Abstract
The current study aimed to investigate diabetic retinopathy (DR) screening and treatment coverages among diabetic patients evaluated through the Brazilian National Health Insurance from 2014 to 2019. The Brazilian Public Health System Information Database was used as the primary data source. DR screening coverage was calculated as the rate of procedures of clinical dilated fundus exam and color fundus photograph over the number of diabetic patients. DR treatment coverage was calculated as the rate of procedures of intravitreal injection, photocoagulation, and panretinal photocoagulation over the number of diabetic patients presumably in need of DR treatment. The overall screening coverage increased from 12.1% in 2014 to 21.2% in 2019 (p < 0.001) with substantial regional discrepancies so that North region was the only one with no changes along the period. The overall treatment coverage increased from 27.7% in 2014 to 44.1% in 2019, with Southeast and Midwest absorbing the demand for service from the North, Northeast and South. Despite an improvement along the past years, both screening and treatment coverages for DR in diabetes patients are ineffective in Brazil. Public health policies should address resources disparities throughout the country aiming to offer same healthcare conditions to patients regardless their geographic location.
Similar content being viewed by others
Introduction
Diabetes mellitus (DM) is one of the most common chronic diseases worldwide with an estimated prevalence of 9.3% of the adult population in 20191. DM is a chronic metabolic disorder characterized by elevated levels of plasma glucose2. The disease can be classified into two main forms of diabetes: type 1 diabetes, when pancreatic beta cells are destroyed and consequently there is no insulin production; and type 2 diabetes, when the organism develops insulin resistance leading to hyperglycemia2. Chronic hyperglycemia due to DM may lead to different micro (nephropathy, neuropathy, and retinopathy) and macrovascular (peripheral artery disease, ischemic heart disease, and stroke) complications. Interventions focused on glucose levels control as well as lifestyle changing are highly recommended in order to reduce those manifestations incidence3.
Diabetes Retinopathy (DR) is the most common and specific DM complication, and one of the leading causes of preventable blindness in the adult population4, 5. DR can be classified as non-proliferative diabetic retinopathy (NPDR) as an early stage, which can be mild, moderate, or severe; as proliferative DR (PDR) as the severe stage; and as clinically significant macular oedema (CSME)6. A recent meta-analysis estimates the prevalence of DR on 22.27% (95% Confidence Interval [CI]: 19.73%–25.03%) globally within the DM population. Moreover, it shows a prevalence of 6.17% (95% CI, 5.43%–6.98%) for severe NPDR and PDR, and 4.07% (95% CI, 3.42%–4.82%) for CSME, those considered vision-threatening conditions7. Vision loss from DR could be prevented with a broad-based systems-level approach: first, by increasing patient’s awareness with specific health education programs; second, by community-level and/or national screening programs for all diabetic patients; and third, by timely referral and treatment for severe stages of DR6.
The ideal screening should include a complete ocular examination with best-corrected visual acuity after refraction and a retinal imaging with wide-field retinal photography and optical coherence tomography under pupil dilatation. This approach, however, is not always feasible even in high-resource settings. Recent guidelines recommend a screening including presenting visual acuity and a retinal examination adequate for DR classification, either a clinical dilated fundus exam or a color fundus photographs, depending on the resource settings6. In terms of treatment, previous randomized clinical trials have shown that up to 98% of blindness due to RD could be prevented by timely treatment with laser photocoagulation therapy, vitrectomy surgery, or intraocular injections of anti-vascular endothelial growth factor (VEGF) inhibitors6, 8,9,10.
Brazil is a country that offers universal, free of charge health insurance financed by the central government (Sistema Único de Saúde—SUS), providing ocular medical attention to the entire Brazilian population including RD screening and treatment. All the national data related to SUS is centered in the Brazilian Public Health System Information Database (DATASUS)11. Previous studies have shown that 3/4 of Brazilian citizens use SUS as their primary health provider while 1/4 rely on private hospitals or private insurance system facilities12,13,14,15. Recently, a study on retinal exams through SUS has shown that about 1/4 of those procedures are performed as diabetic related complications screening16.
