).
Effect of temperature on in vitro seed germination parameters of A. africana. (a) Final Percentage Germination (b) Germination Index (c) Mean Germination Rate (d) time required for 50% germination (T50). Values are presented as means ± standard error. ns-not statistically significant by Tukey’s multiple comparison test and p = 0.05.
Effect of priming duration and priming treatments on in vitro germination of A. africana seeds
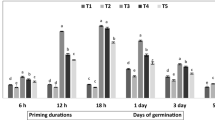
The FGP values of all primed seeds were higher than those of non-primed seeds (control) (Table 1). Among the priming treatments and across all priming durations, the highest FGPs were recorded for seeds primed with \(1.44 \times 10^{ - 3 }\) M GA3, followed by A. africana seeds primed with 0.1 M KNO3, and the lowest FGPs were recorded for hydro-primed seeds (Table 1). The overall highest FGP was 100 ± 0.00% for 12 h priming with \(1.44 \times 10^{ - 3 } \;{\text{M}} \) GA3 and was significantly higher (p < 0.05) than other FGPs across all treatments, except for A. africana seeds primed in \(1.44 \times 10^{ - 3 }\) M GA3 for 18 h (97.5 ± 2.17%) and 24 h (97.5 ± 2.50%) and 0.1 M (87.5 ± 6.29%) and 0.5 M (90.0 ± 7.07%) KNO3 for 6 and 12 h, respectively (Table 1). Priming duration of 12 h resulted in the highest and second highest FGPs in three (100 ± 0.00% in \(1.44 \times 10^{ - 3 }\) M GA3, 90.0 ± 7.07% and 77.5 ± 6.29% in 0.5 and 1.0 M KNO3, respectively) and two (82.5 ± 2.50% in 0.1 M KNO3 and 65.0 ± 2.89% in H2O) treatments, respectively, of all seven priming treatments, and had the highest FGP among all priming durations (Table 1). Highest GI (3.80 ± 0.239) was recorded in seeds primed for 24 h in GA3 and this significantly differed from other GI across all treatments apart from GI for \(1.44 \times 10^{ - 3 } \;{\text{M}}\) GA3 for 6 h (2.97 ± 0.385), 12 h (3.08 ± 0.090), and 18 h (3.25 ± 0.034); \(2.89 \times 10^{ - 4 } \;{\text{M}}\) GA3 for 18 h (2.74 ± 0.238) and 24 h (2.99 ± 0.558); \(2.89 \times 10^{ - 5 }\) M GA3 for 18 h (2.58 ± 0.222) and 24 h (3.31 ± 0.309); 0.1 M KNO3 for 6 h (2.46 ± 0.312); and H2O for 24 h (2.60 ± 0.417) (Table 1). In most cases, seeds primed in 1.0 M KNO3 had the lowest GI, with the lowest values for priming duration of 24 h (0.43 ± 0.093) (Table 1). The GI value improved with increase in priming duration for all concentrations of GA3 and H2O, but the reverse was true for KNO3 (Table 1). The highest MGR was recorded in seeds primed with \(1.44 \times 10^{ - 3 } \;{\text{M}}\) GA3 for 24 h (Table 1). The T50 values for all concentrations of GA3-primed seeds decreased with an increase in priming duration (Table 1). There were no significant differences in T50 values across all GA3 priming concentrations and durations and across hydro-priming at all durations; however, these values were significantly higher (p < 0.05) than T50 for all KNO3 treatments, except for the treatments with 0.1 M concentrations at 6 and 18 h priming durations.
