Abstract
Novel crosslinking bio polyurethane based polymeric solid–solid phase change materials (SSPCM) were synthesized using castor oil (CO) based hyperbranched polyols as crosslinkers. CO-based hyperbranched polyols were synthesized by grafting 1-mercaptoethanol or α–thioglycerol via a thiol-ene click reaction method (coded as COM and COT, respectively). Subsequently, the three SSPCMs were synthesized by a two-step prepolymer method. Polyethylene glycol was used as the phase change material in the SSPCMs, while the CO-based hyperbranched polyols and two types of diisocyanate (hexamethylene diisocyanate (HDI) and 4,4'-diphenylmethane diisocyanate) served as the molecular frameworks. Fourier transform infrared spectroscopy indicated the successful synthesis of the SSPCMs. The solid–solid transition of the prepared SSPCMs was confirmed by X-ray diffraction analysis and polarized optical microscopy. The thermal transition properties of the SSPCMs were analyzed by differential scanning microscopy. The isocyanate and crosslinker types had a significant influence on the phase transition properties. The SSPCM samples prepared using HDI and COT exhibited the highest phase transition enthalpy of 126.5 J/g. The thermal cycling test and thermogravimetric analysis revealed that SSPCMs exhibit outstanding thermal durability. Thus, the novel SSPCMs based on hyperbranched polyols have great potential for application as thermal energy storage materials.
Similar content being viewed by others
Introduction
The continuous increase in fuel prices and efforts toward achieving carbon neutrality in recent years has necessitated the development of sustainable energy sources1,2. From this perspective, thermal energy storage (TES) has become an important part of renewable energy systems3,4,5. To meet the growing demand for cheaper and more efficient TES, phase change materials (PCM) have recently gained attention for their applicability in the development of TES devices6. PCMs exhibit a large latent heat capacity and uniquely maintain a constant temperature during latent heat transfer, which make them suitable for thermal energy management in various applications such as solar energy storage7,8,9, solar desalination10,11,12, building materials13,14,15, and waste heat recovery16,17,18. In the past decade, studies have focused on the suitability of polyethylene glycol (PEG)19, paraffin waxes20, fatty acids21, inorganic salt hydrates22, and the eutectics of these compounds23 as PCMs. However, the development of TES materials using pristine PCMs is challenging, it requires special packaging technology to support the solid–liquid phase transition. Compared to the pristine PCM, solid–solid PCMs (SSPCM) are economical and convenient to produce, face no risk of leakage, and exhibit shape stability24,25,26. Thus, various methods have been studied to fabricate SSPCMs, such as grafting, crosslinking polymerization, and introduction of carbon materials, titanium oxide, black phosphorus, and their hybrids27,28,29,30,31,32,33,34,35.
Polyurethane (PU) is one of the most important polymeric materials that has been widely applied in industries such as automotive, furniture, thermal shielding materials, and sound insulating materials, because it is economical and convenient to produce36,37,38,39. PU is a polymer synthesized through the addition polymerization between a multi-functional alcohol and diisocyanate. As only few types of commercial diisocyanate, the polyols used play a critical role in determining the properties of PUs. Recently, numerous studies on PU-based SSPCMs have been reported in which PEG was used as the PCM material40,41,42. A PU-based SSPCM typically has a crosslinked structure, which limits the dissolution and flow of the PEG soft segment. Several researchers have developed improved polymeric SSPCMs using multifunctional polyol as a crosslinking agent31,43,44,45.
The use of castor oil (CO) as a bio-based polyol, which can replace conventional petrochemical polyols, has attracted considerable attention because of its biodegradability, abundance, nontoxic nature, cost competitiveness, and biodegradability32,46. CO is primarily composed of ricinoleic acid, with a double bond on C–9 and a hydroxyl group on C–12; therefore, it has been frequently applied in studies on bio PU (BPU). However, the applications of CO-based PU have been limited because of its low crosslinking density, which leads to low mechanical properties and low productivity. To overcome such drawbacks, many studies have conducted to facilitate the use of CO by epoxidation47, transesterification48, ozonolysis49, and thiol-ene click reactions50,51. Previously, we have reported the use of the thiol-ene click reaction for grafting 1-mercaptoethanol and α-thioglycerol onto CO52,53.