The purpose of this study is to investigate the diabetic retinopathy screening and treatment coverages among diabetic patients evaluated through the Brazilian National Health Insurance (SUS) throughout the country from 2014 to 2019 as well as to evaluate each region capacity to attend its treatment demand considering intravitreal injection, photocoagulation, and panretinal photocoagulation.
Methods
Brazilian Public Health System Information Database (DATASUS) was used as the primary data source for the current study. DATASUS represents the primary effort of Brazilian Federal Government to collect data from the national health system and includes information from all public health hospitals throughout the country.
Screening procedures of clinical dilated fundus exam and color fundus photographs were retrieved for the current analysis. The numbers of procedure were adjusted accordantly to those performed as diabetic related complications screening. Data on the number of diabetic patients in the country were obtained from the Brazilian Risk Factor Surveillance System for Chronic Diseases (VIGITEL) Program prevalence estimates17. The coverage of screening test among diabetic patients was calculated considering the numerator as the number of diabetic retinopathy screening exams (1/4 of total)12,13,14,15 and the denominator the number of diabetes patients who use SUS (3/4 of total)16, through the formula below:
The total number of treatment procedures including injection, photocoagulation, and panretinal photocoagulation were analyzed according to year, region where the procedure was carried out, and region of the patient’s residence. The treatment coverage among diabetic patients was calculated considering the numerator as the number of diabetic retinopathy treatment procedures and the denominator the number of diabetes patients who use SUS (3/4 of total) presumably in need of diabetic retinopathy treatment (10%), through the formula below:
Spatial treatment coverage was defined as the capacity to attend the treatment demand for each region and it was calculated as the rate of performed procedures in the region over the number of residents from that region submitted to the procedure anywhere through the formula below:
The study was conducted exclusively with publicly available data from DATASUS (https://tabnet.datasus.gov.br) without any type of identification of subjects and it was carried out in accordance with the tenets of the Declaration of Helsinki.
Statistical analyses were performed using Stata/SE Statistical Software, Release 14.0, 2015 (Stata Corp, College Station, Texas, USA). Frequency tables were used for descriptive analysis. Trends along the years were evaluated through univariate generalized linear models. Maps were created using the web application go-cart.io18. p values ≤ 0.05 were considered statistically significant.
Results
Screening
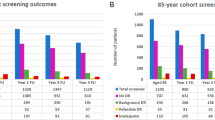
Along the 6 years period from 2014 to 2019, a total of 21,325,380 screening retinal tests were performed through the National Health Insurance (SUS). The total estimated to be associated with diabetic retinopathy screening was 5,331,345 procedures. Figure 1 shows the trends in screening exams coverage of diabetic patients evaluated by SUS along the years.
Trend analysis shows a statistically significant increase on the coverage along the years (p = 0.004). When comparing 2019 to 2014, a change of 71.0% has occurred. The coverages, however, vary substantially according to the country region. Table 1 shows the number of procedures and coverage along the years in the different regions.
South (p = 0.001), Southeast (p = 0.009), Northeast (p = 0.025), and Midwest (p = 0.004) showed increase coverage from 2014 to 2019 of 154.2%, 74.2%, 36.9%, and 36.6%, respectively. No significant changes were observed in the North region (p = 0.663) and it remained as the lowest coverage among all regions along the entire period. Figure 2 shows each state screening coverage in 2019. Individual data for each state along the full study period is presented on Supplementary Table S1.
Considering the total of screening procedures, clinical dilated fundus exam counted for 90.98% and color fundus photographs counted for 9.02%.
Treatment
Along the 6 years period from 2014 to 2019, a total of 275,152 intravitreal injections, 718,342 photocoagulations, and 115,443 panretinal photocoagulations were performed through the National Health Insurance (SUS), summing 1,108,937 treatment procedures. Table 2 shows the number of treatment procedures and coverage along the years in the different regions.
