Abstract
Due to the difficulty in sampling of metastatic tumors, patient selection is commonly based on results of primary tumor samples when metastatic samples are not available. However, due to tumor heterogeneity, metastatic tumors may be different from primary tumors in their phenotypes. The aim of this study was to investigate the expression of EGFR, HER2, and HER3 between primary and lymph node metastatic lesions of colorectal cancer. Paired primary tumors and lymph node metastases from 79 patients with colorectal cancer were retrospectively collected and analyzed for EGFR, HER2, and HER3 expression. High EGFR, HER2, and HER3 expression (2+ and 3+) was found in 64.2%, 66.0%, and 85.0% of primary tumors, and 56.8%, 46.0%, and 76.0% of lymph node metastases, respectively. Correlation rates between primary and metastatic lesions were 67.1%, 63.3%, and 74.7% for EGFR, HER2, and HER3, respectively. Stage IV tumors (with distant metastasis) had higher correlation rates of HER2 expression compared to stage III tumors (without distant metastasis) (P = 0.050). Moderate correlation rates in EGFR, HER2, and HER3 expression were observed between primary and metastatic lesions of colorectal cancer. Tumor stage or existence of distant metastasis could serve as potential predictive markers for the correlation of HER2 expression between primary tumors and lymph node metastases of colorectal cancer.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is the 3rd leading cause of cancer-related death worldwide, which took nearly one million lives in 20181. Tumor metastasis is the major cause of patient death in CRC. As reported in previous studies, 20–25% of the CRC patients showed metastasis when they were firstly diagnosed, and almost half of the patients developed metastasis after progression of the disease2,3. The 5-year survival rate of patients with metastatic CRC (mCRC) was only 13.3% which is much lower than that of localized CRC (73.1%). If left untreated, the median survival period of mCRC was only eight months3,4.
The human epidermal growth factor receptor (HER) family consists of four members: epithelial growth factor receptor (EGFR), HER2, HER3, and HER4. All the HER family members are receptor tyrosine kinases which consist of extracellular domains, transmembrane segments, and endoplasmic tyrosine kinase domains5. The HER family members are activated through formation of homodimers or heterodimers and subsequent phosphorylation of the endoplasmic tyrosine kinase domains6. The roles of HER family members in cancer have been well established. EGFR expression is widely up-regulated among different types of epithelial tumors, and therapies targeting EGFR have been used to treat mCRC for nearly two decades7. Similar to EGFR, overexpression of HER2 is commonly seen in different types of tumors6. Therapies targeting HER2 have been approved for the treatment of HER2-positive breast cancer and HER2-positive gastric cancer8,9, and are currently under investigation in preclinical and clinical studies for the treatment of mCRC10. HER3 activation is thought to be involved in the resistance mechanism of anti-EGFR and anti-HER2 therapies11. In addition, high HER3 expression (immunohistochemistry score 2+ and 3+) was found in 80% of primary lesions and 81% of lymph node metastases of CRC12, and therapy directly targeting HER3 is currently under investigation in phase 2 clinical trial (NCT04479436). Unlike the other HER family members, HER4 is not commonly overexpressed in tumors, and HER4 signaling promotes cell differentiation and apoptosis13.
Previous studies have found significant genetic diversity between and within tumors, namely tumor heterogeneity, which is a key challenge in personalized cancer medicine14,15. Due to the difficulty in tumor sampling, metastatic lesion samples are commonly unavailable, and patient selection in clinical trials or practices is more based on results from primary lesions. However, this may be potentially harmful considering the existence of heterogeneity between primary and metastatic lesions. Several previous studies have compared the expression of EGFR, HER2, or HER3 between primary and distant metastatic lesions of CRC, and the correlation rates ranged from 46.5 to 94.7% for EGFR (46.5%16, 94.7%17, and 60%18), and from 55 to 72.7% for HER2 (55%18, 72.7%19, and 70.2%20).
Lymph node metastasis is the most important prognostic indicator for solid cancer patients, and the distal metastasis of tumor is mostly via lymphatic system21. Although not directly related to drug responsiveness of distant metastatic tumors, the genomic profiles of lymph node metastatic tumors may help understand the evolution of tumor cells during the process of metastasis. Some previous studies compared the EGFR, HER2, or HER3 expressions between primary tumor and lymph node metastases of CRC. Shan19 found a correlation rate of 89.9% (62/69) in HER2 status between primary tumor and lymph node metastasis of CRC. Wei22 compared the immunohistochemistry scores of EGFR, HER2, and HER3 between primary CRC and lymph node metastases, and found moderate to high correlation (EGFR: 69.6%; HER2: 96.4%; HER3: 83.7%). In another study, correlation of low/high HER3 expression between paired primary CRC tumor and lymph node metastases was found in 84 out of 102 (82.4%) patients23. However, it is still difficult to draw a conclusion from these results, considering the limited number of these studies. More investigation is required to provide more evidence. In the present study, we further investigated the protein expression of EGFR, HER2, and HER3 in primary lesions and lymph node metastasis of CRC, which could hopefully shed more light on this problem.