In this study, two types of CO-based hyperbranched polyols were prepared, which were subsequently used in the production of BPU-based SSPCMs. The structural properties of SSPCM were analyzed by Attenuated total reflection (ATR)-Fourier transform infrared (FTIR) spectroscopy, while X-ray diffraction (XRD) analysis and polarized optical microscopy (POM) were conducted to determine the degree of crystallinity. The phase change properties of the SSPCMs were thoroughly examined by differential scanning calorimetry (DSC). After the thermal cycling test, DSC and FT-IR analysis were performed again to verify the thermal reliability of the SSPCMs. Additionally, the thermal stability of the SSPCMs was verified by thermogravimetric analysis (TGA).
Experimental
Materials
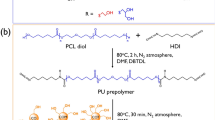
CO was supplied from Yakuri Pure Chemical Co., Ltd. Mercaptoethanol, α-thioglycerol, 2, 2-dimethoxy-2-phenylacetophenone (DMPA), acetic anhydride, pyridine, and dibutyltin dilaurate (DBTDL) were purchased from Sigma-Aldrich (USA). PEG (MW 4000 g mol-1) was supplied from Kanto Chemical Co., Inc. Ethyl acetate (EA), N, N-dimethylformamide (DMF), anhydrous MgSO4 and NaCl were supplied from Daejung Chemical (Korea). 4, 4'-diphenylmethane diisocyanate (MDI) was purchased from Tokyo Chemical Industry Co., Ltd. Hexamethylene diisocyanate (HDI) was supplied from Wako Chemicals. All reagents were used as received without further purification.
Preparation of CO-based hyperbranched polyols
The synthesis of CO-based hyperbranched polyols was carried out according to the method used in our previous studies52,53. The preparation scheme is shown in Scheme 1. CO, thiol, DMPA (as a photoinitiator), and EA (as a solvent) were placed in quartz tubes and rolled in a tube roller for 24 h after placing it in a photochemical reactor equipped with UV-A lamps. The molar ratio of the thiols to C=C double bonds of CO was set to 4:1. After the reaction, the products were washed with distilled water and aqueous NaCl solution at least five times. The obtained polyols were dried using MgSO4, and remained organic solvent was eliminated by rotary evaporation. The obtained polyols were then vacuum dried for 24 h. The CO‐based polyols grafted with 2-mercaptoethanol and α‐thioglycerol were coded as COM and COT, respectively.
Preparation of SSPCMs
As shown in Scheme 2, the synthesis of SSPCMs using PEG as a PCM was conducted using a two-step prepolymer method. First, a predetermined amount of PEG and the two types of diisocyanate (molar ratio of PEG and diisocyanate = 1:2) were separately dissolved in an appropriate amount of DMF, and the diisocyanate solution was added dropwise to the PEG solution with gentle stirring. A few drops of DBTDL (0.05 wt% based on the total weight of final product) were added as a catalyst. This reaction was carried out at 80 °C under N2 atmosphere for 3 h to obtain isocyanate-terminated prepolymers. Second, three types of crosslinkers were dissolved in DMF in adequate molar ratios and slowly added into prepared prepolymer solutions. After 18 h, the reaction mixtures were poured into a PTFE-coated mold and thermally cured at 80 °C for 24 h in a convection oven. The products were then kept in vacuo at room temperature for 24 h before analysis. The sample codes are listed in Table 1 according to the types of diisocyanates and crosslinkers used.
Characterization
ATR-FTIR spectrometer (Nicolet iS50, Thermo Fisher Scientific) was used to determine the structure of the SSPCMs over the range of 500–4000 cm−1. The FTIR spectra were recorded at a resolution of 4 cm−1, and 64 scans were averaged per experiment. Wide-angle XRD patterns were collected using an X-ray diffractometer (Rigaku, SmartLab), and diffractograms were scanned in a 2θ range of 5°‒70° at scan rate of 3°/min. The spherulite morphologies were observed through POM (Olympus BX-51 TX) equipped with a CCD camera. The thermal behavior of the SSPCMs was investigated by DSC (DSC 2010, TA Instrument). An accelerated thermal cycling test was performed for 100 cycles in the temperature range of 0‒100 °C using a thermocycler (MJ research). All samples were tested for 5 consecutive heating–cooling scan from 0 to 100 °C at 5 °C/min under nitrogen atmosphere. TGA of the SSPCMs was performed with nitrogen purging from 30 to 800 °C at 10 °C/min.