Effect of different priming treatments on ex vitro seed emergence
FGPs of GA3-primed seeds were generally higher than those of halo- and hydro-primed seeds, with the highest overall FGP (80.0 ± 5.77%) recorded for \(1.44 \times 10^{ - 3 }\) M GA3 primed seeds (Fig. 3a). Non-primed seeds had the lowest FGP (20.0% ± 2.89%), while the lowest FGP among primed seeds (45.0 ± 5.00%) was for the 1.0 M KNO3 treatment (Fig. 3a). There were no significant differences in FGP among all concentrations of GA3 and H2O and 0.1 M KNO3, but the FGPs of these treatments were significantly higher (p < 0.05) than FGPs for 0.5 M and 1.0 M KNO3 primed and non-primed seeds (Fig. 3a). GI in hormo-primed seeds was significantly higher (p < 0.05) than that in all other treatments, except for hydro-primed seeds, with the highest GI at 3.76 ± 0.434 in the \(1.44 \times 10^{ - 3 }\) M GA3 primed seeds (Fig. 3b). GI values decreased with an increase in KNO3 concentration (Fig. 3b). The lowest GI value was recorded in the non-primed A. africana seeds (Fig. 3b). Fastest MGRs were attained in hormo-primed seeds, but did not significantly differ from MGRs of hydro-primed seeds. However, they did differ from MGRs of halo-primed and non-primed seeds (Fig. 3c). The lowest T50 values were recorded in GA3-primed seeds, followed by hydro-primed seeds (Fig. 3d). In contrast, the longest germination periods with the highest T50 values were for the halo-primed seeds (Fig. 3d). There were no significant differences in the T50 values among all hormo-primed and hydro-primed seeds (Fig. 3d). T50 for all hormo- and hydro-primed seeds was significantly shorter (p < 0.05) than that of all halo-primed and non-primed seeds (Fig. 3d).
Effect of priming treatment on ex vitro seed emergence parameters of A. africana. (a) Final Percentage Germination (b) Germination Index (c) Mean Germination Rate and (d) time required for 50% germination (T50). Values are presented as means ± standard error. Same letters are not significantly different by Tukey’s multiple comparison test and p = 0.05.
Effect of different priming treatments on the early growth of A. africana
A. africana plant growth was highest for seeds primed with GA3 followed by KNO3 and H2O, and lowest for non-primed seeds (Fig. 4). For all the growth parameters analyzed, plants from \(2.89 \times 10^{ - 4 }\) M GA3-primed seeds exhibited the best values, whereas plants from non-primed seeds registered the lowest values for all growth parameters (Fig. 5a–f). All growth parameters of A. africana plants from halo-primed seeds decreased with increasing KNO3 concentrations (Fig. 5a–f).
Comparison of shoot and root lengths of sampled representative A. africana plants derived from hormo-, halo- and hydro primed seeds after three months of growth (a) \(1.44 \times 10^{ - 3 }\) M GA3 (b) \(2.89 \times 10^{ - 4 }\) M GA3 (c) \(2.89 \times 10^{ - 5 }\) M GA3 (d) 1.0 M KNO3 (e) 0.5 M KNO3 (f) 0.1 M KNO3 (g) Distilled water (h) Non treated.
Effect of priming treatment on early growth of A. africana. Growth parameters measured after three months of growth. (a) Shoot length (b) Number of leaves (c) Root length (d) Number of roots (e) Fresh weight (f) Dry weight. Values are presented as means ± standard error. Same letters are not significantly different by Bonferroni’s test and p = 0.05.
The highest average shoot length (333.3 ± 11.71 mm) of A. africana plants from \(2.89 \times 10^{ - 4 }\) M GA3-primed seeds did not vary significantly from the shoot lengths of plants from seeds primed with other concentrations of GA3 and 0.1 M KNO3 but significantly differed (p < 0.05) from the other treatments (Fig. 5a). The highest number of leaves (26.4 ± 1.15) in plants from \(2.89 \times 10^{ - 4 } \;{\text{M}} \) GA3-primed seeds was not significantly different from that of plants from other GA3-primed seeds, but was significantly higher (p < 0.05) than those from hydro- and halo-primed seeds (Fig. 5b). Root lengths of plants from seeds primed with \(2.89 \times 10^{ - 5 }\) and \(1.44 \times 10^{ - 3 }\) M GA3, and 0.1 and 0.5 M KNO3 did not differ significantly from the highest average root lengths (245.0 ± 15.82) of plants from \(2.89 \times 10^{ - 4 } \;{\text{M}}\) GA3-primed seeds, which was significantly higher (p < 0.05) than those from 1.0 M KNO3 primed, hydro-primed, and non-primed seeds (Fig. 5c). The highest number of roots (24.8 ± 1.57) from \(2.89 \times 10^{ - 4 }\) M GA3-primed seeds did not vary significantly from those of other hormo-primed and all halo-primed seeds, but was significantly higher than those from hydro-primed and non-primed seeds (Fig. 5d). Fresh and dry weights of A. africana plants from all hormo-, halo-, and hydro-primed seeds were significantly higher than those from non-primed seeds (Fig. 5e, f). The fresh weights of plants from all primed seeds did not differ significantly (Fig. 5e), whereas the highest dry weight (1.98 ± 0.081 g) from \(2.89 \times 10^{ - 4 }\) M GA3 primed seeds significantly differed from 0.5 M and 1.0 of KNO3 and hydro-primed seeds (Fig. 5f).