Overall, there was a significant increase on the treatment coverage from 2014 to 2019 of 59.2% (p = 0.001). North (p < 0.001), South (p < 0.001), Southeast (p = 0.022), Midwest (p = 0.027), and Northeast (p = 0.043) showed increase coverages of 252.3%, 89.3%, 53.1%, 41.3%, and 28.6%, respectively. Figure 3 shows each state treatment coverage in 2019. Individual data for each state along the full study period is presented on Supplementary Table S2.
Table 3 shows each region spatial treatment coverage for each year according to the performed procedure.
Values below 100% indicates that not enough procedures had been offered in the region so patients needed to perform the procedure in a different region. Most of the time, Southeast and Midwest absorbed the demand for service from the North, Northeast and South. The regional discrepancies become more evident when analyzing individual state data as presented on Supplementary Table S3.
Discussion
The prevalence of DM follows the global population life expectancy increasing, so that the International Diabetes Federation estimates a prevalence of 10.9% of the population affected by 2045, summing more than 700 million diabetic patients1. With the advances on diabetes overall care and awareness of lifestyle changing importance, the lifespan of people living with DM is expected to consequently increases. In that sense, a burden of micro and macrovascular complications associated with disease duration and demand for treatment are expected in the next years.
The most recent report from the Global Burden of Disease (GBD) Study indicates DR as the fifth leading cause of blindness and of moderate to severe vision impairment in adults aged 50 years and older. When evaluating the last 30 years, while significant decreases on the age-standardized global prevalence for blindness due to cataract, uncorrected refractive error, glaucoma, and age-related macular degeneration were noticed, the prevalence of blindness due to DR was the only one to significantly increase from 14.9% in 1990 to 18.5% in 2020. This is a particularly concerning scenario as, in comparison to the leading causes of visual impairment and blindness (i.e. cataract and uncorrected refractive error), the management of DR requires a greater amount of both human and technological resources, including trained ophthalmologists with experience in laser and surgery5.
Screening for DR has been proved to be effective on avoiding visual loss as it detects referable cases for timely full ophthalmic examination and treatment19. Different guidelines have been proposed along the years aiming to reach the ideal screening protocol considering both the exam procedure and the timeline design. In Brazil, a patient will only go through a DR screening procedure at the public health system by referral from a primary care doctor, an endocrinologist or an ophthalmologist. Similarly, a patient will only go through a DR treatment procedure by referral from an ophthalmologist, after a clinical evaluation.
Our study showed a lower coverage of annual DR screening in Brazil with an overall rate of 21.2% of diabetic patients being examined in 2019. No previous studies were performed in other countries with overall national data, however, previous reports with specific communities in Tanzania and in England have shown DR annual screening uptake rates of 28.8% and 82.4%, respectively20, 21. Factors associated with higher compliance to DR screening include older age, higher educational level, and living closer to the health facility20,21,22,23.
Another interest finding on the current analysis is the predominance of clinical dilated fundus exam while color fundus photographs represented only 9.0% of the screening procedures. Fundus photographs in combination with telemedicine protocols have the potential to benefit a large amount of patients improving a screening program cost-effectiveness, particularly in resource-constrained health care settings24, 25. Different health care workers are able to operate retinal imaging devices, not limiting the procedure to highly specialized staff26. Images acquired can then be shared with and graded, remotely, by ophthalmologists or through artificial intelligence algorithms. Several studies have proven the validity of such procedure in comparison to clinical dilated fundus exam and therefore this model should be implanted in order to improve the DR screening programs27,28,29.