Results
Patient clinicopathologic characteristics
Paired primary and lymph node metastatic lesion samples were collected from 107 patients. After quality control, samples from 28 patients were excluded due to lack of tumor cells on slides or incomplete patient information. The median age of the patients was 67 years (range 34–87 years), with 38 (48.1%) males and 41 (51.9%) females. The clinicopathologic characteristics of the 79 patients are summarized in Table 1.
Protein overexpression of EGFR, HER2, and HER3
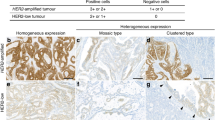
Lymph node metastases are tumor lesions in lymph nodes located in the lymphatic drainage of primary tumor lesions. Overall, lymph node metastases showed relatively lower percentage of high EGFR, HER2, and HER3 expression (immunohistochemistry 2+/3+), compared to primary lesions. In the 79 patients, majority (52, 65.8%) of the primary lesions showed high EGFR expression (2+/3+), compared to 46 (58.2%) in lymph node metastases. High HER2 expression (2+/3+) was found in 52 (65.8%) primary lesions and 33 (41.8%) lymph node metastases. High HER3 expression (2+/3+) was found in 66 (83.5%) of primary lesions and 56 (70.9%) of lymph node metastases. Representative images of EGFR, HER2, and HER3 immunohistochemistry staining are shown in Figs. 1, 2, and 3, respectively.
Representative images (EGFR immunohistochemistry staining). Representative images of immunohistochemistry staining scores (0, 1+, 2+, and 3+) for EGFR in primary tumor (Primary) and lymph node metastasis (LN) of CRC. Low: low magnificent power field (× 40); High: high magnificent power field (× 200).
Representative images (HER2 immunohistochemistry staining). Representative images of immunohistochemistry staining scores (0, 1+, 2+, and 3+) for HER2 in primary tumor (Primary) and lymph node metastasis (LN) of CRC. Low: low magnificent power field (× 40); High: high magnificent power field (× 200).
Representative images (HER3 immunohistochemistry staining). Representative images of immunohistochemistry staining scores (0, 1+, 2+, and 3+) for HER3 in primary tumor (Primary) and lymph node metastasis (LN) of CRC. Low: low magnificent power field (× 40); High: high magnificent power field (× 200).
Correlation of EGFR, HER2, and HER3 scores between primary lesions and lymph node metastases
Table 2 shows a detailed comparison of EGFR, HER2, and HER3 immunohistochemistry scores between primary and metastatic lesions. As shown in Table 2, the correlation rates between primary lesion and lymph node metastases were only 67.1% (53/79) for EGFR, 63.3% (50/79) for HER2, and 74.7% (59/79) for HER3. After converting the scores into high expression (2+/3+) or low expression (0/1+), results showed higher correlation rates: 79.7% (63/79) for EGFR, 73.4% (58/79) for HER2, and 82.3% (65/79) for HER3. In the discordant samples, majority had low expression in lymph node metastases (and high expression in primary lesions): EGFR, 18/26 (69.2%); HER2, 28/29 (96.6%); HER3, 16/20 (80.0%).
As shown in Table 3, stage IV tumors (M1 stage) showed higher correlation rates of HER2 immunohistochemistry scores compared to stage III tumors (M0 stage) (P = 0.050). No significant differences were found in the correlation rates of EGFR or HER3 scores between different subgroups of clinicopathologic characteristics, or in the correlation rates of HER2 between different subgroups of gender, age, tumor type, primary tumor location, differentiation, T stage, and N stage. Furthermore, we also looked into the data of high/low expression of EGFR, HER2, and HER3 and analyzed their relationship with patient information and clinicopathologic parameters. However, no significant difference was observed (see Supplementary Table S1 for details).