Discussion
Temperature is a key factor that significantly affects germination21,22. Temperature directly influences imbibition and biochemical processes involved in germination that regulate metabolism, thus affecting germination rates and percentages21. Several studies have reported the effects of temperature on seed germination in different plants, including medicinal plants23,24,25. According to Baskin and Baskin26, the optimum temperature for many species is between 10 and 20 °C. In our study, low temperatures resulted in low FGPs and GIs for A. africana, and the values increased with temperature to optimal values of 65.0 ± 7.64% and 2.26 ± 0.223, respectively, at 19.8 °C, and further decreased with increase in temperature. This trend has been observed in several other medicinal plant species showing low FPG at low and high temperatures, such as Nepeta binaludensis, Nepeta crassifolia, and Rubia tinctorum23. The percentage germination linearly increases with temperature until an optimum temperature is reached, and then sharply decreases21. Guo, et al.21 further emphasizes that for most perennials, the favorable temperature for germination is 10–20 °C, and the optimum temperature for A. africana lies within this range. As observed, the lowest germination percentages occurred at the highest temperatures. High temperature inhibits germination of seeds in a number of species as it increases the endogenous levels of abscisic acid (ABA) by upregulating genes that biosynthesize ABA and downregulating genes associated with catabolism27,28. Furthermore, high temperatures decrease GA3 content through repression of genes that biosynthesize GA3, thus inhibiting seed germination27,28. The thermoinhibitory effect of ABA has been demonstrated in a number of plant species, including Solanum lycopersicum29, and Pinus bungeana21. The MGR and T50 values increased and decreased, respectively, with increasing temperature. This is presumably because the first phase of seed germination (imbibition) is greatly dependent on temperature and germination increases with increasing temperature30. Imbibition is a critical stage in seed germination, and the process is not only slowed down at low temperatures but also poses a great threat to cell membranes not adapted to low temperature30. Furthermore, the activities of some enzymes, such as dehydrogenases involved in the germination process, were found to increase with temperature30.
The germination parameters for primed seeds for both in vitro and ex vitro experiments were better than those for non-primed seeds. Seed priming is a simple, safe and affordable technique for improving emergence, plant growth and yield31,32,33. Seed priming reduces the effect of abiotic stress during germination leading to higher emergence of seedling and vigorous establishment of seedlings32,33,34. In line with our observations, several studies previously confirmed that priming treatments greatly improved the germination parameters in a number of plants, such as Vicia faba L.35, and lentils36. Seed priming improves several physiological and metabolic processes, including activation of protective enzymes, such as catalase (CAT) and superoxide dismutase (SOD), and accumulation of osmoprotectants37. In a study by Armin, et al.38, KNO3 treatment increased the FGP of sugar-beet seeds by up to 17.87% compared to the control. In another study, priming water melon seeds with KNO3 and water increased FGP and GI39 similar to the observations in our study. Improved germination parameters of seeds with KNO3 priming were also observed for Glycine max40 and Helianthus anuus41 among others. In agreement with our findings for both in vitro and ex vitro investigations, GA3-priming of seeds from other plants, such as Medicago sativa42, and Hibiscus sabdariffa L43. is reported to greatly improve germination.
