Abstract
Hormone replacement therapy (HRT) is widely used to relieve menopausal symptoms; however, it remains unclear whether the use of HRT was associated with gastric cancer. We conducted a systematic review and meta-analysis to synthesize available evidence. This study followed the PRISMA guideline to report meta-analysis. PubMed, Embase, and Cochrane library were searched from conception through 23 February 2022. Eligible studies reporting risk of gastric cancer after HRT were screened and accessed by two independent reviewers. Random-effects meta-analysis was used to calculate pooled risk estimate as relative risk (RR, 95% CI). Pre-established review protocol was registered in PROSPERO (CRD42021281260). Among the 1095 articles identified, we included 11 studies with 1,919,089 women in this meta-analysis. The combined risk estimate (RR, 0.72; 95% CI 0.64–0.81; I2 = 2%) indicated that the use of HRT was associated with a 28% reduction in risk of gastric cancer compared with those who had no HRT exposure. The narrow prediction interval (0.62–0.84) for gastric cancer risk suggested a low between-study variance. In subgroup analysis defined by HRT formulation, there were reduction in risks of gastric cancer after the use of estrogen-only therapy (Pooled RR, 0.63; 95% CI 0.51–0.77, I2 = 0%) and estrogen-progestin therapy (Pooled RR, 0.70; 95% CI 0.57–0.87; I2 = 0%), as compared with non-users. In this systematic review and meta-analysis, the use of HRT was associated with a reduced gastric cancer risk regardless of HRT formulation. Further investigations are warranted to confirm underlying mechanisms.
Similar content being viewed by others

Introduction
Gastric cancer was the sixth commonly diagnosed cancer globally, responsible for 768,793 cancer deaths in 20201. Albeit recent efforts shed light on the reduction of gastric cancer burden1,2,3,4 and the present decreasing trend in incidence rate has been projected to continue5, it was estimated that there will be 1,596,319 gastric cancer cases in 20356.
Hormone replacement therapy (HRT), including various estrogen-only and estrogen–progestin combined regimens, is widely prescribed to relieve menopausal symptoms, such as night sweats, hot flushes and mood swings7. The use of HRT was also linked to osteoporosis prevention8. Previous studies suggested that the use of HRT may be associated with an increased risk of breast cancer and ovarian cancer9,10. On the other hand, several studies have shown a reduction in the risk of esophageal cancer and colorectal cancer in women with HRT11,12,13,14,15.
In 2012, a meta-analysis reported an inverse association between the overall use of HRT and gastric cancer risk16. Since then, more studies have been published but the results were inconsistent17,18,19. In addition, different hormone combinations are available, resulting in concerns about benefits and harms of specific formulations. However, little is known about the associations of different formulations of HRT with the risk of gastric cancer. Achieving individualised treatment and making informed decisions among women with menopausal symptoms require clear and consistent evidence, and therefore, further assessment of gastric cancer risk associated with specific types of HRT are needed. In this study, we sought to perform a systematic review and meta-analysis to summarize the association of HRT use with gastric cancer incidence.
Methods
We performed this systematic review and meta-analysis in accordance with the pre-established review protocol registered in PROSPERO (CRD42021281260). This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guideline (see Supplementary file 1: Table S1–2).
Search strategy and selection criteria
Two reviewers (CYL and HLH) systematically searched human studies reporting the association of hormone replacement therapy and the risk of gastric cancer on the following electronic databases from inception to 23 February 2022: PubMed, Embase, and Cochrane library (the detailed search strategy is described in Supplementary file 1: Table S3–5). We placed no language restrictions. The titles and abstracts of retrieved articles were independently screened by two reviewers (CYL and HLH). Relevant articles were identified using keywords and Mesh terms relating to hormone replacement therapy and gastric cancer. We also reviewed the reference lists of all included studies. For articles in languages other than English, we consulted native speakers for translation. Any disagreement was resolved by consensus.
We included studies if they met the prespecificed criteria: (1) published original researches in human who had no prior cancer diagnosis with data on the use of hormone replacement therapy in relation to the risk of gastric cancer; and (2) study designs were randomised controlled trials, cohort studies, or case–control studies. Exclusion criteria included studies assessing hormone replacement therapy and cancer mortality; and cross-sectional studies, reviews, case reports, letters, and animal studies. We applied no restrictions on the route of hormone replacement therapy administration. The primary outcome was risk of gastric cancer after hormone replacement therapy. In this review, only studies that provided hazard ratio (HR), relative risk (RR), or odds ratio (OR) with 95% confidence intervals (CIs); or provide sufficient data that would allow the risk estimate to be calculated were eligible for inclusion.