Result and discussion
Preparation of polyols
COM and COT were successfully synthesized under the optimized reaction conditions obtained from our previous studies52,53. The FTIR spectra of the newly prepared polyols are shown in Fig. S1. As stated in the supporting information, the chemical structures of the synthesized COM and COT are consistent with the results of our previous studies52,53. The hydroxyl values of CO, COM, and COT measured according to the ASTM D1957-86 standard were 160, 270, and 380 mg KOH g−1, respectively.
Structural analysis of the SSPCMs
The chemical structures of the synthesized SSPCMs were analyzed by FTIR spectroscopy, and the results are presented in Fig. 1. The disappearance of the isocyanate peak at 2265 cm−1 in all the SSPCM spectra indicates that the PUs were successfully synthesized. The newly formed absorbance bands at 3341 and 1539 cm−1 correspond to the N–H stretching and amide vibration within the PU bond, respectively. The FTIR analysis did not show a significant difference in the spectra in terms of the functionality of the crosslinker or the type of isocyanate.
Crystalline structure analysis of the SSPCMs
The crystal structures of the SSPCMs were analyzed by XRD analysis, and the results are presented in Fig. 2. Two strong diffraction peaks were detected in all the SSPCMs spectra, indicating that the SSPCMs had crystalline structures. PEG is known to exhibit sharp diffraction peaks at 19.1° (120) and 23.3° (032) implying that it is a semi-crystalline polymer with high crystallinity. The XRD patterns of the SSPCMs also showed peaks at 19.1° (120) and 23.3° (032), suggesting that the crystallinity of SSPCM is dominated by crystallinity of PEG. Compared to the XRD pattern of PEG, the SSPCMs had a lower peak intensity and a broader full width at half maximum. The crosslinked structure formed by hyperbranched polyols partially confined the PEG chain, which led to a decrease in the degree of crystallinity. Meanwhile, a significant difference in the degree of crystallinity was observed depending on the isocyanate type used. For PEG, the peak corresponding to the (032) plane in the XRD pattern had a higher intensity than that to the (120) plane. However, for the SSPCMs prepared using MDI, the intensity of the peak corresponding to the (032) plane decreased; for the SSPCMs prepared using HDI, the crystal structure of the pristine PEG was retained as the functionality of the crosslinker increased. The reduced intensity of the peak corresponding to the non-equatorial (032) plane of the SSPCMs prepared using MDI implied that thinner PEG lamellae and tilt of the PEG chain in the lamellae were generated with the formation of the crosslinked structures54. This was more prominent for the SSPCMs prepared using MDI because the rigid benzene ring further confines the PEG chain41.
For further analysis of the crystal structure of the synthesized SSPCMs, the crystal morphologies of the pristine PEG and the SSPCMs were recorded through POM, and the results are shown in Fig. 3. All SSPCM samples demonstrated cross-extinction patterns caused by polarized light. As shown in Fig. 3a, the crystal of pristine PEG grew to several millimeters in size, while the synthesized SSPCMs demonstrated a decrease in the crystal size because the crosslinked structure limited the motion of the soft segment. The crystal structure remained unchanged when the temperature of the SSPCMs was raised to the phase transition temperature. However, as the temperature reached the transition point, the crystal structure began to disappear and eventually no crystal features could be observed in the POM image (Fig. 3e and i). As shown in Fig. 4, the SSPCMs remained in the solid state even at a high temperature close to the phase transition temperature. The solid–liquid phase transition did not occur in all the synthesized SSPCMs, even at temperature higher than the transition point.
Phase transition properties of the SSPCMs
DSC analysis was performed to examine the phase transition properties of the synthesized SSPCMs. The five consecutive DSC scans after heating and cooling of the pristine PEG and SSPCMs are presented in Figs. S2 and 5, respectively. For the pristine PEG, the solid–liquid phase transition resulted in very sharp exothermic and endothermic peaks in the range of 20–70 °C. The DSC scans for all SSPCMs also exhibited distinct exothermic and endothermic peaks, suggesting that the SSPCMs had reversible thermal storage and release properties. A consecutive heating–cooling test was performed to verify the potential reusability of the SSPCMs. The DSC thermograms remained unchanged during the test. The phase transition properties based on the five consecutive DSC scans are summarized in Table 2. The exothermic and endothermic enthalpies of all SSPCM structures were significantly lower than those of the pristine PEG. This implies that the heat storage and release capacities of the synthesized SSPCMs were lower than those of the pristine PEG. The solid–solid phase transition of the SSPCMs is due to the transformation of the PEG soft segment from a crystalline to an amorphous state. The PEG chains in the SSPCM structures were strongly confined to a finite interspace because of the crosslinked network formed by the hyperbranched crosslinker. Thus, the crystallization of the PEG chain was limited and certain PEG chains were prevented from crystallization in the phase transition process. Therefore, the crystal domain of the SSPCMs was reduced compared to that of the pristine PEG, resulting in reduced heat storage and release capacities. These inferences are supported by XRD analysis and POM results previously discussed in this study.