We observed that seed germination responses to priming were in the order GA3 > KNO3 > H2O. Similar to our observation, in a study on the medicinal plant Foeniculum vulgare, it was reported that GA3 was also superior to other priming agents used, including KNO344. Tahaei, et al.44 explained that GA3 improves germination by upregulating α-amylase activity, eventually improving the metabolism of starch and sugar solubility. Furthermore, GA3 activates embryo growth, reserve mobilization, and endosperm layer weakening, thus greatly improving germination45,46. Additionally, exogenous GA3 was observed to greatly influence radicle protrusion in germinating Arabidopsis seeds46. In agreement with our results, Singh et al.47 also observed that although both KNO3 and H2O priming of seeds improved germination parameters, FGP for KNO3 was better than that for H2O in cow pea. This could have been possible because KNO3 supplied nitrate to the seeds and caused exosmosis that eliminated all germination inhibiting substances47. A similar finding was also reported for sorghum seeds primed with KNO348. Seed priming with KNO3 is known to enhance germination, improve seedling growth, seedling vigor and drought tolerance through increased water imbibition, and activation of enzymes (amylases, xylanase, and dehydrogenases) and numerous ROS-scavenging antioxidants32. At the imbibition stage, seeds take up increased oxygen amount, resulting in accumulation of ROS shifting the redox state49. KNO3 increases the activity of antioxidant enzymes such as SOD, CAT, ascorbate oxidase (AOX), and peroxidase (POX) in seedlings49.
Similar to our in vitro germination study, Damalas, et al.35 reported that faba bean germination parameters were affected by priming duration. In their study, hydro-priming durations of 8 and 16 h had very high FGP and GI, which declined at longer priming durations of 24 and 48 h. Contrary to their findings, in our study, seeds hydro-primed for longer durations showed slightly improved germination, but for KNO3 priming treatments, germination parameters declined at higher concentrations and longer treatment durations. The decline in germination in both our in vitro and ex vitro investigations with increasing concentrations of KNO3 was possibly due to increasing external osmotic pressure, which affected imbibition by the seeds, leading to decreased FGP, decreased GI and MGR, and a longer T50 duration. Oliveira, et al.39 also reported decreased melon seed FGP and GI with increasing salt stress. Osmotic stress affects starch hydrolysis energy production, thus affecting germination39,50. Furthermore, in line with our observation, Ruttanaruangboworn, et al.51 also reported a better germination response of Oryza sativa L. when primed with a lower concentration (1%) of KNO3 than with KNO3 at a higher concentration (2%). Generally, germination parameters improved with increasing GA3 concentration, although there were no significant differences among the GA3-treated seeds for both in vitro and ex vitro investigations. Increasing the concentration of GA3 improves the metabolic and physiological processes during germination. As in our study, priming of Capsicum annum L. seeds in \(1.44 \times 10^{ - 3 }\) M GA3 resulted in the highest FGP of 85.98%52. Inconsistent with our findings, germination of Leymus chinensis seeds was best when primed with GA3 at a concentration of \(5.05 \times 10^{ - 5 }\) M53. Such disparities could be attributed to differences in the species and seed conditions.
Comparing the in vitro and ex vitro germination parameters, the in vitro germination parameters were improved for both primed and non-primed A. africana seeds. Finch-Savage and Bassel54 pointed out that soil is such an intricate environment that exerts considerable stress on germinating seeds and seedlings. Seeds and seedlings are therefore vulnerable to such complexity, including mechanical impedance54.
A. africana seed priming improved plant growth for all priming solutions, with all primed seeds recording increased plant growth compared to non-primed seeds. This observation is in agreement with findings from a number of previous studies35,39,55,56. In fact, Zhu, et al.57 recorded increased root lengths, and fresh and dry stem weights of two Brassica napus L. varieties for all priming solutions when treated with five different priming agents that included GA3. Compared to non-primed seeds, priming causes increased cell division at the apical meristem of roots of seedlings, which eventually promotes growth and development58.
Across all measured parameters, GA3-primed seeds produced plants with the highest growth compared to halo- and hydro- primed seeds. These observations were similar to those of previous research findings39,56. The superiority of GA3 over halo- and hydro-priming could be because GA3 breaks dormancy in seeds, promoting germination, increasing intermodal lengths and cell division in the cambial zone, and also causes an increase in leaf size56,59.
