Abstract
Various studies have shown the importance of using different types of Zooplankton biomasses as an additional substance in the diet of fish. In addition, the drainage water of the fish cultures could be used in plant irrigation. In this study, biomasses of water flea Daphnia magna and Gammarus pulex collected and tested, for the first time, their effect against pathogenic microorganisms and on plant germination. The results showed significant antibacterial activity of D. magna and G. pulex against Staphylococcus aureus and Pseudomonas aeruginosa bacteria, as well as antifungal activity against Alternaria solani and Penicillium expansum, which gives the possibility to be used as biocontrol against these bacteria and plant pathogenic fungi. Furthermore, both animals showed positive activity in the germination rate of Vicia faba seed, reaching 83.0 ± 3.5 and 86.0 ± 3.8%, respectively. In conclusion, the biomasses of D. magna and G. pulex are promising and effective agents for their use in the medical field against some pathogenic microbes and as stimulators of plant growth.
Similar content being viewed by others
Introduction
Zooplankton plays a critical role in aquaculture, influencing survival of livestock under harsh environmental conditions and growth depending on various conditions1. In addition, zooplankton contributes to the aquatic food chain as an intermediator, feeding upon bacteria, fungi, and algae, while turning fed by numerous invertebrates, birds, and fishes2. Zooplankton are used as an environmental indicator for water quality, pollution, and the eutrophication situation3. Recently, plankton biotechnology has gained extensive significance, because of its rich constituents involved in active primary and secondary metabolites4. The current applied studies on zooplankton communities mainly focused on their composition as a rich source of pigments, vitamins, essential fatty acids, proteins, carbohydrates, and biologically active primary and secondary metabolites5. Many studies mentioned the importance of using Daphnia magna (Straus, 1820) and Gammarus pulex (Linnaeus, 1758) as an alternative substance for protein in the fish diet at different fish farms2,6,7,8,9,10,11,12.
The water flea D. magna is a micro-crustacean zooplankton widespread in freshwater bodies. Recently, it was used for feeding fish fry in aquaculture as it has a high nutritional composition that varies according to the culture medium and viability degree of phytoplankton2,11,12,13,14. The crustacean amphipod G. pulex is widespread worldwide11 and one of the most common freshwater macroinvertebrates. G. pulex has a central role in organic matter degradation15,16, and risk assessment17, besides its contribution to the food net18. It contains high levels of protein, fats, and amino acids19, thus Gammarus meal enhances the feed intake, immune response, stress resistance, and growth performance in fish20. Gammarus is used as a cheap alternative substrate for animal protein in the diet of highest value fish21. Therefore, it is important to explore the effect of D. magna and G. pulex biomass against the most common pathogenic microbes that could infect either the fed-fish or humans as the end-consumer. In addition, it is important to study their effect on plant germination, because the drainage water of fish farms is loaded with biomass and is recycled for irrigating plants.
Pathogenic microbes and infectious diseases have become one of the major problems in the medical field, leading to the death of many people worldwide22. Antibiotics resistance is counted as one of the most parameters affecting human health, particularly after the emergence of multi-drug-resistant (MDR) pathogens23,24. The wrong usage of antibiotics and the deficiency of capabilities and scientific tools to improve such drugs had led scientists to explore alternative natural molecules with potential antimicrobial activity25.
Several types of bacteria and fungi can enter, individually or together, vital matrix called biofilm through a microbial-molecular communication system that regulates the virulence traits of these microbes and introducing infections by biofilm-forming microbes via medical devices26. The biofilm is a complex network of lipids and proteins used to protect microbes against a broad range of antibiotics and the host immune system27,28,29. Thus, it is necessary to search for new treatment substrates such as natural molecules to provide a new vision in treatment and limit the spread of MDR pathogens28,30,31,32.
Different species of fungi cause the major common plant diseases, mostly controlled by synthetic fungicides33. Whereas repeated fungicide leads to the rapid evolution of fungal resistance against the fungicide, creating a high demand for exploring a natural alternative molecule34.
Broad beans or faba beans (Vicia faba) are considered one of the most important winter crops with high nutritional value, especially in Egypt35. The main fungal diseases infecting Vicia faba are wilting, root rot, leaf spot, and even plant death caused by species of diverse fungal genera such as Alternaria, Fusarium, Ascochyta, Colletotrichum, Rhizopus, Pythium, Rhizoctonia, and Clonostachys36,37. Hence, finding an eco-friendly substrate will be helpful in biocontrol plant diseases.