Regarding the influence of the functionality of the crosslinker on the changes in the SSPCMs endothermic enthalpy, the degree of latent heat for both MDI- and HDI-based SSPCM followed the order; CO < COM < COT. Studies have revealed several factors that influence the SSPCM phase transition properties, including soft segment content by weight, crystalline state of the soft segments, and steric hindrance of the crosslinking points. With an increase in the functionality of the crosslinker, the synthesized SSPCM can accommodate more PEG, thereby increasing the latent heat storage capacity. For better understanding of the phase transition properties of the SSPCMs on the basis of the functionality of the crosslinker, the relative latent heat efficiency was calculated using the following equation:
where \({\Delta }H_{m.SSPCM}\) and \({\Delta }H_{m.PEG}\) indicate the melting enthalpies of SSPCMs and the pristine PEG, respectively, and \(\omega_{PEG}\) is the mass fraction of PEG in the SSPCM. Table 2 presents the relative latent heat efficiency of the SSPCM. The \(\eta\) value indicates the influence of the framework structure, where higher values suggest less heat loss of the PEG in the SSPCM. Both MDI- and HDI-based SSPCMs exhibited \(\eta\) values in the order of CO > COM > COT, suggesting that the increased level of steric hindrance caused by the increased crosslinking density led to an increase in the number of soft segments that cannot form the crystal structure during the phase transition process.
Considering the influence of the isocyanate type on the endothermic enthalpy of the SSPCMs, the latent heat of the HDI-based SSPCM was higher than that of the MDI-based SSPCM when an identical crosslinker was used. The \(\eta\) value of H.CO, H.COM, and H.COT were shown to be increased by 3.0%, 3.8%, and 3.5%, respectively, compared to M.CO, M.COM, and M.COT, which is due to the presence of a rigid benzene ring in MDI that prevents PEG crystallization.
The analysis of the phase transition properties of the SSPCMs by DSC demonstrated that using hyperbranched polyols led to an increase in latent heat efficiency, although the relative latent heat efficiency decreased due to an increase in the crosslinking density. Steric hindrance arising from the structural characteristics of isocyanate is another factor influencing the latent heat efficiency of the SSPCMs.
The results of the crystalline structure and phase transition property analyses confirmed that the synthesized SSPCMs exhibited repetitive heat storage and release characteristics without any leakage. The phase change mechanisms of the SSPCMs crosslinked with CO and COT are shown in Fig. 6. The crosslinked networks formed by the CO-based hyperbranched polyols served as the molecular framework, which prevented the dissolution and flow of the PEG soft segment. Compared with the CO-based SSPCM, the COT-based SSPCM exhibited higher thermal energy storage and release efficiencies because a greater amount of the PCM material can be integrated into it. Additionally, Table 3 listed the comparison of phase change properties of the H.COM and H.COT with those of other crosslinked polymeric SSPCMs in the literature. As clearly shown in the tabulated data, the latent heat capacity of the SSPCMs synthesized in present work is higher than the most of SSPCMs reported earlier 40,41,55,56,57,58,59,60,61,62,63,64.
Thermal reliability of SSPCM
To verify the thermal stability of the SSPCMs, an accelerated thermal cycling test (100 cycles from 0 to 100 °C) was performed. Even after the 100th thermal cycle, no weight reduction was observed in the SSPCMs, which indicates that they exhibit thermal reliability even after repeated use within the phase transition temperature range. This result verified the applicability of the SSPCMs as PCM materials.
Figure 7 shows the FTIR results for the SSPCMs after 100 cycles of the thermal cycling test. The initial FTIR curves for the SSPCMs are represented by dotted lines. Even after 100 cycles of the thermal cycling test, the FTIR curves for the SSPCMs displayed almost identical shapes and locations of the absorption peaks, suggesting that neither thermal decomposition nor structural changes occurred during repetitive thermal cycling.