Similar to our findings, increased growth of plants from KNO3 primed seeds has been previously reported38,55,60,61. Thejeshwini, et al.56 pointed out that growth of plants from KNO3 primed seeds was comparable to that of plants from GA3-primed seeds. Seed priming with KNO3 greatly improved soybean plant height, dry weight, seedling shoot, and root lengths62. In another study, KNO3 priming improved plant height, number of leaves, and leaf area among other growth parameters in rice55. Adnan, et al.60 explained the increased growth observed in plants from KNO3 primed seeds as a result of the nitrates that regulate growth and translocate photo-assimilates to specific plant parts, improving growth and yield. Hydro-priming improves the growth of a number of plants35,60. Hydro-priming increases shoot length, root length, and number of roots among other parameters in sorghum60. The shoots of hydro-primed seeds show higher amylase enzyme activity that enhances the hydrolysis of shoot transitory starch, providing more glucose and enabling more growth58.
Conclusion
In this study, in vitro germination level of non-primed A. africana seeds was low across all investigated temperatures. Hydro-, halo-, and hormonal priming greatly improved both in vitro germination and ex vitro emergence of A. africana seeds. For the in vitro setup, seeds primed with \(1.44 \times 10^{ - 3 }\) M GA3 had the highest FGP and GI, and the shortest T50 across all priming durations. Seeds primed in KNO3 had better germination parameters at shorter priming durations compared to longer priming durations. Furthermore, the highest overall FGP was observed for seeds primed for 12 h in \(1.44 \times 10^{ - 3 }\) M GA3. Ex vitro seed emergence was significantly enhanced for seeds primed with GA3 compared to non-primed seeds. In addition, the ex vitro A. africana seed emergence was significantly enhanced with a decrease in KNO3 concentration. Priming A. africana seeds with H2O, KNO3, and GA3 improved their growth parameters. After three months of treatment with \(2.89 \times 10^{ - 4 }\) M GA3, A. africana seeds produced plants with the longest shoot and root lengths, highest number of leaves and roots, and highest fresh and dry weights. In our study, we did not determine the base and ceiling temperatures for seed germination of A. africana, and we recommend further study in this regard. Seed priming of A. africana is a feasible approach to greatly improve germination. This is the first study investigating the effects of temperature and priming treatments on the germination and emergence of A. africana seeds.
References
Okello, D. et al. An in vitro propagation of Aspilia africana (Pers.) CD Adams, and evaluation of its anatomy and physiology of acclimatized plants. Front. Plant Sci. https://doi.org/10.3389/fpls.2021.704896 (2021).
Ajeigbe, K., Onifade, A., Omotoso, D., Enitan, S. & Olaleye, S. Anti-ulcerogenic activity of Aspilia africana leaf extract: Roles of gastric acid, oxidative stress and neutrophil infiltration. Afr. J. Biomed. Res. 17, 193–201 (2014).
Okoli, C. et al. Anti-inflammatory activity of hexane leaf extract of Aspilia africana CD Adams. J. Ethnopharmacol. 109, 219–225 (2007).
Okello, D. & Kang, Y. Exploring antimalarial herbal plants across communities in Uganda based on electronic data. Evid. Based Complement. Altern. Med. 2019, 1–27 (2019).
Okello, D., Lee, J. & Kang, Y. Ethnopharmacological potential of Aspilia africana for the treatment of inflammatory diseases. Evid. Based Complement. Altern. Med. 2020, 1–11 (2020).
Niyonizigiye, I. et al. Characterization and in vitro cytotoxicity of phytochemicals from Aspilia africana obtained using green extraction techniques. S. Afr. J. Bot. 128, 231–238 (2020).
Komakech, R., Matsabisa, M. G. & Kang, Y. The wound healing potential of Aspilia africana (Pers.) CD Adams (Asteraceae). Evid. Based Complement. Altern. Med. 2019, 1–12 (2019).
Kumar, B., Gupta, E., Mali, H., Singh, H. & Akash, M. Constant and alternating temperature effects on seed germination potential in Artemisia annua L. J. Crop Improv. 27, 636–642 (2013).
Verma, S. K., Kumar, B., Ram, G., Singh, H. & Lal, R. Varietal effect on germination parameter at controlled and uncontrolled temperature in Palmarosa (Cymbopogon martinii). Ind. Crops Prod. 32, 696–699 (2010).