Data extraction and quality assessment
Two reviewers (CYL and HLH) independently reviewed all identified articles to extract the following data using a standardised observation form: name of first author, publication year, country, study design, study period, age, sample size, information on hormone replacement therapy, numbers of outcomes, adjustment, and risk estimates. A third reviewer (YCJ) performed verification. The Newcastle–Ottawa Quality Assessment Scale (NOS) was used to assess the methodological quality of all included studies20.
Data synthesis and analysis
In this study, pooled estimates of relative risks were synthesised using random-effects meta-analysis, considering both within- and between-study variation. Methodological and clinical heterogeneity was assessed by I2 statistic to quantify the percentage of variation attributable to between-study heterogeneity. The I2 was categorised as low (≤ 50%), moderate (51–75%), and high (> 75%) heterogeneity21. Predictive intervals describing the heterogeneity in random-effects meta-analysis were estimated to inform the potential future treatment effect in 95% of all populations22. Visual inspection of Begg funnel plot and Egger test were performed to evaluate potential publication bias and small-study effects23. Trim and fill method was performed if publication bias existed24. Subgroup analyses were performed based on different types of HRT (estrogen-only HRT or estrogen-progestin HRT) for the risk of gastric cancer. Additional sensitivity analysis was performed to assess the robustness of the primary meta-analysis by using a fixed effect model to rerun the analysis. To assess the impact of individual studies on primary analysis, we conducted leave-one-out meta-analysis by omitting one study at a time25. In the present study, exact p values are provided unless p < 0.0001. Data were analysed using STATA version 16.1 (College Station, TX, USA).
Results
Literature search
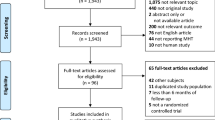
As shown in Fig. 1, 1095 potentially relevant articles were identified after initial literature search from PubMed, Embase and Cochrane library. Among the 1095 articles, 1089 remained after removing six duplicated articles. We then excluded 1077 irrelevant articles after the review of titles and abstracts. One article was included from the references lists26, leaving 13 articles for full-text review. An additional two studies were excluded because of overlapping data sources27,28. Of studies with the same data source, the study with longer duration was included in the analysis. Finally, 11 studies were included in qualitative assessment and meta-analysis17,18,19,26,29,30,31,32,33,34,35.
Characteristics of included studies
Table S6 shows the key characteristics of the included studies. In total, seven cohort studies17,18,26,29,30,31,32, three case–control studies19,34,35, and one nested case–control study33 were eligible for inclusion in the meta-analysis. The studies were published between 2003 and 2021 and included cohorts from 16 countries (Canada, China, Denmark, France, Germany, Greece, Italy, Japan, Korea, Netherlands, Norway, Singapore, Spain, Sweden, United Kingdom, and The United States). The participant number ranged from 652 to 1,160,351, which resulted in a total of 1,919,089 participants included in this meta-analysis. Study quality scores, assessed by NOS, were between low and high. Table S7–8 presents the study quality scores (see Supplementary file 1).
Meta-analysis and subgroup analysis
The pooled risk estimate from 11 studies with 1,919,089 participants in the meta-analysis showed that, as compared with non-users, individuals who received HRT had a 28% lower risk (RR, 0.72; 95% CI 0.64–0.81; I2 = 2%) of gastric cancer (Fig. 2). After accounting for between-study variance, the prediction interval (0.62–0.84) indicated that a future study is likely to yield an inverse association between HRT and risk of gastric cancer (Figure S1). In subgroup analyses defined by the type of HRT, the pooled RR was 0.63 (95% CI 0.51–0.77, I2 = 0%) for individuals who had estrogen-only HRT, comparing with non-users (Fig. 3A). There was a 30% lower risk of gastric cancer among individuals (RR, 0.70; 95% CI 0.57–0.87; I2 = 0%, comparing combined estrogen-progestin therapy vs. non-users) (Fig. 3B).
Sensitivity analysis and publication bias
In additional analysis, the risk estimate was generally consistent after we reran the meta-analysis with a fixed-effect model (Pooled RR, 0.72; 95% CI 0.64–0.80; I2 = 0%) (Figure S2). In leave-one-out meta-analysis, no study had a substantial impact on the pooled risk estimate (Supplementary Figure S3). The funnel plot asymmetry (Supplementary Figure S4) and Egger test (p = 0.416) of the association between the use of HRT and risk of gastric cancer suggested that there was no publication bias and small-study effects; and therefore, trim-and-fill method was not conducted.