The discovery of natural antimicrobials might meet the consumer demand for environmentally friendly materials avoiding chemicals with harmful side effects38,39. There has been a promising trend for scientific research and industrial applications of biotechnology and marine compounds40. In this context, this study was conducted to studying for the first time the antibacterial, antifungal, and antibiofilm activities of the dried biomasses of G. pulex, and D. magna to be used safely in the fish diet and medical field, and limit the spread of some pathogenic bacterial and fungal microbes in addition to investigating the effect of these biomasses on plant seed germination. This report will open the door for further applications and valorization for other types of zooplankton as an inexpensive and safe alternative substrate.
Materials and methods
Tested animals
Daphnia magna and Gammarus pulex were collected from northern Egypt, Lake Mariout (31° 7.5′ N, 29° 47.1′ E) in June 2019; the salinity in this part of the lake is rather stable around 8‰39.
Cladoceran species, Daphnia magna, and Amphipods, Gammarus spp., were collected with a standard plankton net of 200 µm mesh size, which was lowered vertically to a shallow bottom then pulled up to the water surface5,7. Then the net content was placed into plastic containers filled with lake water and identify D. magna and G. pulex through dissecting microscope. The samples were identified by traditional morphological and biochemical methods. The identified D. magna and G. pulex species were kept in 1001 transplant plastic holding containers that had dechlorinated tap water, with a salinity of 8% as in nature, for 24 h acclimatization before starting the cultivation experiments. After cultivation for a certain period under standard conditions, according to the methods described by Abo-Taleb et al.11 both species were dried at 70 °C for 48 h to be ready for further experiments2.
Chemical analyses of the tested animals
The chemical composition of D. magna and G. pulex including moisture, carbohydrate, crude protein, fibers, crude fat, calories, and ash, was analyzed according to the standard methods of AOAC (1990)6. The moisture content was estimated by drying the samples to constant weight at 70 °C in a drying oven for 48 h. Whereas the nitrogen content was measured using an Automatic Kjeldahl system (UDK 139, VELP Scientifica). However, the lipid content was determined by ether extraction in multiunit extraction Soxhlet apparatus. The ash was determined by combusting dry samples in a muffle furnace at 550 °C for 3 h. Vitamins A, B2, B6, B12, D, E, and folic acid, besides the antioxidants such as tannic acid and β-carotene, have been determined in the cultivated D. magna and G. pulex to evaluate their nutritional value5.
Biological Activities of D. magna and G. pulex
Antibacterial and MIC determination
Antibacterial activities of D. magna and G. pulex were evaluated using an agar well diffusion assay on Muller-Hinton agar plates according to CLSI guidelines against human pathogenic bacterial strains Pseudomonas aeruginosa ATCC 27,853 and Escherichia coli ATCC8739, which represented Gram-negative bacteria, whereas Bacillus subtilis ATCC 6051 and Staphylococcus aureus ATCC 6538 were used to represent Gram-positive bacteria. The inhibition zone diameter was specified in millimeter (mm)41. The Micro-dilution method in micro-titer plates (MTP) was applied to determine the minimum inhibitory concentration (MIC) of active D. magna and G. pulex against tested pathogenic bacteria. Accordingly, D. magna and G. pulex were diluted in 1% DMSO at various concentrations (0.009–5.0 mg mL−1, w/v) and tested against the pathogenic bacterial strains. Briefly, 1:100 (v/v) overnight cultures of the test strains were added to 200 µl of Mueller–Hinton broth media dispersed in MTP wells with/without D. magna and G. pulex. Then, the plates were incubated with shaking at 120 rpm at 37 °C for 24 h. The lowest concentration of D. magna and G. pulex which inhibited bacterial growth was considered the MIC42,43. All experiments were performed in triplicates.
Antibiofilm assay
To analyze the anti-biofilm activity of the two animal species, a Microtiter plate (MTP) assay was used. D. magna and G. pulex were tested against two known biofilm-producing strains: Pseudomonas aeruginosa ATCC 27,853 and Staphylococcus aureus ATCC 6538. Sub-inhibitory concentrations (0.01–1.0 mg mL−1) of D. magna and G. pulex were loaded in a flat bottom MTP, containing Tryptic-Soy broth media (TSB) supplemented with 1% glucose and mixed well. The tested strains were cultured and incubated overnight in TSB at 37 °C and after incubation diluted to reach the turbidity of 1.5 × 108 CFU mL−1 then inoculated into MTP wells containing different concentrations of the tested biomasses and incubated at 37 °C for 48 h44. After the incubation period, the growth density was measured at OD 620 nm. Subsequently, the floated cells were transferred without troubling the formed biofilm and the plates were washed three times with sterilized phosphate-buffered saline (PBS) pH 7.4 to remove excess residue of floated unbounded cells. The biofilms in all wells were fixed with 200 µl of methanol 95% for 10 min. Then, crystal violet (CV 0.3% w/v) was added to each well using a multi-channel micropipette (CAPP, Germany), and the plates were incubated for 15 min at room temperature. After that, the excess CV was removed, and the wells were gently washed with sterile distilled water. For the quantitative detection of biofilm formation, the adherent biofilm-bounded CV was assayed by eluting in 30% acetic acid and measured at OD 540 nm using an automated microplate reader (Tecan, Elx800-USA). The treated wells were compared with that of the untreated control. the untreated well inoculated with the test organism is considered the positive control while the negative control is the uninoculated media. All control and experiment groups were performed in triplicates.