Discussion
The HER family members, EGFR, HER2, and HER3, are key treatment targets in mCRC, and anti-HER therapies are currently in clinical trials or already used in clinical practice5,7,10. In our study, we investigated the protein expression of those factors in paired primary lesions and lymph node metastases. Results showed moderate to high percentages of high EGFR, HER2, and HER3 protein expression (immunohistochemistry 2+ and 3+), ranging from 65.8% to 83.5% in primary lesions and 41.8% to 70.9% in lymph node metastases. However, the percentages of positive EGFR, HER2, and HER3 expression in CRC varied greatly in previous studies22,24,25,26. As summarized by Wei22, percentages of positive EGFR, HER2, and HER3 expression in CRC ranged from 20 to 95%, 3% to 82%, and 36% to 89%, respectively in different studies. The great variations were possibly caused by differences in patient population, antibody, and scoring criteria. For example, many of the studies used the percentage of positive-stained cells by immunohistochemistry to define positivity/negativity of HER family members17,27,28,29,30,31. Therefore, we limit our discussion within studies using similar scoring criteria to our study (4-tiered scoring criteria based on staining intensity).
Regarding EGFR expression, study by Wei22 reported high EGFR expression (2+ and 3+) in 29% of primary lesions and 14% of lymph node metastases. In the study by Yarom16, high EGFR expression was observed in 39.7% of primary tumors. In our study, high EGFR expression (2+ and 3+) was more commonly seen: 65.8% in primary tumor and 58.2% in lymph node metastases. Regarding HER2 expression, high HER2 expression was only found in 1.8% (1/55) of both primary and metastatic lesions in Wei’s study22. Using a slightly stricter criteria (2+ in > 50% tumor cells, instead of 10%), Shan19 found high HER2 expression in 11.6% of primary tumors and 10.1% of lymph node metastases. In addition, studies by Li JL18, Styczen26, Drecoll32, Fotiades33, Farzand34, Kavanagh35, Li Q36, Ramieri37, Schuell38, Seo39, Gao40, and Stahler41 reported high HER2 expression in 54.5%, 63.6%, 14%, 33.3%, 54.8%, 10%, 15.5%, 10%, 4%, 6%, 50%, and 14.4% of primary tumors of CRC, respectively. Our results showed high HER2 expression in 65.8% of primary lesions and 41.8% of lymph node metastases, which are higher than the previous studies. For HER3 expression, two studies by Ledel12,23 reported high HER3 expression in 80% and 70% of primary lesions, and 81% and 75% of lymph node metastases, respectively. Study by Styczen26 found high HER3 expression in 72.7% of primary tumors. Studies by Stahler41, Seo39, and Ledel42 reported high HER3 expression in 67%, 69%, and 67% of primary tumors, respectively, and our study showed high HER3 expression in 83.5% of primary tumors and 70.9% of metastases. In all, although the percentages of high HER3 expression were relatively close between previous studies and our findings, the percentages of high EGFR and HER2 expression varied greatly even when similar scoring criteria were used. This could possibly be explained by the different antibodies and experimental conditions used in those studies, highlighting the importance of standardization of reagents and experimental conditions in this field.
Our results also showed lower percentages of high EGFR, HER2, and HER3 expression in lymph node metastases compared to primary tumors (EGFR: 58.2% vs. 65.8%; HER2: 41.8% vs. 65.8%; HER3: 70.9% vs. 83.5%), which was also observed in the EGFR and HER2 expression results reported in previous studies16,18,19,20,22,26. However, unlike our results, high HER3 expression was more commonly seen in metastases rather than primary tumors in previous reports12,23,26.