A DR screening cost-effectiveness is also highly influenced by the frequency of the clinical examinations and/or retinal imaging30. Fixed annual screening programs have the challenge of the ever-increasing number of diabetic (and therefore) eligible patients requiring higher budgets each year31,32,33 and the challenge of lower patient compliance as a large proportion of screened patients who has the same result of no detected retinopathy every year tends to skip the visits on the following years34. Several studies in European countries have shown successful results on extending the screening interval from annual to every 2 or 3 years for patients with no evidence of retinopathy at the exam35,36,37. In that sense, differentiating patients into low-risk and high-risk groups to determine the ideal timeline of screening has the potential to further improve cost-effectiveness, mainly in low-resources settings.
Previous studies performed with individual sampling have shown DR treatment coverages among diabetic patients ranging from of 20% in Oman to 79% in Australia38, 39. Even though we searched DATASUS database for therapeutic procedures that are usually part of the treatment of diabetic retinopathy, the applied method carries a limitation that overestimates the real number of patients treated for diabetic retinopathy, since there are other retinal diseases treated with the same procedures; unfortunately, it is not possible to identify each patients’ diagnosis on DATASUS database. Hence, the number of procedures correspond to the whole amount of patients with a myriad of retinal diseases treated with the above mentioned procedures, including not only DR but also age-related macular degeneration and retinal vascular occlusions, retinal tears, among others. Additionally, because no patient is identified, and since the same patient may have undergone more than one therapeutic procedure, the rate of coverage is further overestimated. Consequently, the poor coverage rate found in the present study is probably even lower, which further aggravates the DR treatment coverage landscape in Brazil’s public health system.
Our search did not include vitrectomy as a therapeutic procedure for DR patients because this kind of surgery is performed in only a few services throughout Brazil’s public health system, and it is not mainly performed for DR treatment, but rather for rhegmatogenous retinal detachment. Additionally, currently there is no reliable data on the rate of procedures performed to address the treatment of DR. Elsewhere, only a small fraction of such procedures are performed for the treatment of DR in underserved countries40; finally, most diabetic vitrectomies are performed along with laser pan photocoagulation, which has already been considered in our search.
Significant disparities according to the region were observed. Brazil is a country with continental extension with substantial socioeconomic regional disparities that also influences the ophthalmologist’s distribution throughout the country. According to the World Health Organization (WHO), the ideal scenario of ocular health care within a population is a rate of at least 1 ophthalmologist per 17,000 habitants. The most recent census promoted by the Brazilian Council of Ophthalmology in 2019 shows that the country counts with 20,455 ophthalmologists which results in 1 professional per 9,224 population, in accordance to the WHO recommendation. When analyzing each region, however, the rates range from 1 per 7599 in the Southeast to 1 per 12,084 in the North region41. The disparities on concentration of ophthalmologists in the country may impact both the frequency of procedures performed and the spatial coverage in each region. A recent study on risk of developing DM in the next 10 years in the Brazilian population indicated a significantly higher risk in the North region so that the current observed disparities are expected to increase if no intervention is designed42.
In conclusion, despite an improvement along the past 6 years, both screening and treatment for diabetic retinopathy in diabetes patients are ineffective in Brazil, with substantial differences among the regions. Screening programs should focus on patients awareness, flexible timeline planning according to risk, and use of telemedicine protocols including fundus photograph and artificial intelligence. Moreover, public health policies should address the unequal distribution of human and technological resources throughout the country in order to offer the same health care conditions of screening and treatment to patients regardless their geographical location.
References
Saeedi, P. et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 157, 107843 (2019).
American Diabetes Association. Classification and diagnosis of diabetes: Standards of medical care in Diabetes-2021. Diabetes Care 44, S15–S33 (2021).
Lovic, D. et al. The growing epidemic of diabetes mellitus. Curr. Vasc. Pharmacol. 18, 104–109 (2020).
Khalil, H. Diabetes microvascular complications - A clinical update. Diabetes Metab. Syndr. 11, S133–S139 (2017).
GBD. Blindness and Vision Impairment Collaborators. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob. Health 9, e144–e160 (2019).
Wong, T. Y. et al. Guidelines on diabetic eye care: The international council of ophthalmology recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology 125, 1608–1622 (2018).