The activity against plant pathogenic fungi
The antifungal activity of D. magna and G. pulex was performed by the agar well method45 against plant pathogenic fungi strains Fusarium oxysporum RCMB (008 001 “2”), Penicillium expansum RCMB (001 001 “1”), Alternaria solani RCMB (009 003), Aspergillus niger RCMB (002 007 “2”), Aspergillus fumigatus RCMB (002 008 “2”), and Rhizoctonia solani RCMB (031 001). Briefly, 10 mg mL−1 of D. magna or G. pulex crude powder were placed separately into the wells of the plates. The plates were incubated for 5 days at 30 °C. Antifungal activity was evaluated by measuring the diameter of the inhibition zone for each strain. Each strain was tested in triplicates.
The seed germinations test
The faba bean cv. Giza 716 was obtained from the Legume Research Department, Field Crop Institute, Agricultural Research Center, Egypt. The seeds of Vicia faba cv. Giza 716 identification was confirmed by the Botanical herbarium, Botany and Microbiology Department, Faculty of Science Al-Azhar University. The collection of plant material complies with the institutional, national, and international guidelines and legislation. equal size were surface sterilized by submersion in 10% sodium hypochlorite solution for 10 min, soaked in distilled water at 25 °C for 24 h, and then allowed to germinate in Petri dishes. One piece of filter paper and 5 mL of different concentrations (1, 0.5, 0.25, 0.125, 0.0625, and 0.031 mg mL−1) of D. magna and G. pulex were placed in distilled water in other Petri dishes. The germinating seeds were transferred to filter paper with 10 seeds per dish and a minimum distance of 1 cm between each seed (three replicates for each treatment). Distilled water was used for the control (untreated) experiments. For the germination rate and root growth investigation, the seeds were allowed to germinate for one week, then the seed germination percentage was calculated, and the seedling root length was measured. Four replicates were carried out for each treatment. Treated and control seeds were evaluated for record the percentage of seed germination with the following formula46:
Statistical analysis
Three replicates were performed for each assay, and the standard error was calculated. all resulting values were the averages of three independent replicates. The differences between a sample and the corresponding control were analyzed by using Student’s t-test and the differences were considered significant if the p values were ≤ 0.05.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Results and discussions
Chemical analyses of tested animals
The chemical content of the two tested animals was analyzed to determine their prospective efficiency for industrial applications. Biochemical composition of D. magna and G. pulex is given in Table 1 based on three independent readings. The results point to higher chemical content of D. magna in protein, fibers, nitrogen-free extract, and both types of energy, while G. pulex had higher chemical content in fats, ash, and carbohydrate (Table 1). The chemical composition of Gammarus can be affected by several factors such as age, season, habitats, region, and drying process after harvesting21. Based on their high content of protein and other ingredients, D. magna and G. pulex were the proper alternatives for fish meal in feeding fish larvae47.
Vitamins and antioxidant content
D. magna and G. pulex contain vitamins and antioxidants required to improve the health status of fish larvae. The vitamins contained in 100 g of D. magna were Vitamin A (279.53 IU), B2 (218.44 mg), B6 (120.84 mg), B12 (222.61 mg), D (22.59 mg), E (98.12 mg), and folic acid (139.08 µg). The antioxidant found in 100 g of D. magna were β-carotene (6,811.90 IU) and tannic acid (137.65 mg).
The vitamins contained in 100 g of G. pulex were: Vitamin A (11 IU), B2 (613.80 mg), B6 (321.76 mg), B12 (288.24 mg), D (48.37 mg), E (183.25 µg), and folic acid (477.23 µg). The antioxidant found in 100 g of G. pulex were β-carotene (18,740.60 IU) and tannic acid (273.98 mg) (Fig. 1).
Therefore, the major constituents of D. magna and G. pulex biomasses were vitamin-A and β-carotene. The vitamin content in D. magna obtained from this study was lower than that mentioned by El-Feky et al.2, although the antioxidant contents were higher. On the other hand, the vitamin and antioxidant contents of G. pulex of this study showed a lower variation than mentioned by Abo-Taleb et al.11.