Motsa, M. M., Slabbert, M., Van Averbeke, W. & Morey, L. Effect of light and temperature on seed germination of selected African leafy vegetables. S. Afr. J. Bot. 99, 29–35 (2015).
Koger, C. H., Reddy, K. N. & Poston, D. H. Factors affecting seed germination, seedling emergence, and survival of texasweed (Caperonia palustris). Weed Sci. 52, 989–995 (2004).
Akramghaderi, F., Soltani, A. & Sadeghipour, H. Cardinal temperature of germination in medical pumpkin (Cucurbita pepo conver. pepo var. styriaca), borago (Borago officinalis L.) and black cumin (Nigella sativa L.). Asian J. Plant Sci. 2, 101–109 (2008).
Forti, C. et al. Molecular dynamics of pre-germinative metabolism in primed eggplant (Solanum melongena L.) seeds. Hortic. Res. 7, 1–12 (2020).
Du, B. et al. Rice seed priming with sodium selenate: Effects on germination, seedling growth, and biochemical attributes. Sci. Rep. 9, 1–9 (2019).
Rajjou, L. et al. Seed germination and vigor. Annu. Rev. Plant Biol. 63, 507–533 (2012).
Hussain, S. et al. Benefits of rice seed priming are offset permanently by prolonged storage and the storage conditions. Sci. Rep. 5, 1–12 (2015).
Oko, O., Asuquo, O., Agiang, E. & Osim, E. Neuroendocrine and behavioural responses of Japanese quails to dietary Aspilia africana leaf meal and extracts. J. Livest. Sci. (ISSN online 2277–6214) 8, 43–51 (2017).
Okello, D. et al. Effects of commercial soils on germination, early growth, and chlorophyll content of Aspilia africana, a medicinal plant. J. Plant Biotechnol. 48, 115–122 (2021).
Farooq, M., Basra, S., Ahmad, N. & Hafeez, K. Thermal hardening: A new seed vigor enhancement tool in rice. J. Integr. Plant Biol. 47, 187–193 (2005).
Reka, A. A. et al. Chemical, mineralogical and structural features of native and expanded perlite from Macedonia. Geol. Croat. 72, 215–221 (2019).
Guo, C., Shen, Y. & Shi, F. Effect of temperature, light, and storage time on the seed germination of Pinus bungeana Zucc. ex Endl.: The role of seed-covering layers and abscisic acid changes. Forests 11, 300 (2020).
Yang, L.-E. et al. Cold stratification, temperature, light, GA3, and KNO3 effects on seed germination of Primula beesiana from Yunnan, China. Plant Divers. 42, 168–173 (2020).
Bannayan, M., Nadjafi, F., Rastgoo, M. & Tabrizi, L. Germination properties of some wild medicinal plants from Iran. Seed Technol. 28, 80–86 (2006).
Kamaha, C. & Maguire, J. Effect of temperature on germination of six winter wheat cultivars. Seed Sci. Technol. 20, 181–185 (1992).
Zeinati, E., Soltani, A., Galeshi, S. & Sadati, S. Cardinal temperatures, response to temperature and range of thermal tolerance for seed germination in wheat (Triticum aestivum L.) cultivars. Electron. J. Crop Prod. 3, 23–42 (2010).
Baskin, C. C. & Baskin, J. M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination (Elsevier, 1998).
Vishal, B. & Kumar, P. P. Regulation of seed germination and abiotic stresses by gibberellins and abscisic acid. Front. Plant Sci. 9, 838 (2018).
Toh, S. et al. High temperature-induced abscisic acid biosynthesis and its role in the inhibition of gibberellin action in Arabidopsis seeds. Plant Physiol. 146, 1368–1385 (2008).
Geshnizjani, N., Ghaderi-Far, F., Willems, L. A., Hilhorst, H. W. & Ligterink, W. Characterization of and genetic variation for tomato seed thermo-inhibition and thermo-dormancy. BMC Plant Biol. 18, 1–12 (2018).
Szczerba, A. et al. Effect of low temperature on germination, growth, and seed yield of four soybean (Glycine max L.) cultivars. Agronomy 11, 800 (2021).