Teo, Z. L. et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: Systematic review and meta-analysis. Ophthalmology 28, 1580–1591 (2021).
Ferris, F. L. How effective are treatments for diabetic retinopathy?. JAMA 269, 1290–1291 (1993).
Cheung, N., Mitchell, P. & Wong, T. Y. Diabetic retinopathy. Lancet 376, 124–136 (2010).
El Rami, H., Barham, R., Sun, J. K. & Silva, P. S. Evidence-based treatment of diabetic retinopathy. Semin Ophthalmol. 32, 67–74 (2017).
Bittencourt, S. A., Camacho, L. A. B. & Leal, M. C. Hospital information systems and their application in public health. Cad Saude Publica 22, 19–30 (2006).
Paim, J., Travassos, C., Almeida, C., Bahia, L. & Macinko, J. The Brazilian health system: History, advances, and challenges. Lancet 377, 1778–1797 (2011).
Viacava, F. & Bellido, J. G. Health, access to services and sources of payment, according to household surveys. Cien Saude Colet. 21, 351–370 (2016).
Massuda, A., Hone, T., Leles, F. A. G., de Castro, M. C. & Atun, R. The Brazilian health system at crossroads: Progress, crisis and resilience. BMJ Glob Health 3, e000829 (2018).
Silva, B. et al. Dual use of public and private health care services in Brazil. Int J. Environ. Res. Public Health 19, 1829 (2022).
Malerbi, F. K. et al. Retinal exams requested at Primary Care Unit: indications, results and alternative strategies of evaluation. Einstein São Paulo 18, eGS4913 (2020).
Gastner, M. T., Seguy, V. & More, P. Fast flow-based algorithm for creating density-equalizing map projections. Proc. Natl. Acad. Sci. U. S. A. 115, E2156–E2164 (2018).
Monteiro, C. A. et al. Monitoramento de fatores de risco para doenças crônicas por entrevistas telefônicas [Surveillance of risk factors for chronic diseases through telephone interviews]. Rev. Saude Publica 39, 47–57 (2005).
Vujosevic, S. et al. Screening for diabetic retinopathy: new perspectives and challenges. Lancet Diabetes Endocrinol. 8, 337–347 (2020).
Mumba, M., Hall, A. & Lewallen, S. Compliance with eye screening examinations among diabetic patients at a Tanzanian referral hospital. Ophthalmic Epidemiol. 14, 306–310 (2007).
Moreton, R. B. R., Stratton, I. M., Chave, S. J., Lipinski, H. & Scanlon, P. H. Factors determining uptake of diabetic retinopathy screening in Oxfordshire. Diabet Med. 34, 993–999 (2017).
Meyer, R. E. & Herrick, H. Prevalence of diabetes-related eye disease in North Carolina: Findings from the North Carolina behavioral risk factor surveillance system. N. C. Med. J. 72, 413–416 (2011).
Mukamel, D. B., Bresnick, G. H., Wang, Q. & Dickey, C. F. Barriers to compliance with screening guidelines for diabetic retinopathy. Ophthalmic Epidemiol. 6, 61–72 (1999).
Jones, S. & Edwards, R. T. Diabetic retinopathy screening: a systematic review of the economic evidence. Diabet Med. 27, 249–256 (2010).
Avidor, D., Loewenstein, A., Waisbourd, M. & Nutman, A. Cost-effectiveness of diabetic retinopathy screening programs using telemedicine: A systematic review. Cost Eff. Resour. Alloc. 18, 16 (2020).
Tan, C. H., Kyaw, B. M., Smith, H., Tan, C. S. & Tudor Car, L. Use of Smartphones to detect diabetic retinopathy: Scoping review and meta-analysis of diagnostic test accuracy studies. J. Med. Internet Res. 22, e16658 (2020).
Gupta, A., Cavallerano, J., Sun, J. K. & Silva, P. S. Evidence for telemedicine for diabetic retinal disease. Semin. Ophthalmol. 32, 22–28 (2017).