Antibacterial activity and MIC determination
The inhibitory action of D. magna and G. pulex biomasses are indicated by the diameter of the inhibition zone formed (Table 2). The results indicated that G. pulex has better inhibitory effects against Gram-positive and Gram-negative pathogenic bacteria than D. magna. The inhibitory action for D. magna and G. pulex biomasses may be due to the reaction of the biomasses with bacterial protein by combining the thiol (-SH) group leading to the inactivation of proteins and bacterial growth. Therefore, it is necessary to test the MIC of D. magna and G. pulex for each bacterial strain (Table 2). bioagents with lowest MIC values is candidate to be effective antimicrobial agents48. Results showed that the MIC for D. magna was 2.0 mg mL−1 against both P. aeruginosa and S. aureus. Moreover, MIC for G. pulex was 2.0 mg mL−1 against P. aeruginosa and S. aureus, respectively. Hence, biomasses have the same activity against Gram positive and negative bacteria. However, the mechanism of bacterial growth reduction through the interaction of D. magna and G. pulex still needs further studies.
The antibiofilm activity
In this work, the antibiofilm activity of the tested biomass exhibited varied effects against different bacterial strains (Fig. 2). Accordingly, G. pulex and D. magna biomass affected the biofilm formation of Pseudomonas aeruginosa at concentrations lower than the inhibitory concentration, while the biomass didn't exhibit any antibiofilm activity against S. aureus. MIC values of the D. magna and G. pulex against P. aeruginosa and S. aureus were 2.0 mg mL−1. The sub-lethal concentrations of D. magna and G. pulex exhibited antibiofilm activity against P. aeruginosa with significant differences (p < 0.05) in a dose-dependent manner where the concentrations 1, 0.5, 0.25, and 0.125 mg mL−1 of D. magna were reducing the biofilm formation of P. aeruginosa with 91.43, 78.31, 60.63, and 42.04%, respectively, while the same concentrations didn't affect the biofilm formation of S. aureus (Fig. 2a). On the other hand, the concentrations of 1, 0.5, and 0.25 mg mL−1 of G. pulex reduced the biofilm formation of P. aeruginosa by 90.38, 88.41, and 60.71% respectively, whereas the concentrations 1 and 0.5 mg mL−1 only affect the biofilm formation of S. aureus by 49.1 and 33.9% (Fig. 2b). The ability of these biomasses to reduce the biofilm formation of this opportunistic pathogens in a dose-dependent manner at sub-inhibitory concentrations motivating the researcher for more exploration to investigate the active compounds in these biomasses that may interfere with the biofilm formation pathway or the virulence regulation system in bacteria such as quorum sensing system. Our findings make these compounds promising molecules that could use as biofilm preventive agents. Interestingly, the primary stage of biofilm inhibition was observed at the MIC values41. The previous study explained the antibiofilm activity which showed deformation, external cell roughness, and cell wall shrinkage of bacterial cells49.
The activity against plant pathogenic fungi
This study investigated the activity of D. magna and G. pulex biomasses against six plant pathogenic fungi (A. solani, A. niger, A. fumigatus, F. oxysporum, P. expansum, and R. solani). The results showed that D. magna and G. pulex had antifungal activity against two plant pathogenic fungi (A. solani and P. expansum) at 10 mg mL−1 (Table 3). The inhibition zone diameters formed by the effect of D. magna biomass were 10.31 ± 0.88 and 10.41 ± 0.51 mm for A. solani and P. expansum, respectively, while G. pulex exhibited antifungal activity with inhibition zone diameters of 10.88 ± 0.72 and 9.61 ± 0.43 mm for the same fungal strains A. solani and P. expansum, respectively. The biomasses may inhibit the fungal growth by affecting cellular functions, causing distortion in fungal hyphae, or preventing the expansion of conidia and conidiophores, consequently leading to cell death50. These different responses of tested plant pathogenic fungi may be due to the resistance of some strains and the sensitivity of others51 . The sensitivity of fungal plant pathogens may be altered by the sensitive target site by mutant or changes in amino acids52. The activity of D. magna and G. pulex biomasses against Alternaria solani and Penicillium expansum which cause severe damage to plants may contribute to the production of a safe bio fungicide in the future to protect the plant from this fungus.