Arun, M. N. et al. Plant Stress Physiology-Perspectives in Agriculture (IntechOpen, 2022).
Ali, L. G., Nulit, R., Ibrahim, M. H. & Yien, C. Y. S. Efficacy of KNO3, SiO2 and SA priming for improving emergence, seedling growth and antioxidant enzymes of rice (Oryza sativa), under drought. Sci. Rep. 11, 1–11 (2021).
Harris, D. et al. On-farm seed priming: Using participatory methods to revive and refine a key technology. Agric. Syst. 69, 151–164 (2001).
Mondal, S. & Bose, B. Seed priming: An interlinking technology between seeds, Seed germination and seedling establishment. in Plant Reproductive Ecology-Recent Advances (ed. Rustagi, A., & Chaudhry, B.) 107–122 (IntechOpen, 2021).
Damalas, C. A., Koutroubas, S. D. & Fotiadis, S. Hydro-priming effects on seed germination and field performance of faba bean in spring sowing. Agriculture 9, 201 (2019).
Sağlam, S., Sibel, D., Gamze, K. & Gürbüz, A. Hydropriming increases germination of lentil (Lens culinaris Medik.) under water stress. Not. Sci. Biol. 2, 103–106 (2010).
Yan, M. Seed priming stimulate germination and early seedling growth of Chinese cabbage under drought stress. S. Afr. J. Bot. 99, 88–92 (2015).
Armin, M., Asgharipour, M. & Razavi-Omrani, M. The effect of seed priming on germination and seedling growth of watermelon (Citrullus lanatus). Adv. Environ. Biol. 4, 501–505 (2010).
da Silva Oliveira, C. E. et al. Seed priming improves the germination and growth rate of melon seedlings under saline stress. Ciênc. Rural https://doi.org/10.1590/0103-8478cr20180588 (2019).
Mohammadi, G. The effect of seed priming on plant traits of late-spring seeded soybean (Glycine max L.). Am. Eurasian J. Agric. Environ. Sci. 5, 322–326 (2009).
Kaya, M. D., Okçu, G., Atak, M., Cıkılı, Y. & Kolsarıcı, Ö. Seed treatments to overcome salt and drought stress during germination in sunflower (Helianthus annuus L.). Eur. J. Agron. 24, 291–295 (2006).
Younesi, O. & Moradi, A. Effect of priming of seeds of Medicago sativa" Bami" with gibberellic acid on germination, seedlings growth and antioxidant enzymes activity under salinity stress. J. Hortic. Res. 22, 167–174 (2014).
Abiri, R. et al. Quantitative assessment of indica rice germination to hydropriming, hormonal priming and polyethylene glycol priming. Chil. J. Agric. Res. 76, 392–400 (2016).
Tahaei, A., Soleymani, A. & Shams, M. Seed germination of medicinal plant, fennel (Foeniculum vulgare Mill), as affected by different priming techniques. Appl. Biochem. Biotechnol. 180, 26–40 (2016).
Chunthaburee, S., Sanitchon, J., Pattanagul, W. & Theerakulpisut, P. Alleviation of salt stress in seedlings of black glutinous rice by seed priming with spermidine and gibberellic acid. Not. Bot. Horti Agrobot. Cluj-Napoca 42, 405–413 (2014).
Gallardo, K. et al. Proteomics of Arabidopsis seed germination. A comparative study of wild-type and gibberellin-deficient seeds. Plant Physiol. 129, 823–837 (2002).
Singh, A., Dahiru, R., Musa, M. & Sani Haliru, B. Effect of Osmopriming duration on germination, emergence, and early growth of Cowpea (Vigna unguiculata (L.) Walp.) in the Sudan Savanna of Nigeria. Int. J. Agron. 2014, 1–4 (2014).
Singh, A., Dahiru, R. & Musa, M. Osmopriming duration influence on germination, emergence and seedling growth of sorghum. Seed Technol. 34, 111–118 (2012).
Hernández, J. A., Díaz-Vivancos, P., Acosta-Motos, J. R. & Barba-Espín, G. J. S. Potassium nitrate treatment is associated with modulation of seed water uptake, Antioxidative Metabolism and Phytohormone Levels of Pea Seedlings. Seeds 1, 5–15 (2021).