Figure 8 shows the five consecutive DSC heating–cooling scans for the SSPCMs after the 100-cycle thermal cycling test; the phase transition characteristics are listed in Table 4. All the SSPCMs demonstrated distinct exothermic and endothermic peaks even after thermal cycling test and exhibited with a relatively uniform thermal behavior. These results confirmed that the SSPCM structures retained their reversible heat storage and release properties. However, the melting enthalpies decreased for all samples, and the degree of reduction of relative latent heat efficiency varied based on the isocyanate and crosslinker types. Figure 9 shows the initial relative latent heat efficiency of the SSPCMs followed by the final relative latent heat efficiency and the degree of reduction in the relative latent heat efficiency after thermal cycling. MDI-based SSPCMs exhibited a greater decrease in efficiency than HDI-based SSPCMs. Regardless of the isocyanate type, the reduction of efficiency gradually became smaller as the crosslinker functionality increased; M.COT and H.COT demonstrated reductions by 4.1% and 3.3%, respectively. Thus, hyperbranched polyols are feasible for use as crosslinkers for the production of highly efficient and durable SSPCMs.
TGA was performed to evaluate the thermal stability of the SSPCMs; the results are presented in Fig. 10. The weight of the pristine PEG and SSPCMs remained unchanged up to approximately 280 °C, implying that the SSPCMs were highly stable in the phase transition temperature range, facilitating TES application. Moreover, all the SSPCMs exhibited thermal decomposition at temperatures higher than those for the pristine PEG, which can be attributed to the crosslinked molecular structure of the SSPCMs. The derivative TGA curves in Fig. 10b shows that the SSPCMs exhibited two phases degradation behavior in contrast to the phase 1 thermal degradation behavior of the pristine PEG. Phase 1 is marked by the thermal degradation of the urethane bonds in the SSPCMs, whereas phase 2 is represented by the degradation of PEG37,65. Also, the maximum decomposition temperature of all the SSPCMs was higher than that of PEG, exhibiting the outstanding thermal stability of the SSPCMs.
Conclusion
In this study, two types of hyperbranched polyols, COM and COT, with an increased hydroxyl value, were prepared via the thiol-ene click reaction with CO. A new SSPCM series for TES was successfully prepared using PEG as the PCM in the SSPCMs, while CO, COM, and COT provided the molecular framework. FTIR analysis of the SSPCMs revealed the formation of PU structures, which confirmed the successful synthesis of the SSPCMs. Moreover, the results of the XRD analysis and POM indicated the solid–solid phase transition. The results of the DSC analysis revealed that the isocyanate and crosslinker types had a significant influence on the phase transition properties. The H.COT exhibited the highest phase transition enthalpy at 126.5 J/g. Furthermore, the results of the thermal cycling test and TGA demonstrated the outstanding durability of the SSPCMs. Thus, the novel SSPCMs based on hyperbranched polyols has great potential to be applied in TES materials.
References
Zhou, D., Zhao, C.-Y. & Tian, Y. Review on thermal energy storage with phase change materials (PCMs) in building applications. Appl. Energy 92, 593–605 (2012).
Sharma, R., Ganesan, P., Tyagi, V., Metselaar, H. & Sandaran, S. Developments in organic solid–liquid phase change materials and their applications in thermal energy storage. Energy Convers. Manag. 95, 193–228 (2015).
Alva, G., Lin, Y. & Fang, G. An overview of thermal energy storage systems. Energy 144, 341–378 (2018).
Regin, A. F., Solanki, S. & Saini, J. Heat transfer characteristics of thermal energy storage system using PCM capsules: A review. Renew. Sustain. Energy Rev. 12, 2438–2458 (2008).
Kuravi, S., Trahan, J., Goswami, D. Y., Rahman, M. M. & Stefanakos, E. K. Thermal energy storage technologies and systems for concentrating solar power plants. Prog. Energy Combust. Sci. 39, 285–319 (2013).
Prieto, C. & Cabeza, L. F. Thermal energy storage (TES) with phase change materials (PCM) in solar power plants (CSP). Concept and plant performance. Appl. Energy 254, 113646 (2019).
Al-Jethelah, M., Tasnim, S. H., Mahmud, S. & Dutta, A. Nano-PCM filled energy storage system for solar-thermal applications. Renew. Energy 126, 137–155 (2018).
Mofijur, M. et al. Phase change materials (PCM) for solar energy usages and storage: An overview. Energies 12, 3167 (2019).