Seed germinations test
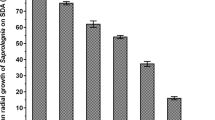
The experiment showed a positive effect with G. pulex and D. magna on seed germination presence of V. faba. D. magna and G. pulex increased the germination percentage with significant differences (P < 0.01) when compared with the control at 1, 0.5, and 0.25 mg mL−1 (Fig. 3). D. magna enhanced the germination rate by 90, 83, and 80%, respectively (Fig. 3b), while with G. pulex the increase in germination rate was 86.6, 83, and 76.6%, respectively (Fig. 3c). Furthermore, D. magna showed high enhancement of root length with the highest effect recorded at 1 mg mL−1 of 222%, while G. pulex increased root length by 111% compared to control. On the other hand, the 0.031 mg mL−1 concentration did not show any positive effect on seed germination by either D. magna or G. pulex. These results relate to several studies which reported that seaweed extracts improved the V. faba morphological characters such as shoot and root lengths. The use of water flea, D. magna, and the crustacean amphipod, G. pulex, as complementary protein and lipid sources in the transitional diet for Common Carp (Cyprinus carpio L.) nurseries53 affect the growth and yield of faba bean (V. faba L.) positively. These components are involved in the primary metabolism of building and maintaining plant cells and viability, resulting in growth enhancement. The more inductive effect of D. magna and G. pulex on Vicia faba vegetative growth parameters including shoot height, was explained by the presence of folic acid in D. magna and G. pulex. For more, D. magna and G. pulex used as nutraceuticals through enhancement biochemical mechanisms and production of secondary metabolites54. The previous study carried by Cauchie, et al.,55. al., Chitosan plays an effective role in the enhancement of plant growth and inductive defense factors in many plants through regulation of the most important metabolic processes as Carbon and Nitrogen Metabolism56. Another reason for the induction effect of Chitosan contents of D. magna and G. pulex on Vicia faba vegetative growth is that Chitosan reduced water uptake by minimizing plant transpiration. Moreover, nutrients, mineral vitamins, and antioxidants resulted in the regulation of Vicia faba metabolic processes, especially against stress conditions57,58.
Effect of different concentrations of D. magna and G. pulex on faba bean (V. faba) seed germination rate and shoot and root length. (a) Seeds in the petri dish with control, D. magna, and G. pulex. (b) The effect of different concentrations of D. magna. (c) The effect of different concentrations of G. pulex. Significant differences are indicated by * (P < 0.01).
Conclusion
In this study, isolated biomass of D. magna and G. pulex showed obvious ability to inhibit several pathogens involving Gram-positive and Gram-negative bacteria, as well as inhibition against some plant pathogenic fungi. The current report considers the first biotechnological study of the current micro and macroinvertebrates. The biomass from D. magna and G. pulex exhibited the possibility of applying them in the medical and agricultural fields limiting the spread of some pathogenic microbes and enhancing plant germination.
Data availability
All authors declare that the data supporting the findings of this study are available within the article.
References
Maas, A. E., Miccoli, A., Stamieszkin, K., Carlson, C. A. & Steinberg, D. K. Allometry and the calculation of zooplankton metabolism in the subarctic Northeast Pacific Ocean. J. Plankton Res. 43, 413–427 (2021).
El-feky, M. & Abo-Taleb, H. Effect of feeding with different types of nutrients on intensive culture of the water flea, Daphnia magna Straus, 1820. Egypt. J. Aqua. Biol. Fish. 24, 655–666 (2020).
Heneash, A. M., Alprol, A. E., Abd El-Hamid, H. T., Khater, M. & El Damhogy, K. A. Assessment of water pollution induced by anthropogenic activities on zooplankton community in Mariout Lake using statistical simulation. Arab. J. Geosci. 14, 1–21 (2021).
Hejazi, M. A. & Wijffels, R. H. Milking of microalgae. Trends Biotechnol. 22, 189–194 (2004).
Pachiappan, P., Santhanam, P., Begum, A. & Prasath, B. B. in Basic and applied phytoplankton biology 1–24 (Springer, 2019).
Mona, M. H., Rizk, E.-S.T., El-feky, M. & Elawany, M. E. Effect of nutritional quality of rotifers and copepods on sea bream (Sparus aurata) fry fish productivity. Egypt. J. Exp. Biol. 15, 135–142 (2019).
Ashour, M., Abo-Taleb, H., Abou-Mahmoud, M. & El-Feky, M. Effect of the integration between plankton natural productivity and environmental assessment of irrigation water, El-Mahmoudia Canal, on aquaculture potential of Oreochromis niloticus. Turk. J. Fish. Aquat. Sci. 18, 1163–1175 (2018).
E Hassan, S., M Azab, A., A Abo-Taleb, H. & M El-Feky, M. Effect of replacing fish meal in the fish diet by zooplankton meal on growth performance of Dicentrarchus labrax (Linnaeus, 1758). Egyptian Journal of Aquatic Biology and Fisheries 24, 267–280 (2020).