Discussion
In this study, we used non-overlapping data from ten studies comprising 1,919,089 women to conduct a systematic review and meta-analysis and examine the association between HRT use and the risk of gastric cancer. The pooled results showed that women who used HRT were at a 28% lower risk of gastric cancer, compared to non-users. Estrogen-only therapy was associated with a 37% reduction in gastric cancer incidence and combined estrogen-progestin therapy reduced gastric cancer risk by 30%. The estimations for prediction intervals were in line with the main analysis. In sensitivity analysis, the results did not change substantially when using the fixed-effect meta-analysis.
Our findings are broadly in line with the previous meta-analysis, including seven studies published before 201116. The current meta-analysis incorporated the most updated evidence and conducted stratified analysis according to specific types of HRT, which were not considered in the previous meta-analysis. Our results suggested that both estrogen-only therapy and combined estrogen–progestin therapy were associated with lower gastric cancer incidence, providing new insight to the assessment of benefits and risks among individuals who had been prescribed with estrogen-only HRT after hysterectomy. The prevalence of HRT use has reduced significantly after the Women’s Health Initiative study suggested that HRT use was associated with a number of adverse health outcomes36. Despite the fact that HRT remains a management option for women with menopausal symptoms and its benefits may outweigh the harms37,38, many may focus on side effects without considering all the available evidence when making choices. Nevertheless, our findings that HRT was associated with a lower risk of gastric cancer does not ipso facto imply that HRT should be prescribed as primary preventive measure. Finally, due to paucity of data, we were unable to perform analysis stratified by dosage and duration. Given that stomach cancer is the seventh most common cancer in women, affecting 1 in 80 women during their lifetime39, our findings justify further preclinical research and explorations on the link between HRT use and gastric cancer risk according to the dosage and duration of use.
Although the causal pathway has not been well established, our findings provided evidence that hormone use may lead to a favourable outcome for primary gastric cancer prevention. There are several mechanisms through which exposure to HRT may lead to a reduction of gastric cancer risk. The presence of estrogen receptors-beta (ERβ) have been demonstrated in gastric adenocarcinoma40, and exogenous hormone binding could result in inhibition of cancer cell growth and induce apoptosis41. The findings of a Korea study using human gastric cancer cell lines implied inhibitory effects of HRT on the ERβ-positive gastric cancer42. Indeed, loss of ERβ expression was associated with poor gastric cancer survival40. In addition, a selective estrogen receptor modulator was suggested to promote gastric carcinogenesis via antiestrogenic effects in breast cancer survivors43. A previous study using data from the Swedish Cancer Registry showed a shorter latency of gastric cancer development in breast cancer survivors who had tamoxifen, as compared with non-users44.
To our knowledge, this study is the most updated and comprehensive meta-analysis that examines the association of HRT use with the risk of gastric cancer. The present study is also the first to provide subgroup analyses based on HRT formulation. This meta-analysis has some limitations. First, eight of the 11 included studies used self-reported questionnaires to assess the usage of HRT18,19,26,29,30,31,34,35. However, among these eight studies, five studies were prospective design, which may minimize the bias from misclassification since any misreporting may be random and unrelated to outcomes. Second, observational studies are susceptible to residual confounding. In this analysis, the majority of the included studies adjusted for major risk factors for gastric cancer: cigarette smoking was adjusted in nine out of 11 studies17,18,19,26,29,30,31,33,35, alcohol consumption was adjusted in five studies17,26,30,33,35, and body mass index (BMI) or obesity was adjusted in eight studies17,18,19,29,30,31,33,35. Third, despite rigorously searching for the literature, only three of 11 studies included were from gastric cancer high incidence countries (China, Japan, and Korea)26,31,32. Third, the relatively small cancer case number of the included studies may limit statistical power. Nevertheless, we provided prediction intervals indicating the potential findings of future studies. Fourth, the current available evidence does not allow us to conduct subgroup analysis based on cancer histology, location or H. pylori infection status. Given the heterogeneity in gastric cancer, the results of the present study may not represent a specific type of gastric cancer. Lastly, the possibility of health user bias cannot be entirely excluded, in which HRT users may have different lifestyle behaviors from non-users. The adjustment for cigarette smoking, alcohol consumption, and BMI in most included studies may lessen the concerns regarding health user bias. While the current evidence suggests that HRT may be of clinical benefit in the reduction of gastric cancer risk, the findings do not necessarily support HRT use for the purpose of cancer prevention. Further clinical assessment is needed to consider the balance between benefits and harms of HRT use in the setting of chronic disease prevention.
Conclusion
In conclusion, this meta-analysis of observational studies showed that the use of HRT was associated with a lower risk of gastric cancer regardless of HRT formulation. Further studies are needed to investigate the mechanisms and to explore the associations by various dosage and duration.