Tumor heterogeneity is a key challenge for cancer medicine. Although targeted therapies often lead to robust initial responses, it is almost inevitable for the tumors to develop resistance and relapse, which is partially caused by intra-tumor and temporal heterogeneity (evolution of tumor cells under the pressure of treatment)14,15,43,44,45. The discrepancies in the phenotypes of tumor cells observed between primary and metastatic lesions also reflect both spatial (inter-tumor) and temporal heterogeneity15. In our study, we also investigated the heterogeneity of EGFR, HER2, and HER3 expression between primary lesions and lymph node metastases. Results showed moderate correlation rates in the scores of EGFR (67.1%), HER2 (63.3%), and HER3 (74.7%). For EGFR, Wei22 reported a 69.6% correlation rate of EGFR scores between primary tumors and lymph node metastases, which is close to our findings (67.1%). For HER2 scores, Wei22 reported a correlation rate of 96.4%. Shan19 investigated the correlation of HER2 positive (immunohistochemistry 2+/3+) and negative (0/1+) between primary tumors and lymph node metastases, and reported a correlation rate of 89.2%. Since the study populations of Wei22, Shan19, and our study were all Chinese population and our study used the same scoring criteria as the previous study by Wei22, the higher correlation rates found in previous studies by Wei22 and Shan19 may be partially related to the small number of HER2 high expression cases in these studies (1 and 8, respectively) which could potentially lead to bias. For HER3, in the studies by Wei22 and Ledel23, correlation of high expression (2+/3+)/low expression (0/1+) of HER3 was investigated, and results showed correlation rates of 83.7% and 82.4%, respectively, which were similar to our findings (82.3%). In all, unlike distant metastasis which showed great variations in correlation rates (EGFR: 46.5% to 94.7%16,17,18; HER2: 55% to 72.7%18,19,20), good agreement was observed in the correlation rates of EGFR expression (67.1% to 69.6%) and HER3 expression (82.3% to 83.7%) between primary tumors and lymph node metastases. Compared to our study results (63.3%), previous studies found much higher correlation rates of HER2 expression (96.4% and 89.2%), which might be due to the bias caused by small numbers of HER2 high expression cases in these studies19,22. Investigations in large patient cohorts are required to determine the correlation rate of HER2 expression between primary tumors and lymph node metastases. Furthermore, the significant proportions of uncorrelated EGFR, HER2, and HER3 expression levels between primary tumors and lymph node metastases indicate that it may not be appropriate to use genetic profiles of lymph node metastasis to guide treatment plans, given that genetic profiles of primary tumor are more widely-used to guide patient selection of targeted therapies in clinical trials46,47,48,49,50. Several possible methods could be used to minimize the proportion of uncorrelated cases, e.g. standardization of antibodies, experimental conditions and scoring criteria, and investigations based on large patient cohorts. However, due to the intrinsic differences in the EGFR, HER2, and HER3 expression levels between primary tumors and lymph node metastases, uncorrelated cases may still exist even after applying these methods.
In hope of finding possible relationships between clinicopathologic characteristics and correlation of EGFR, HER2, and HER3 expression, we further analyzed the number of correlated cases in different patient subgroups. Interestingly, significantly higher correlation of HER2 scores were found in stage IV tumors (M1 stage) compared to stage III tumors (M0 stage), although no significant differences were found between the correlation of clinicopathologic characteristics and EGFR or HER3 expression, or between HER2 expression and clinicopathologic characteristics other than tumor stage and M stage. To the best of our knowledge, our study is the first to analyze the relationship between clinicopathologic characteristics and the correlation of EGFR, HER2, and HER3 expression between primary and lymph node metastatic lesions of mCRC. More investigations are required to find more possible markers to predict the correlation of these factors.
Although majority of the studies used immunohistochemistry to evaluate the expression of EGFR, HER2, and HER3 in CRC, some previous investigations used other technologies, e.g. fluorescence in situ hybridization (FISH), next-generation sequencing (NGS), silver in situ hybridization (SISH), reverse transcription-polymerase chain reaction (RT-PCR), SNP-array, and microarray. In an early study by Ooi51, HER2 overexpression measured by immunohistochemistry showed an 100% correlation with HER2 amplification detected by FISH. In addition, HER2 overexpression in primary tumors also showed an 100% correlation with the HER2 expression of tumor cells in lymph node metastasis and/or liver metastasis51. However, only 25% of the paired lymph node metastases showed EGFR-overexpressing cancer cells in the 12 cases with EGFR-overexpressing primary CRC tumors51. In a previous study by Molinari29, EGFR amplification or Chromosome 7 polysomy was detected using FISH, and results showed an 84.6% (11/13) correlation rate between primary tumors and lymph node metastases. Shimada52 performed comprehensive genomic sequencing by NGS to detect HER2 amplification in mCRC, and the results showed a strong correlation (an 100% correlation rate) with the HER2 status determined by immunohistochemistry and FISH. Similarly, in two previous studies by Gong53 and EI-Deiry54, HER2 amplification was identified using NGS in patients with CRC, and the reported positive rates were 5.1% (7/138) and 5.7% (94/1653), respectively. In another study, HER2 amplification was measured by SISH, and results showed an 8.4% (16/191) positive rate of HER2 amplification in advanced CRC55. Yen31 used RT-PCR to measure EGFR mRNA levels, and EGFR mRNA overexpression was found in 78.9% (75/95) of CRC tumors, which was higher than the EGFR protein overexpression rate (61.1%, 58/95) measured by immunohistochemistry. In another study using RT-PCR to measure EGFR mRNA expression, a significant correlation was observed in EGFR mRNA expression levels between primary tumor and liver metastasis56. Del Carmen28 investigated EGFR expression in sporadic CRC tumors and found a strong correlation between EGFR expression measured by immunohistochemistry and EGFR gene copy number determined by FISH, SNP-array, and microarray.