Abo-Taleb, H. A. et al. Effect of a new feed Daphnia magna (Straus, 1820), as a fish meal substitute on growth, feed utilization, histological status, and economic revenue of grey mullet, Mugil cephalus (Linnaeus 1758). Sustainability 13, 7093 (2021).
Abo-Taleb, H. A. et al. Growth performance, feed utilization, gut integrity, and economic revenue of grey mullet, Mugil cephalus, fed an increasing level of dried zooplankton biomass meal as fishmeal substitutions. Fishes 6, 38 (2021).
A Abo-Taleb, H. et al. Isolation and cultivation of the freshwater amphipod Gammarus pulex (Linnaeus, 1758), with an evaluation of its chemical and nutritional content. Egypt. J. Aqua. Biol. Fish. 24, 69–82 (2020).
Liu, Z., Malinowski, C. R. & Sepúlveda, M. S. Emerging trends in nanoparticle toxicity and the significance of using Daphnia as a model organism. Chemosphere, 132941 (2021).
Herawati, V. E., Nugroho, R. & Hutabarat, J. in IOP Conference Series: Earth and Environmental Science. 012004 (IOP Publishing).
da Silva Campos, C. V. F. et al. Production of Daphnia similis Claus, 1876 using wastewater from tilapia cultivation in a biofloc system. Aquacul. Int. 28, 403–419 (2020).
Shahid, N., Becker, J. M., Krauss, M., Brack, W. & Liess, M. Adaptation of Gammarus pulex to agricultural insecticide contamination in streams. Sci. Total Environ. 621, 479–485 (2018).
Palmia, B. et al. Effects of drying and re-wetting on litter decomposition and nutrient recycling: a manipulative experiment. Water 11, 708 (2019).
Vigneron, A. et al. Evolution of cadmium tolerance and associated costs in a Gammarus fossarum population inhabiting a low-level contaminated stream. Ecotoxicology 24, 1239–1249 (2015).
Macneil, C., Dick, J. T. & Elwood, R. W. The dynamics of predation on Gammarus spp (Crustacea: Amphipoda). Biol. Rev. 74, 375–395 (1999).
Köprücü, K. & Özdemir, Y. Apparent digestibility of selected feed ingredients for Nile tilapia (Oreochromis niloticus). Aquaculture 250, 308–316 (2005).
Rufchaei, R., Hoseinifar, S. H., Mirzajani, A. & Van Doan, H. Dietary administration of Pontogammarus maeoticus extract affects immune responses, stress resistance, feed intake and growth performance of Caspian roach (Rutilus caspicus) fingerlings. Fish Shellfish Immunol. 63, 196–200 (2017).
Harlıoğlu, M. M. & Farhadi, A. Importance of Gammarus in aquaculture. Aquacult. Int. 26, 1327–1338 (2018).
Jones, K. E. et al. Global trends in emerging infectious diseases. Nature 451, 990–993 (2008).
Hashem, A. H. et al. Bacillus megaterium-mediated synthesis of selenium nanoparticles and their antifungal activity against Rhizoctonia solani in Faba bean plants. J. Fungi 7, 195 (2021).
Nassar, O., Desouky, S. E., El-Sherbiny, G. M. & Abu-Elghait, M. Correlation between phenotypic virulence traits and antibiotic resistance in Pseudomonas aeruginosa clinical isolates. Microb. Pathog. 162, 105339 (2022).
Khan, S. T., Musarrat, J. & Al-Khedhairy, A. A. Countering drug resistance, infectious diseases, and sepsis using metal and metal oxides nanoparticles: current status. Colloids Surf., B 146, 70–83 (2016).
Waters, E. M., Rowe, S. E., O’Gara, J. P. & Conlon, B. P. Convergence of Staphylococcus aureus persister and biofilm research: can biofilms be defined as communities of adherent persister cells?. PLoS Pathog. 12, e1006012 (2016).
El-gamal, M. S., Desouky, S. E., Hassan, S. E. & Elghait, M. A. Phenotypic and biochemical characterization of specific virulence determinants regulated By Agr quorum sensing system in Staphylococcus aureus. Al Azhar Bull. Sci. 9, 217–226 (2017).
Okba, M. M. et al. UPLC-ESI-MS/MS profiling of the underground parts of common Iris species in relation to their anti-virulence activities against Staphylococcus aureus. J. Ethnopharmacol. 282, 114658 (2022).