Abbreviations
- CI:
-
Confidence intervals
- ERß:
-
Estrogen receptor beta
- HR:
-
Hazard ratio
- HRT:
-
Hormone replacement therapy
- NOS:
-
Newcastle–Ottawa scale
- OR:
-
Odds ratio
- RR:
-
Relative risk
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 71, 209–249 (2021).
Thrift, A. P. & El-Serag, H. B. Burden of gastric cancer. Clin. Gastroenterol. Hepatol. 18, 534–542 (2020).
Zhang, X. et al. Endoscopic screening in Asian countries is associated with reduced gastric cancer mortality: A meta-analysis and systematic review. Gastroenterology 155, 347–354 (2018).
Huang, H. L. et al. Effect and cost-effectiveness of national gastric cancer screening in Japan: A microsimulation modeling study. BMC Med. 18, 257 (2020).
Arnold, M. et al. Is gastric cancer becoming a rare disease? A global assessment of predicted incidence trends to 2035. Gut 69, 823–829 (2020).
Ferlay, J. et al. Global Cancer Observatory: Cancer Tomorrow. Lyon, France: International Agency for Research on Cancer (2020). https://gco.iarc.fr/tomorrow. Accessed 18 Nov 2021.
Lobo, R. A. Hormone-replacement therapy: Current thinking. Nat. Rev. Endocrinol. 13, 220–231 (2017).
Wells, G. et al. Meta-analyses of therapies for postmenopausal osteoporosis. V. Meta-analysis of the efficacy of hormone replacement therapy in treating and preventing osteoporosis in postmenopausal women. Endocrine Rev. 23, 529–539 (2002).
Collaborative Group on Epidemiological Studies of Ovarian Cancer. Menopausal hormone use and ovarian cancer risk: Individual participant meta-analysis of 52 epidemiological studies. Lancet 385, 1835–1842 (2015).
Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: Individual participant meta-analysis of the worldwide epidemiological evidence. Lancet 394, 1159–1168 (2019).
Lagergren, K., Lagergren, J. & Brusselaers, N. Hormone replacement therapy and oral contraceptives and risk of oesophageal adenocarcinoma: A systematic review and meta-analysis. Int. J. Cancer 135, 2183–2190 (2014).
Johnson, J. R. et al. Menopausal hormone therapy and risk of colorectal cancer. Cancer Epidemiol. Biomark. Prev. 18, 196–203 (2009).
Jang, Y. C., Huang, H. L. & Leung, C. Y. Association of hormone replacement therapy with mortality in colorectal cancer survivor: A systematic review and meta-analysis. BMC Cancer 19, 1199 (2019).
Chlebowski, R. T. et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. N. Engl. J. Med. 350, 991–1004 (2004).
Grodstein, F., Newcomb, P. A. & Stampfer, M. J. Postmenopausal hormone therapy and the risk of colorectal cancer: A review and meta-analysis. Am. J. Med. 106, 574–582 (1999).
Camargo, M. C. et al. Sex hormones, hormonal interventions, and gastric cancer risk: A meta-analysis. Cancer Epidemiol. Biomark. Prev. 21, 20–38 (2012).
Brusselaers, N., Maret-Ouda, J., Konings, P., El-Serag, H. B. & Lagergren, J. Menopausal hormone therapy and the risk of esophageal and gastric cancer. Int. J. Cancer 140, 1693–1699 (2017).
Wang, Z. et al. Reproductive factors, hormone use and gastric cancer risk: The Singapore Chinese Health Study. Int. J. Cancer 138, 2837–2845 (2016).
Lope, V. et al. Menstrual and reproductive factors and risk of gastric and colorectal cancer in Spain. PLoS One 11, e0164620 (2016).
Wells, G., Shea, B., & O’Connell, D. Proceedings of the Third Symposium on Systematic Reviews. Beyond the Basics: Improving Quality and Impact. Oxford: The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analysis. (2000).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558 (2002).
IntHout, J., Ioannidis, J. P., Rovers, M. M. & Goeman, J. J. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 6, e010247 (2016).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997).
Duval, S. & Tweedie, R. Trim and fill: A simple funnel-plot- based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56, 455–463 (2000).
Patsopoulos, N. A., Evangelou, E. & Ioannidis, J. P. Sensitivity of between-study heterogeneity in meta-analysis: Proposed metrics and empirical evaluation. Int. J. Epidemiol. 37, 1148–1157 (2008).