In summary, our study found moderate proportions of high EGFR, HER2, and HER3 expression in primary tumors and lymph node metastases of patients with mCRC. Further analysis showed moderate correlations of the scores of EGFR, HER2, and HER3 between primary and metastatic lesions. In addition, our study for the first time showed that tumor stage and M stage could serve as predictive markers for the correlation of HER2 scores between primary tumors and lymph node metastases of mCRC. More studies with larger sample size are required to further validate those findings.
Methods
Patient samples
Paired primary tumor and lymph node metastasis samples were retrospectively collected from archived formalin-fixed and paraffin embedded (FFPE) samples of patients who were treatment-naïve and underwent surgery in Sichuan Provincial People's Hospital from January 2019 to June 2019. All the lymph node metastasis samples were from lymph nodes within the lymphatic drainage of the primary tumor, which were collected together with the primary tumor samples during the surgery. The histological type of the tumor was determined after reviewing by two pathologists. Clinicopathologic characteristics were also collected. The pathological TNM staging of the tumors was based on the guideline developed by Japanese Society for Cancer of the Colon and Rectum57. Subsequent immunohistochemistry staining was conducted in November 2021. This study was approved by the Medical Ethics Committee of Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital (#484, Year 2021) and in compliance with Declaration of Helsinki. Informed consent was waived by the Medical Ethics Committee since all the archival samples were retrospectively collected and all the data were collected and analyzed anonymously.
Immunohistochemistry staining
The paired primary tumor and lymph node metastasis samples were sectioned at 4 μm and placed on the same slide. After de-paraffinization, hydration, and antigen retrieval using boiling, slides were stained using primary antibody against EGFR (RMA-0804, MXB Biotechnologies), HER2 (ab16662, Abcam), or HER3 (ab93739, Abcam). After staining with secondary antibody (ab6721, Abcam), slides were counterstained with hematoxylin and visualized using 3,3′-diaminobenzidine (DAB). Tumor samples which were stained positive in previous tests were used as positive control, and negative controls were prepared by replacing primary antibody with phosphate-buffered saline.
Scoring criteria
The immunohistochemistry staining results were then independently scored by two pathologists (J.X. and F.L.) using pre-defined scoring criteria which were based on the 4-tiered scoring system suggested by Hofmann58 and criteria used for EGFR, HER2, and HER3 scoring in previous studies12,22. Detailed criteria are listed as follows: 0, completely negative staining or faint staining < 10% of tumor cells; 1+, faint staining of tumor cell membranes (or very faint staining in only part of the membrane for HER3); 2+, moderate staining of entire cell membrane (or in entire or basolateral membrane for HER3); 3+, strong staining of entire cell membrane (or in entire or basolateral membrane for HER3). High expression of the biomarkers was defined as immunohistochemistry scores of 2+ or 3+, while low expression was defined as immunohistochemistry scores of 0 or 1+. The pathologists were blinded to the patient characteristics information, and slides with insufficient tumor tissue in either primary lesion or lymph node metastasis were excluded in the scoring step.
Statistical analysis
Statistical analysis was performed using SPSS 19.0 statistical software (IBM Corporation, USA). The χ2 test was used to analyze possible relationships between the immunohistochemistry score (or high/low expression of biomarkers) and patient clinicopathologic characteristics. P < 0.05 was considered statistically significant.
Data availability
All the data were included in the article or Supplementary Information.
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424. https://doi.org/10.3322/caac.21492 (2018).
Villalba, M., Evans, S. R., Vidal-Vanaclocha, F. & Calvo, A. Role of TGF-beta in metastatic colon cancer: It is finally time for targeted therapy. Cell Tissue Res. 370, 29–39. https://doi.org/10.1007/s00441-017-2633-9 (2017).
De Greef, K. et al. Multisciplinary management of patients with liver metastasis from colorectal cancer. World J. Gastroenterol. 22, 7215–7225. https://doi.org/10.3748/wjg.v22.i32.7215 (2016).
Joachim, C. et al. Overall survival of colorectal cancer by stage at diagnosis: Data from the Martinique Cancer Registry. Medicine 98, e16941. https://doi.org/10.1097/MD.0000000000016941 (2019).
Kumar, R. et al. HER family in cancer progression: From discovery to 2020 and beyond. Adv. Cancer Res. 147, 109–160. https://doi.org/10.1016/bs.acr.2020.04.001 (2020).
Sergina, N. V. & Moasser, M. M. The HER family and cancer: emerging molecular mechanisms and therapeutic targets. Trends Mol. Med. 13, 527–534. https://doi.org/10.1016/j.molmed.2007.10.002 (2007).