Okba, M. M., El-Shiekh, R. A., Abu-Elghait, M., Sobeh, M. & Ashour, R. HPLC-PDA-ESI-MS/MS profiling and anti-biofilm potential of eucalyptussideroxylon flowers. Antibiotics 10, 761 (2021).
Alzahrani, A. Y., Ammar, Y. A., Salem, M. A., Abu-Elghait, M. & Ragab, A. Design, synthesis, molecular modeling, and antimicrobial potential of novel 3-[(1H-pyrazol-3-yl) imino] indolin-2-one derivatives as DNA gyrase inhibitors. Arch. Pharm. 355, 2100266 (2022).
Alzahrani, A. Y. et al. Development of novel indolin-2-one derivative incorporating thiazole moiety as DHFR and quorum sensing inhibitors: Synthesis, antimicrobial, and antibiofilm activities with molecular modelling study. Bioorg. Chem. 119, 105571 (2022).
Desouky, S. E. et al. Secondary metabolites of actinomycetales as potent quorum sensing inhibitors targeting gram-positive pathogens: in vitro and in silico study. Metabolites 12, 246 (2022).
Chandrasekaran, M., Thangavelu, B., Chun, S. C. & Sathiyabama, M. Proteases from phytopathogenic fungi and their importance in phytopathogenicity. J. Gen. Plant Pathol. 82, 233–239 (2016).
Compant, S., Duffy, B., Nowak, J., Clément, C. & Barka, E. A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 71, 4951–4959 (2005).
Karkanis, A. et al. Faba bean cultivation–revealing novel managing practices for more sustainable and competitive European cropping systems. Front. Plant Sci. 9, 1115 (2018).
Haddoudi, I. et al. Occurrence of fungal diseases in faba bean (Vicia faba L.) under salt and drought stress. Eur. J. Plant Pathol. 159, 385–398 (2021).
Coca-Morante, M. & Mamani, F. Control of leaf spot diseases on ecotypes of faba bean (Vicia faba L.) produced in the Andean region of Bolivia. Am. J. Plant Sci. 2012 (2012).
Boakye, Y. D., Osafo, N., Danquah, C. A., Adu, F. & Agyare, C. Antimicrobial agents: Antibacterial agents, anti-biofilm agents, antibacterial natural compounds, and antibacterial chemicals. Antimicrob. Antibiot. Resistance, Antibiofilm Strateg. Activity Methods, 75 (2019).
Shreadah, M. A., Masoud, M. S., Khattab, A.-R.M. & El Zokm, G. M. Nutrient fluxes and sediments composition in El Mex Bay and surround drains, Alexandria Egypt. Am. J. Anal. Chem. 6, 513 (2015).
Lee, J., Durst, R. & Wrolstad, R. AOAC official method 2005.02: total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method. Official methods of analysis of AOAC International 2 (2005).
Abdelhameed, R. M., Abu-Elghait, M. & El-Shahat, M. Hybrid three MOFs composites (ZIF-67@ ZIF-8@ MIL-125-NH2): Enhancement the biological and visible-light photocatalytic activity. J. Environ. Chem. Eng. 8, 104107 (2020).
Desouky, S. E., El-Gamal, M. S., Mohammed, A. F. & Abu-Elghait, M. A. Determination of some virulence factors in Staphylococcus spp. isolated from clinical samples of different Egyptian patients. World Appl. Sci. J. 32, 731–740 (2014).
Elbahnasawy, M. A., Shehabeldine, A. M., Khattab, A. M., Amin, B. H. & Hashem, A. H. Green biosynthesis of silver nanoparticles using novel endophytic Rothia endophytica: Characterization and anticandidal activity. J. Drug Deliv. Sci. Technol. 62, 102401 (2021).
Mohamed, A. A., Abu-Elghait, M., Ahmed, N. E. & Salem, S. S. Eco-friendly mycogenic synthesis of ZnO and CuO nanoparticles for in vitro antibacterial, antibiofilm, and antifungal applications. Biol. Trace Elem. Res. 199, 2788–2799 (2021).
Chavez-Esquivel, G. et al. Antimicrobial activity of graphite oxide doped with silver against Bacillus subtilis, Candida albicans, Escherichia coli, and Staphylococcus aureus by agar well diffusion test: Synthesis and characterization. Mater. Sci. Eng., C 123, 111934 (2021).
Abdel-Azeem, E. A. & Elsayed, B. A. Phytotoxicity of silver nanoparticles on Vicia faba seedlings. NY Sci. J 6, 148–155 (2013).
Jobling, A. & Jobling, C. Conversion of bone to edible products. Proceedings-Easter School in Agricultural Science, University of Nottingham (1983).