Javadi, F., Metselaar, H. & Ganesan, P. Performance improvement of solar thermal systems integrated with phase change materials (PCM), a review. Sol. Energy 206, 330–352 (2020).
Al-harahsheh, M., Abu-Arabi, M., Mousa, H. & Alzghoul, Z. Solar desalination using solar still enhanced by external solar collector and PCM. Appl. Therm. Eng. 128, 1030–1040 (2018).
Abu-Arabi, M., Al-harahsheh, M., Mousa, H. & Alzghoul, Z. Theoretical investigation of solar desalination with solar still having phase change material and connected to a solar collector. Desalination 448, 60–68 (2018).
Yousef, M. S. & Hassan, H. Energetic and exergetic performance assessment of the inclusion of phase change materials (PCM) in a solar distillation system. Energy Convers. Manag. 179, 349–361 (2019).
Osterman, E., Tyagi, V., Butala, V., Rahim, N. A. & Stritih, U. Review of PCM based cooling technologies for buildings. Energy Build. 49, 37–49 (2012).
Souayfane, F., Fardoun, F. & Biwole, P.-H. Phase change materials (PCM) for cooling applications in buildings: A review. Energy Build. 129, 396–431 (2016).
Zavrl, E. & Stritih, U. Improved thermal energy storage for nearly zero energy buildings with PCM integration. Sol. Energy 190, 420–426 (2019).
Royo, P. et al. High-temperature PCM-based thermal energy storage for industrial furnaces installed in energy-intensive industries. Energy 173, 1030–1040 (2019).
Li, D., Wang, J., Ding, Y., Yao, H. & Huang, Y. Dynamic thermal management for industrial waste heat recovery based on phase change material thermal storage. Appl. Energy 236, 1168–1182 (2019).
Du, K., Calautit, J., Eames, P. & Wu, Y. A state-of-the-art review of the application of phase change materials (PCM) in mobilized-thermal energy storage (M-TES) for recovering low-temperature industrial waste heat (IWH) for distributed heat supply. Renew. Energy https://doi.org/10.1016/j.renene.2020.12.057 (2020).
Sundararajan, S., Samui, A. B. & Kulkarni, P. S. Versatility of polyethylene glycol (PEG) in designing solid–solid phase change materials (PCMs) for thermal management and their application to innovative technologies. J. Mater. Chem. A 5, 18379–18396 (2017).
Kumar, K., Sharma, K., Verma, S. & Upadhyay, N. Experimental investigation of graphene-paraffin wax nanocomposites for thermal energy storage. Mater. Today Proc. 18, 5158–5163 (2019).
Wang, R., Ren, M., Gao, X. & Qin, L. Preparation and properties of fatty acids based thermal energy storage aggregate concrete. Constr. Build. Mater. 165, 1–10 (2018).
Wong-Pinto, L.-S., Milian, Y. & Ushak, S. Progress on use of nanoparticles in salt hydrates as phase change materials. Renew. Sustain. Energy Rev. 122, 109727 (2020).
Atinafu, D. G., Dong, W., Huang, X., Gao, H. & Wang, G. Introduction of organic-organic eutectic PCM in mesoporous N-doped carbons for enhanced thermal conductivity and energy storage capacity. Appl. Energy 211, 1203–1215 (2018).
Atinafu, D. G., Dong, W., Berardi, U. & Kim, S. Phase change materials stabilized by porous metal supramolecular gels: Gelation effect on loading capacity and thermal performance. Chem. Eng. J. 394, 124806 (2020).
Jebasingh, B. E. & Arasu, A. V. A comprehensive review on latent heat and thermal conductivity of nanoparticle dispersed phase change material for low-temperature applications. Energy Storage Mater. 24, 52–74 (2020).
Wu, S., Yan, T., Kuai, Z. & Pan, W. Thermal conductivity enhancement on phase change materials for thermal energy storage: A review. Energy Storage Mater. 25, 251–295 (2020).
Zhou, Y. et al. Polyurethane-based solid-solid phase change materials with in situ reduced graphene oxide for light-thermal energy conversion and storage. Chem. Eng. J. 338, 117–125 (2018).
Zhou, Y. et al. Polyurethane-based solid-solid phase change materials with halloysite nanotubes-hybrid graphene aerogels for efficient light-and electro-thermal conversion and storage. Carbon 142, 558–566 (2019).