El Bali, M., Bakkach, J. & Bennani Mechita, M. Colorectal cancer: From genetic landscape to targeted therapy. J. Oncol. 2021, 9918116. https://doi.org/10.1155/2021/9918116 (2021).
Maadi, H., Soheilifar, M. H., Choi, W. S., Moshtaghian, A. & Wang, Z. Trastuzumab mechanism of action; 20 years of research to unravel a dilemma. Cancers https://doi.org/10.3390/cancers13143540 (2021).
Zhu, Y., Zhu, X., Wei, X., Tang, C. & Zhang, W. HER2-targeted therapies in gastric cancer. Biochem. Biophys. Acta. 1876, 188549. https://doi.org/10.1016/j.bbcan.2021.188549 (2021).
Greally, M., Kelly, C. M. & Cercek, A. HER2: An emerging target in colorectal cancer. Curr. Probl. Cancer 42, 560–571. https://doi.org/10.1016/j.currproblcancer.2018.07.001 (2018).
Mishra, R., Patel, H., Alanazi, S., Yuan, L. & Garrett, J. T. HER3 signaling and targeted therapy in cancer. Oncol. Rev. 12, 355. https://doi.org/10.4081/oncol.2018.355 (2018).
Ledel, F., Stenstedt, K., Hallstrom, M., Ragnhammar, P. & Edler, D. HER3 expression in primary colorectal cancer including corresponding metastases in lymph node and liver. Acta Oncol. 54, 480–486. https://doi.org/10.3109/0284186X.2014.983654 (2015).
Jones, F. E. HER4 intracellular domain (4ICD) activity in the developing mammary gland and breast cancer. J. Mammary Gland Biol. Neoplasia 13, 247–258. https://doi.org/10.1007/s10911-008-9076-6 (2008).
McGranahan, N. & Swanton, C. Clonal heterogeneity and tumor evolution: Past, present, and the future. Cell 168, 613–628. https://doi.org/10.1016/j.cell.2017.01.018 (2017).
Burrell, R. A., McGranahan, N., Bartek, J. & Swanton, C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature 501, 338–345. https://doi.org/10.1038/nature12625 (2013).
Yarom, N. et al. EGFR expression variance in paired colorectal cancer primary and metastatic tumors. Cancer Biol. Ther. 10, 416–421. https://doi.org/10.4161/cbt.10.5.12610 (2010).
Italiano, A. et al. Epidermal growth factor receptor (EGFR) status in primary colorectal tumors correlates with EGFR expression in related metastatic sites: Biological and clinical implications. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 16, 1503–1507. https://doi.org/10.1093/annonc/mdi282 (2005).
Li, J. L. et al. Clinical significance of HER2 and EGFR expression in colorectal cancer patients with ovarian metastasis. BMC Clin. Pathol. 19, 3. https://doi.org/10.1186/s12907-019-0085-8 (2019).
Shan, L., Lv, Y., Bai, B., Huang, X. & Zhu, H. Variability in HER2 expression between primary colorectal cancer and corresponding metastases. J. Cancer Res. Clin. Oncol. 144, 2275–2281. https://doi.org/10.1007/s00432-018-2744-z (2018).
Lee, W. S. et al. Comparison of HER2 expression between primary colorectal cancer and their corresponding metastases. Cancer Med. 3, 674–680. https://doi.org/10.1002/cam4.228 (2014).
Ferris, R. L., Lotze, M. T., Leong, S. P., Hoon, D. S. & Morton, D. L. Lymphatics, lymph nodes and the immune system: Barriers and gateways for cancer spread. Clin. Exp. Metas. 29, 729–736. https://doi.org/10.1007/s10585-012-9520-2 (2012).
Wei, Q. et al. EGFR, HER2 and HER3 expression in primary colorectal carcinomas and corresponding metastases: Implications for targeted radionuclide therapy. Oncol. Rep. 25, 3–11 (2011).
Ledel, F., Hallstrom, M., Ragnhammar, P., Ohrling, K. & Edler, D. HER3 expression in patients with primary colorectal cancer and corresponding lymph node metastases related to clinical outcome. Eur. J. Cancer 50, 656–662. https://doi.org/10.1016/j.ejca.2013.11.008 (2014).
Scartozzi, M. et al. Epidermal growth factor receptor (EGFR) downstream signalling pathway in primary colorectal tumours and related metastatic sites: Optimising EGFR-targeted treatment options. Br. J. Cancer 97, 92–97. https://doi.org/10.1038/sj.bjc.6603847 (2007).
Ljuslinder, I. et al. ErbB 1-4 expression alterations in primary colorectal cancers and their corresponding metastases. Anticancer Res. 29, 1489–1494 (2009).