Jagtap, G., Dhopte, S. & Dey, U. Bio-efficacy of different antibacterial antibiotic, plant extracts and bioagents against bacterial blight of soybean caused by Pseudomonas syringae pv. glycinea. Sci. J. Microbiol. 1, 1–9 (2012).
Hamed, A. et al. Meleagrin from marine fungus Emericella dentata Nq45: crystal structure and diverse biological activity studies. Nat. Prod. Res. 35, 3830–3838 (2021).
He, L., Liu, Y., Mustapha, A. & Lin, M. Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol. Res. 166, 207–215 (2011).
Koenraadt, H., Somerville, S. C. & Jones, A. Characterization of mutations in the beta-tubulin gene of benomyl-resistant field strains of Venturia inaequalis and other plant pathogenic fungi. Phytopathology 82, 1348–1354 (1992).
Leroux, P. et al. Mechanisms of resistance to fungicides in field strains of Botrytis cinerea. Pest Manag. Sci. 58, 876–888 (2002).
Suantika, G. et al. The use of cyanobacteria arthrospira platensis and cladoceran daphnia magna as complementary protein and lipid sources in transitional diet for common carp (Cyprinus carpio L.) Nursery. Natl. Resour. 7, 423 (2016).
Caicedo-López, L. H. et al. Elicitores: implicações bioéticas para a agricultura e a saúde humana. Revista Bioética 29, 76–86 (2021).
Cauchie, H.-M., Jaspar-Versali, M.-F., Hoffmann, L. & Thomé, J.-P. in Annales de Limnologie-International Journal of Limnology. 223–231 (EDP Sciences).
Zhang, X., Li, K., Xing, R., Liu, S. & Li, P. Metabolite profiling of wheat seedlings induced by chitosan: Revelation of the enhanced carbon and nitrogen metabolism. Front. Plant Sci. 8 (2017).
Bittelli, M., Flury, M., Campbell, G. S. & Nichols, E. J. Reduction of transpiration through foliar application of chitosan. Agric. For. Meteorol. 107, 167–175 (2001).
Rady, M. M. et al. Exogenous gibberellic acid or dilute bee honey boosts drought stress tolerance in Vicia faba by rebalancing osmoprotectants, antioxidants, nutrients, and phytohormones. Plants 10, 748 (2021).
Acknowledgements
The researchers extend their sincere thanks to the Egyptian Ministry of Higher Education and Scientific Research for its continuous support, and all the results in the current research are some of the outputs of a project funded from the budget of the Minister of Scientific Research office (a project of national strategy program for genetic engineering and biotechnology, phase III). The research project is titled “Innovation of new non-conventional methods for the use of plankton and their extracts as alternative natural sources for animal protein in fish diets and the medical enhancements” (Project ID: 44 H/2019).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
A.M.K.: Conceptualization, Methodology of antimicrobial, Resources, Investigation, software, writing-original draft. H.A.A.-T.: Conceptualization, Methodology of plankton isolation, writing-review and original draft, editing Resources. A.M.A.: Resources, Investigation of antifungal activity, writing-review. M.A.M.E.-T.: Methodology of plankton isolation, software, writing-review. M.M.M.E.-F.: Conceptualization, Methodology of Plankton identification, Resources. M.A.-E.: Conceptualization, Methodology, Investigation of antibiofilm activity, software, writing-review and original draft, editing and submitting the paper to the journal.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khattab, A.M., Abo-Taleb, H.A., Abdelaziz, A.M. et al. Daphnia magna and Gammarus pulex, novel promising agents for biomedical and agricultural applications. Sci Rep 12, 13690 (2022). https://doi.org/10.1038/s41598-022-17790-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-17790-z
This article is cited by
-
Hindering the biofilm of microbial pathogens and cancer cell lines development using silver nanoparticles synthesized by epidermal mucus proteins from Clarias gariepinus
BMC Biotechnology (2024)
-
Interception of Epoxide ring to quorum sensing system in Enterococcus faecalis and Staphylococcus aureus
AMB Express (2023)
-
Optimization of oil yield of Pelargonium graveolens L'Hér using Box-Behnken design in relation to its antimicrobial activity and in silico study
Scientific Reports (2023)
-
Potential impacts of Ascophyllum nodosum, Arthrospira platensis extracts and calcium phosphite as therapeutic nutrients for enhancing immune response in pepper plant against Fusarium wilt disease
Biomass Conversion and Biorefinery (2023)
-
Gum Arabic-assisted biomass synthesis of bimetallic ZnO-CuO nanoparticles using gamma rays for controlling potato post-harvest tuber rots-causing Alternaria solani: towards improving food safety
Biomass Conversion and Biorefinery (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.