Huemer, J., Wagner, S. K. & Sim, D. A. The evolution of diabetic retinopathy screening programmes: A chronology of retinal photography from 35 mm slides to artificial intelligence. Clin. Ophthalmol. 14, 2021–2035 (2020).
Kalogeropoulos, D., Kalogeropoulos, C., Stefaniotou, M. & Neofytou, M. The role of tele-ophthalmology in diabetic retinopathy screening. J. Optom. 13, 262–268 (2020).
Scanlon, P. H. Screening intervals for diabetic retinopathy and implications for care. Curr. Diabetes Rep. 17, 96 (2017).
Scanlon, P. H. et al. Development of a cost-effectiveness model for optimisation of the screening interval in diabetic retinopathy screening. Health Technol. Assess. 19, 1–116 (2015).
Leese, G. P. et al. Progression of diabetes retinal status within community screening programs and potential implications for screening intervals. Diabetes Care 38, 488–494 (2015).
Taylor-Phillips, S. et al. Extending the diabetic retinopathy screening interval beyond 1 year: Systematic review. Br. J. Ophthalmol. 100, 105–114 (2016).
Scanlon, P. H. The English national screening programme for sight-threatening diabetic retinopathy. J. Med. Screen. 15, 1–4 (2008).
Agardh, E. & Tababat-Khani, P. Adopting 3-year screening intervals for sight-threatening retinal vascular lesions in type 2 diabetic subjects without retinopathy. Diabetes Care 34, 1318–1319 (2011).
Jones, C. D., Greenwood, R. H., Misra, A. & Bachmann, M. O. Incidence and progression of diabetic retinopathy during 17 years of a population based screening program in England. Diabetes Care 35, 592–596 (2012).
Looker, H. C. et al. Predicted impact of extending the screening interval for diabetic retinopathy: The Scottish diabetic retinopathy screening programme. Diabetologia 56, 1716–1725 (2013).
Khandekar, R., Al Lawatii, J., Mohammed, A. J. & Al Raisi, A. Diabetic retinopathy in Oman: A hospital based study. Br. J. Ophthalmol. 87, 1061–1064 (2003).
Keel, S. et al. The prevalence of diabetic retinopathy in Australian adults with self-reported Diabetes: The national eye health survey. Ophthalmology 124, 977–984 (2017).
Okonkwo, O. N. et al. Indications and outcomes of vitrectomy surgery in a series of 1000 black African eyes. BMJ Open Ophthalmol. 4, e000083 (2019).
Conselho Brasileiro de Oftalmologia. As condições de saúde ocular no Brasil. (2019). Available in: https://www.cbo.com.br/novo/publicacoes/condicoes_saude_ocular_brasil2019.pdf Accessed 28 November 2021.
Nascimento, L. G. et al. A new Brazilian regional scenario of Type 2 diabetes risk in the next ten years. Prim. Care Diabetes 15, 1019–1025 (2021).
Author information
Authors and Affiliations
Contributions
Author A.G.F. designed the study; collected, analysed, and interpreted data; and wrote the main manuscript text. Authors A.N.F. and R.B. collected and interpreted data; and wrote the main manuscript text. Author F.K.M. designed the study; analysed data and interpreted data; and wrote the main manuscript text. All authors reviewed the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fernandes, A.G., Ferraz, A.N., Brant, R. et al. Diabetic retinopathy screening and treatment through the Brazilian National Health Insurance. Sci Rep 12, 13941 (2022). https://doi.org/10.1038/s41598-022-18054-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-18054-6
This article is cited by
-
Present and future screening programs for diabetic retinopathy: a narrative review
International Journal of Retina and Vitreous (2024)
-
The use of social simulation modelling to understand adherence to diabetic retinopathy screening programs
Scientific Reports (2024)
-
Prevalence of diabetic retinopathy in Brazil: a systematic review with meta-analysis
Diabetology & Metabolic Syndrome (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.