Styczen, H. et al. HER-2 and HER-3 expression in liver metastases of patients with colorectal cancer. Oncotarget 6, 15065–15076. https://doi.org/10.18632/oncotarget.3527 (2015).
Goos, J. A. et al. Epidermal growth factor receptor (EGFR) and prostaglandin-endoperoxide synthase 2 (PTGS2) are prognostic biomarkers for patients with resected colorectal cancer liver metastases. Br. J. Cancer 111, 749–755. https://doi.org/10.1038/bjc.2014.354 (2014).
Del Carmen, S. et al. Prognostic implications of EGFR protein expression in sporadic colorectal tumors: Correlation with copy number status, mRNA levels and miRNA regulation. Sci. Rep. 10, 4662. https://doi.org/10.1038/s41598-020-61688-7 (2020).
Molinari, F. et al. Differing deregulation of EGFR and downstream proteins in primary colorectal cancer and related metastatic sites may be clinically relevant. Br. J. Cancer 100, 1087–1094. https://doi.org/10.1038/sj.bjc.6604848 (2009).
Hecht, J. R. et al. Lack of correlation between epidermal growth factor receptor status and response to Panitumumab monotherapy in metastatic colorectal cancer. Clin. Cancer Res. 16, 2205–2213. https://doi.org/10.1158/1078-0432.CCR-09-2017 (2010).
Yen, L. C. et al. Activating KRAS mutations and overexpression of epidermal growth factor receptor as independent predictors in metastatic colorectal cancer patients treated with cetuximab. Ann. Surg. 251, 254–260. https://doi.org/10.1097/SLA.0b013e3181bc9d96 (2010).
Drecoll, E. et al. Expression analysis of heat shock protein 90 (HSP90) and Her2 in colon carcinoma. Int. J. Colorectal Dis. 29, 663–671. https://doi.org/10.1007/s00384-014-1857-3 (2014).
Fotiades, P. P. et al. HER2/CHR 17 tissue microarray chromogenic in situ hybridization analysis in colon adenocarcinoma: Multiple gene signals vs protein expression. J. B. U. O. N. 19, 109–114 (2014).
Farzand, S., Siddique, T., Saba, K. & Bukhari, M. H. Frequency of HER2/neu overexpression in adenocarcinoma of the gastrointestinal system. World J. Gastroenterol. 20, 5889–5896. https://doi.org/10.3748/wjg.v20.i19.5889 (2014).
Kavanagh, D. O. et al. Is overexpression of HER-2 a predictor of prognosis in colorectal cancer?. BMC Cancer 9, 1. https://doi.org/10.1186/1471-2407-9-1 (2009).
Li, Q., Wang, D., Li, J. & Chen, P. Clinicopathological and prognostic significance of HER-2/neu and VEGF expression in colon carcinomas. BMC Cancer 11, 277. https://doi.org/10.1186/1471-2407-11-277 (2011).
Ramieri, M. T. et al. Detection of HER2 amplification using the SISH technique in breast, colon, prostate, lung and ovarian carcinoma. Anticancer Res. 30, 1287–1292 (2010).
Schuell, B., Gruenberger, T., Scheithauer, W., Zielinski, C. & Wrba, F. HER 2/neu protein expression in colorectal cancer. BMC Cancer 6, 123. https://doi.org/10.1186/1471-2407-6-123 (2006).
Seo, A. N. et al. HER3 protein expression in relation to HER2 positivity in patients with primary colorectal cancer: clinical relevance and prognostic value. Virchows Arch. 466, 645–654. https://doi.org/10.1007/s00428-015-1747-2 (2015).
Gao, F. J. et al. Lgr5 over-expression is positively related to the tumor progression and HER2 expression in stage pTNM IV colorectal cancer. Int. J. Clin. Exp. Pathol. 7, 1572–1579 (2014).
Stahler, A. et al. Prevalence and influence on outcome of HER2/neu, HER3 and NRG1 expression in patients with metastatic colorectal cancer. Anticancer Drugs 28, 717–722. https://doi.org/10.1097/CAD.0000000000000510 (2017).
Ledel, F., Stenstedt, K., Hallstrom, M., Ragnhammar, P. & Edler, D. HER3 expression is correlated to distally located and low-grade colon cancer. Acta Oncol. 55, 875–880. https://doi.org/10.3109/0284186X.2015.1131334 (2016).
Burrell, R. A. & Swanton, C. Tumour heterogeneity and the evolution of polyclonal drug resistance. Mol. Oncol. 8, 1095–1111. https://doi.org/10.1016/j.molonc.2014.06.005 (2014).