Wei, X. et al. Photo-and electro-responsive phase change materials based on highly anisotropic microcrystalline cellulose/graphene nanoplatelet structure. Appl. Energy 236, 70–80 (2019).
Cao, H. et al. The influence of hydrogen bonding on N-methyldiethanolamine-extended polyurethane solid–solid phase change materials for energy storage. RSC Adv. 7, 11244–11252 (2017).
Sundararajan, S., Samui, A. B. & Kulkarni, P. S. Thermal energy storage using poly (ethylene glycol) incorporated hyperbranched polyurethane as solid–solid phase change material. Ind. Eng. Chem. Res. 56, 14401–14409 (2017).
Qian, Y. et al. Enhanced thermal-to-flexible phase change materials based on cellulose/modified graphene composites for thermal management of solar energy. ACS Appl. Mater. Interfaces 11, 45832–45843 (2019).
Du, X. et al. Ti3C2T x@ PDA-integrated polyurethane phase change composites with superior solar-thermal conversion efficiency and improved thermal conductivity. ACS Sustain. Chem. Eng. 8, 5799–5806 (2020).
Wang, C. et al. Graphene oxide stabilized polyethylene glycol for heat storage. Phys. Chem. Chem. Phys. 14, 13233–13238 (2012).
Sarı, A., Biçer, A. & Alkan, C. Thermal energy storage characteristics of poly (styrene-co-maleic anhydride)-graft-PEG as polymeric solid–solid phase change materials. Sol. Energy Mater. Sol. Cells 161, 219–225 (2017).
Lee, Y. J., Park, C. K. & Kim, S. H. Fabrication of castor-oil/polycaprolactone based bio-polyurethane foam reinforced with nanocellulose. Polym. Compos. 39, 2004–2011 (2018).
Lee, J. H., Park, S. H. & Kim, S. H. Fabrication of bio-based polyurethane nanofibers incorporated with a triclosan/cyclodextrin complex for antibacterial applications. RSC Adv. 10, 3450–3458 (2020).
Park, S. H., Oh, K. W. & Kim, S. H. Reinforcement effect of cellulose nanowhisker on bio-based polyurethane. Compos. Sci. Technol. 86, 82–88 (2013).
Park, S. H., Ryu, Y. S. & Kim, S. H. Effect of modified silica nanoparticle on the properties of bio-based polyurethane ultrafine fibers. J. Mater. Sci. 50, 1760–1769 (2015).
Peng, K. et al. Preparation and properties of β-cyclodextrin/4, 4′-diphenylmethane diisocyanate/polyethylene glycol (β-CD/MDI/PEG) crosslinking copolymers as polymeric solid–solid phase change materials. Sol. Energy Mater. Sol. Cells 145, 238–247 (2016).
Liu, Z. et al. Solvent-free synthesis and properties of novel solid–solid phase change materials with biodegradable castor oil for thermal energy storage. Sol. Energy Mater. Sol. Cells 147, 177–184 (2016).
Du, X., Wang, H., Wu, Y., Du, Z. & Cheng, X. Solid–solid phase-change materials based on hyperbranched polyurethane for thermal energy storage. J. Appl. Polym. Sci. https://doi.org/10.1002/app.45014 (2017).
Cao, Q. & Liu, P. Hyperbranched polyurethane as novel solid–solid phase change material for thermal energy storage. Eur. Polym. J. 42, 2931–2939 (2006).
Sundararajan, S., Samui, A. B. & Kulkarni, P. S. Synthesis and characterization of poly (ethylene glycol)(PEG) based hyperbranched polyurethanes as thermal energy storage materials. Thermochim. Acta 650, 114–122 (2017).
Liao, L., Cao, Q. & Liao, H. Investigation of a hyperbranched polyurethane as a solid-state phase change material. J. Mater. Sci. 45, 2436–2441 (2010).
Lee, J. H. & Kim, S. H. Fabrication of silane-grafted graphene oxide and its effect on the structural, thermal, mechanical, and hysteretic behavior of polyurethane. Sci. Rep. 10, 1–13 (2020).
Chen, J. et al. Highly efficient epoxidation of vegetable oils catalyzed by a manganese complex with hydrogen peroxide and acetic acid. Green Chem. 21, 2436–2447 (2019).
Encinar, J. M., Nogales-Delgado, S., Sánchez, N. & González, J. F. Biolubricants from rapeseed and castor oil transesterification by using titanium isopropoxide as a catalyst: Production and characterization. Catalysts 10, 366 (2020).