Kaneko, S., Tamakoshi, A., Ohno, Y., Mizoue, T. & Yoshimura, T. Menstrual and reproductive factors and the mortality risk of gastric cancer in Japanese menopausal females. Cancer Causes Control 14, 53–59 (2003).
La Vecchia, C. et al. Menstrual and reproductive factors and gastric-cancer risk in women. Int. J. Cancer 59, 761–764 (1994).
Lindblad, M., Rodriguez, L. G., Chandanos, E. & Lagergren, J. Hormone replacement therapy and risks of oesophageal and gastric adenocarcinomas. Br. J. Cancer 94, 136–141 (2006).
Duell, E. J. et al. Menstrual and reproductive factors, exogenous hormone use, and gastric cancer risk in a cohort of women from the European Prospective Investigation Into Cancer and Nutrition. Am. J. Epidemiol. 172, 1384–1393 (2010).
Freedman, N. D. et al. The association of menstrual and reproductive factors with upper gastrointestinal tract cancers in the NIH-AARP cohort. Cancer Interdiscip. Int. J. Am. Cancer Soc. 116, 1572–1581 (2010).
Freedman, N. D. et al. Menstrual and reproductive factors and gastric cancer risk in a large prospective study of women. Gut 56, 1671–1677 (2007).
Nam, J. H. et al. The effect of menopausal hormone therapy on gastrointestinal cancer risk and mortality in South Korea: A population-based cohort study. BMC Gastroenterol. 21, 1–2 (2021).
Green, J. et al. Menopausal hormone therapy and risk of gastrointestinal cancer: Nested case–control study within a prospective cohort, and meta-analysis. Int. J. Cancer 130, 2387–2396 (2012).
Frise, S., Kreiger, N., Gallinger, S., Tomlinson, G. & Cotterchio, M. Menstrual and reproductive risk factors and risk for gastric adenocarcinoma in women: Findings from the Canadian national enhanced cancer surveillance system. Ann. Epidemiol. 16, 908–916 (2006).
Fernandez, E. et al. Hormone replacement therapy and cancer risk: A systematic analysis from a network of case–control studies. Int. J. Cancer 105, 408–412 (2003).
Grossman, D. C. et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement. JAMA 318, 2224–2233 (2017).
Rymer, J., Brian, K. & Regan, L. HRT and breast cancer risk. BMJ 367, 25 (2019).
Hamoda, H. & Moger, S. Looking at HRT in perspective. BMJ 377, o1425 (2022).
Fitzmaurice, C. et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: A systematic analysis for the global burden of disease study. JAMA Oncol. 4, 1553–1568 (2018).
Xu, C. Y. et al. Prognostic role of estrogen receptor α and estrogen receptor β in gastric cancer. Ann. Surg. Oncol. 17, 2503–2509 (2010).
Qin, J. et al. The direct effect of estrogen on cell viability and apoptosis in human gastric cancer cells. Mol. Cell. Biochem. 395, 99–107 (2014).
Kim, M. J. et al. Effects of 17β-estradiol and estrogen receptor antagonists on the proliferation of gastric cancer cell lines. J. Gastr. Cancer 13, 172–178 (2013).
Rahman, M. S. & Cao, J. Estrogen receptors in gastric cancer: Advances and perspectives. World J. Gastroenterol. 22, 2475–2482 (2016).
Chandanos, E. et al. Tamoxifen exposure in relation to gastric adenocarcinoma development. Eur. J. Cancer 44, 1007–1014 (2008).
Author information
Authors and Affiliations
Contributions
H.L.H. and C.Y.L. conceived and designed the study and protocol registration. Y.C.J. and C.Y.L. contributed equally to this work. H.L.H. and C.Y.L. refined the protocol registration. C.Y.L. and H.L.H. performed the literature search, data extraction, quality assessment. H.L.H. and C.Y.L. contributed to statistical analysis and interpretation of data. Y.C.J., H.L.H., and C.Y.L. wrote and revised the manuscript critically for important intellectual content. H.L.H., and C.Y.L. had full access to all the data in the study and had final responsibility for the decision to submit for publication. All authors approved the final version before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jang, YC., Leung, C.Y. & Huang, HL. Association of hormone replacement therapy with risk of gastric cancer: a systematic review and meta-analysis. Sci Rep 12, 12997 (2022). https://doi.org/10.1038/s41598-022-17345-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-17345-2
This article is cited by
-
Reproductive factors, hormonal interventions, and gastric cancer risk in the Stomach cancer Pooling (StoP) Project
Cancer Causes & Control (2024)
-
Clinicopathologic differences of gastric neoplasms between Helicobacter pylori-infected and -naïve patients: a multicenter retrospective analysis
Journal of Gastroenterology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.