Marcos Filho, J. Fisiologia de sementes de plantas cultivadas: Fealq. FEALQ, Piracicaba, Brazil (2015).
Ruttanaruangboworn, A., Chanprasert, W., Tobunluepop, P. & Onwimol, D. Effect of seed priming with different concentrations of potassium nitrate on the pattern of seed imbibition and germination of rice (Oryza sativa L.). J. Integr. Agric. 16, 605–613 (2017).
Tombegavani, S. S., Zahedi, B., Mousavi Fard, S. & Ahmadpour, A. Response of germination and seedling growth of pepper cultivars to seed priming by plant growth regulators. Int. J. Hortic. Sci. Technol. 7, 59–68 (2020).
Ma, H.-Y. et al. A multi-year beneficial effect of seed priming with gibberellic acid-3 (ga 3) on plant growth and production in a perennial grass, Leymus chinensis. Sci. Rep. 8, 1–9 (2018).
Finch-Savage, W. E. & Bassel, G. W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 67, 567–591 (2016).
Bose, B., Srivastava, A. & Siddique, A. Impact of nitrate salt hardened seeds and sowing dates on seedling stand, growth, yield attributes, nitrogen and stress metabolism of rice. Int. J. Agric. Environ. Biotechnol. 9, 381–392 (2016).
Thejeshwini, B., Manohar Rao, A., Hanuman Nayak, M. & Sultana, R. Effect of seed priming on plant growth and bulb yield in onion (Allium cepa L.). Int. J. Curr. Microbiol. Appl. Sci. 8, 1242–1249 (2019).
Zhu, Z. H. et al. Effects of seed priming treatments on the germination and development of two rapeseed (Brassica napus L.) varieties under the co-influence of low temperature and drought. Plos one 16, e0257236 (2021).
Hasanuzzaman, M. & Fotopoulos, V. Priming and Pretreatment of Seeds and Seedlings (Springer, 2019).
Shukla, N. et al. Effect of different concentrations of GA3 and NAA and their methods of application on growth and yield of onion (Allium cepa L.). Progress. Hortic. 42, 111–113 (2010).
Adnan, M. et al. Seed priming; An effective way to improve plant growth. EC Agric. 6, 01–05 (2020).
Ghobadi, M., Shafiei-Abnavi, M., Jalali-Honarmand, S., Ghobadi, M. & Mohammadi, G. Does KNO3 and hydropriming improve wheat (Triticum aestivum L.) seeds germination and seedlings growth?. Ann. Biol. Res. 3, 3156–3160 (2012).
Ahmadvand, G., Soleimani, F., Saadatian, B. & Pouya, M. Effects of seed priming on germination and emergence traits of two soybean cultivars under salinity stress. J. Basic Appl. Sci. Res. 3, 234–241 (2012).
Acknowledgements
This study was supported under the framework of the International Cooperation Program (Korea-South Africa Cooperative Research Project for Excavation of Candidate Resources of Complementary and Alternative Medicine) managed by the National Research Foundation of Korea (Grant No. 2017093655 and KIOM: D17470). Additionally, this work was also supported by the Development of Foundational Techniques for the Domestic Production of Herbal Medicines (K18405), the Development of Sustainable Application for Standard Herbal Resources (KSN2013320), and the Korea Institute of Oriental Medicine through the Ministry of Science and ICT, South Korea.
Author information
Authors and Affiliations
Contributions
D.O. conceived the research idea, designed the experimental plan, participated in every stage and all parts of the research work, did the statistical analyses, and wrote the manuscript. R.K. collected the experimental data. RG collected the plant materials and wrote the manuscript. E.R., Y.C. and F.O. read, revised and improved the manuscript. Y.K. provided the technical guidance, supervised the whole research work, read and improved the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Okello, D., Komakech, R., Gang, R. et al. Influence of various temperatures, seed priming treatments and durations on germination and growth of the medicinal plant Aspilia africana. Sci Rep 12, 14180 (2022). https://doi.org/10.1038/s41598-022-18236-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-18236-2
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.