Sun, J., Aly, K. I. & Kuckling, D. Synthesis of hyperbranched polymers from vegetable oil based monomers via ozonolysis pathway. J. Polym. Sci. Part A Polym. Chem. 55, 2104–2114 (2017).
Feng, Y. et al. A solvent-free and scalable method to prepare soybean-oil-based polyols by thiol–ene photo-click reaction and biobased polyurethanes therefrom. ACS Sustain. Chem. Eng. 5, 7365–7373 (2017).
Lee, J. H., Park, C. K., Jung, J. S. & Kim, S. H. Synthesis of vegetable oil-based hyperbranched polyol via thiol-yne click reaction and their application in polyurethane. Prog. Org. Coat. 164, 106700 (2022).
Park, C. K., Lee, J. H., Kim, I. S. & Kim, S. H. Castor oil-based polyols with gradually increasing functionalities for biopolyurethane synthesis. J. Appl. Polym. Sci. 137, 48304 (2019).
Lee, J. H., Kim, S. H. & Oh, K. W. Bio-based polyurethane foams with castor oil based multifunctional polyols for improved compressive properties. Polymers-Basel 13, 576 (2021).
Wang, H. et al. Confined crystallization of polyethylene oxide in nanolayer assemblies. Science 323, 757–760 (2009).
Chen, C., Liu, W., Yang, H., Zhao, Y. & Liu, S. Synthesis of solid–solid phase change material for thermal energy storage by crosslinking of polyethylene glycol with poly (glycidyl methacrylate). Sol. Energy 85, 2679–2685 (2011).
Chen, C., Liu, W., Wang, H. & Peng, K. Synthesis and performances of novel solid–solid phase change materials with hexahydroxy compounds for thermal energy storage. Appl. Energy 152, 198–206 (2015).
Huang, X. et al. Preparation and characterization of pentaerythritol/butane tetracarboxylic acid/polyethylene glycol crosslinking copolymers as solid-solid phase change materials. J. Macromol. Sci. Part A 53, 500–506 (2016).
Mu, S. et al. Preparation and thermal properties of cross-linked poly (acrylonitrile-co-itaconate)/polyethylene glycol as novel form-stable phase change material for thermal energy storage. Mater. Lett. 171, 23–26 (2016).
Qin, Y. et al. Structure-property correlation of poly (ethylene glycol) based form stable phase change materials with different crosslinking structure. Sol. Energy Mater. Sol. Cells 203, 110192 (2019).
Harlé, T., Nguyen, G. T., Ledesert, B., Mélinge, Y. & Hebert, R. L. Cross-linked polyurethane as solid-solid phase change material for low temperature thermal energy storage. Thermochim. Acta 685, 178191 (2020).
Sundararajan, S., Samui, A. B. & Kulkarni, P. S. Crosslinked polymer networks of poly (ethylene glycol)(PEG) and hydroxyl terminated poly (dimethyl siloxane)(HTPDMS) as polymeric phase change material for thermal energy storage. Sol. Energy 181, 187–194 (2019).
Zhou, Y. et al. Graphene oxide/polyurethane-based solid–solid phase change materials with enhanced mechanical properties. Thermochim. Acta 658, 38–46 (2017).
Yang, Y., Kong, W. & Cai, X. Solvent-free preparation and performance of novel xylitol based solid-solid phase change materials for thermal energy storage. Energy Build. 158, 37–42 (2018).
Kong, W., Fu, X., Yuan, Y., Liu, Z. & Lei, J. Preparation and thermal properties of crosslinked polyurethane/lauric acid composites as novel form stable phase change materials with a low degree of supercooling. RSC Adv. 7, 29554–29562 (2017).
Gaboriaud, F. & Vantelon, J. Mechanism of thermal degradation of polyurethane based on MDI and propoxylated trimethylol propane. J. Polym. Sci. Polym. Chem. Ed. 20, 2063–2071 (1982).
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2016R1A6A1A03013422 and 2021R1A6A3A01086560).
Author information
Authors and Affiliations
Contributions
J.H.L. wrote the main manuscript under the supervision of S.H.K. All authors contributed to discussions of the results. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, J.H., Kim, S.H. Synthesis and characterization of biopolyurethane crosslinked with castor oil-based hyperbranched polyols as polymeric solid–solid phase change materials. Sci Rep 12, 14646 (2022). https://doi.org/10.1038/s41598-022-17390-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-17390-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.