Marusyk, A., Janiszewska, M. & Polyak, K. Intratumor heterogeneity: The rosetta stone of therapy resistance. Cancer Cell 37, 471–484. https://doi.org/10.1016/j.ccell.2020.03.007 (2020).
Ocana, A., Amir, E. & Pandiella, A. HER2 heterogeneity and resistance to anti-HER2 antibody-drug conjugates. Breast Cancer Res. 22, 15. https://doi.org/10.1186/s13058-020-1252-7 (2020).
Cunningham, D. et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N. Engl. J. Med. 351, 337–345. https://doi.org/10.1056/NEJMoa033025 (2004).
Siena, S. et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): A multicentre, open-label, phase 2 trial. Lancet Oncol. 22, 779–789. https://doi.org/10.1016/S1470-2045(21)00086-3 (2021).
Gan, H. K. et al. A phase I, first-in-human study of GSK2849330, an anti-HER3 monoclonal antibody, in HER3-expressing solid tumors. Oncologist 26, e1844–e1853. https://doi.org/10.1002/onco.13860 (2021).
Adams, R. et al. Inhibition of EGFR, HER2, and HER3 signalling in patients with colorectal cancer wild-type for BRAF, PIK3CA, KRAS, and NRAS (FOCUS4-D): A phase 2–3 randomised trial. Lancet Gastroenterol. Hepatol. 3, 162–171. https://doi.org/10.1016/S2468-1253(17)30394-1 (2018).
Meulendijks, D. et al. First-in-human phase I study of lumretuzumab, a glycoengineered humanized anti-HER3 monoclonal antibody, in patients with metastatic or advanced HER3-positive solid tumors. Clin. Cancer Res. 22, 877–885. https://doi.org/10.1158/1078-0432.CCR-15-1683 (2016).
Ooi, A. et al. Protein overexpression and gene amplification of HER-2 and EGFR in colorectal cancers: An immunohistochemical and fluorescent in situ hybridization study. Mod. Pathol. 17, 895–904. https://doi.org/10.1038/modpathol.3800137 (2004).
Shimada, Y. et al. Utility of comprehensive genomic sequencing for detecting HER2-positive colorectal cancer. Hum. Pathol. 66, 1–9. https://doi.org/10.1016/j.humpath.2017.02.004 (2017).
Gong, J., Cho, M., Sy, M., Salgia, R. & Fakih, M. Molecular profiling of metastatic colorectal tumors using next-generation sequencing: A single-institution experience. Oncotarget 8, 42198–42213. https://doi.org/10.18632/oncotarget.15030 (2017).
El-Deiry, W. S. et al. Molecular profiling of 6,892 colorectal cancer samples suggests different possible treatment options specific to metastatic sites. Cancer Biol. Ther. 16, 1726–1737. https://doi.org/10.1080/15384047.2015.1113356 (2015).
Nam, S. K. et al. BRAF, PIK3CA, and HER2 oncogenic alterations according to KRAS mutation status in advanced colorectal cancers with distant metastasis. PLoS ONE 11, e0151865. https://doi.org/10.1371/journal.pone.0151865 (2016).
Kuramochi, H. et al. Epidermal growth factor receptor (EGFR) mRNA levels and protein expression levels in primary colorectal cancer and corresponding liver metastases. Cancer Chemother. Pharmacol. 65, 825–831. https://doi.org/10.1007/s00280-009-1087-5 (2010).
Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma: the 3d English Edition [Secondary Publication]. J. Anus Rectum Colon 3, 175–195, https://doi.org/10.23922/jarc.2019-018 (2019).
Hofmann, M. et al. Assessment of a HER2 scoring system for gastric cancer: Results from a validation study. Histopathology 52, 797–805. https://doi.org/10.1111/j.1365-2559.2008.03028.x (2008).
Author information
Authors and Affiliations
Contributions
F.L. provided clinical samples and performed the experiments. P.Y. participated in the experiments, performed data analysis and drafted the manuscript. F.L. and J.X. performed data interpretation. Y.Z., J.C., R.D., and H.C. participated in data collection. Y.W. supervised data collection. J.X. and P.C. designed and supervised the overall study. All the authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ye, P., Li, F., Wei, Y. et al. EGFR, HER2, and HER3 protein expression in paired primary tumor and lymph node metastasis of colorectal cancer. Sci Rep 12, 12894 (2022). https://doi.org/10.1038/s41598-022-17210-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-17210-2
This article is cited by
-
HER2-Positive Metastatic Colorectal Cancer
Current Treatment Options in Oncology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.