Abstract
Behçet’s disease (BD) is a chronic inflammatory disease. Low levels of plasma high-density lipoprotein cholesterol (HDL-C) are associated with Crohn’s disease, another chronic inflammatory disease. However, the effects of low HDL-C levels on BD are unclear. We investigated the effects of HDL-C levels, and variability therein, on the risk for BD. We used the Korean National Health Insurance System database to identify 5,587,754 adults without a history of BD who underwent ≥ 3 medical examinations between 2010 and 2013. Mean HDL-C levels at each visit were used to calculate variability independent of the mean (VIM) and the coefficient of variation (CV). There were 676 new cases of BD (0.012%). The risk for BD was increased in participants with highly variable and low mean HDL-C levels. In a multivariate-adjusted model, the hazard ratios (95% confidence intervals) for BD incidence were 1.335 (1.058–1.684) in a high mean/high VIM group, 1.527 (1.211–1.925) in a low mean/low VIM group, and 2.096 (1.67–2.63) in a low mean/high VIM group compared to a high mean/low VIM group. Low mean HDL-C levels, and high variability therein, are independent risk factors for BD.
Similar content being viewed by others
Introduction
Behçet’s disease (BD) is a chronic inflammatory disease that causes oral and genital ulcers, uveitis, and skin lesions1. BD is a multisystem vasculitis that affects the mucosal lining, eyes, and joints as well as the nervous, cardiovascular, and gastrointestinal systems. The incidence and prevalence of BD are highest in areas near the ancient Silk Road, particularly Korea. The annual incidence rate of BD in Korea is 3976/100,000 population, much higher than in other countries2,3. Although the pathomechanism of BD is not clear, abnormalities of the immune system are probably involved4.
Lipid abnormalities increase the risk for atherosclerosis, and low levels of high-density lipoprotein cholesterol (HDL-C) in particular significantly increase the risk for cardiovascular disease5,6. Variability in cholesterol levels has been recognized as a risk factor for many diseases7,8,9,10. Low mean and highly variable HDL-C levels are associated with an increased risk for diabetes, myocardial infarction (MI), stroke, and mortality7,11.
The main pathological findings in BD are vasculitis and endothelial damage. Endothelial dysfunction leads to focal lipid deposition in the arterial intima layer12,13. In BD patients, the levels of atherosclerotic and inflammatory markers, such as tumor necrosis factor-α (TNF-α), lipoprotein-associated phospholipase A2 (Lp-PLA2), and homocysteine, are increased14. Recent studies have demonstrated low HDL-C levels in patients with Crohn’s disease, a chronic inflammatory disease15, but the relationship between HDL-C levels and BD is unclear. We investigated the effects of HDL-C levels (mean levels and variability therein) on the risk for BD in a national, population-based cohort of over 5 million people.
Results
Baseline characteristics of the study population
Table 1 summarizes the baseline characteristics of participants in groups based on HDL-C mean and CV. Participants were divided into four groups: high-mean/low-variability, high-mean/high-variability, low-mean/low-variability, and low-mean/high-variability groups. Compared to participants in the other groups, those in the low-mean/high-variability group had higher age, BMI, waist circumference, triglyceride levels, and prevalence of DM and hypertension as well as lower total cholesterol levels and income.
Risk for Behçet’s disease according to HDL-C levels and variability
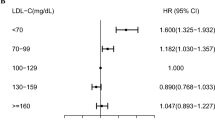
There were 676 new cases of BD (0.01%) during the follow-up period. In the Kaplan–Meier survival analysis with BD diagnosis as the dependent variable, participants in the lowest quartile of HDL-C levels showed the lowest disease-free survival rate (Fig. 1). After adjusting for age, sex, BMI, alcohol use, smoking, exercise, income, DM, hypertension, and mean HDL-C levels, HRs for BD diagnosis were higher in decile (D) groups D1–6 of mean HDL-C compared to D10 (Fig. 2a) and in D4–10 of VIM compared to D1 (Fig. 2b). After adjusting for age, sex, alcohol use, smoking, exercise, income, BMI, DM, hypertension, and mean HDL-C levels, the HRs (95% CIs) for BD diagnosis were 1.335 (1.058–1.684) in the high-mean/high-variability group, 1.527 (1.211–1.925) in the low-mean/low-variability group, and 2.096 (1.670–2.630) in the low-mean/high-variability group compared to the high-mean/low-variability group (Table 2).
Incidence rates (IRs), hazard ratios (HRs), and 95% confidence intervals (CIs) of Behçet’s disease by deciles of high-density lipoprotein cholesterol mean (a) and variability (b). Adjusted for age, sex, body mass index, alcohol use, smoking, exercise, income, diabetes mellitus, hypertension, and mean high-density lipoprotein cholesterol.
Discussion
We found that a low mean level of HDL-C, and high variability, are associated with an increased risk for BD.
Although the pathophysiology of BD is not clear, infections in genetically predisposed individuals appear to play a role4,16. The genetic factors that increase the risk for BD include HLA-B*51 allele and single-nucleotide polymorphisms in interleukin 10, 23R, and 12RB24,12,16. Triggers for BD include bacterial infections (such as Streptococcus sanguis, Helicobacter pylori, and Mycoplasma), viral infections (such as herpes simplex virus type 1, Epstein-Barr virus, hepatitis, and cytomegalovirus), and abnormal autoantigens (such as 60 kDa and 70 kDa heat-shock proteins, S-antigen, interphotoreceptor retinoid-binding protein, α-tropomyosin, and αβ-crystallin)4,16. Imbalances among natural killer cells, gamma delta T cells, and neutrophils may also be involved in the development of BD. Endothelial dysfunction and neutrophilic vascular inflammation cause thrombosis in BD patients. CD4 + T-helper cell subtypes 1, 2, 17, and 22, regulatory T cells, and cytokines involved in the adaptive immune system are also important in the pathogenesis of BD16.
Decreased levels of protective HDL-C reduce the activity and expression of endothelial nitric oxide synthase, leading to oxidative stress and damage to the vascular endothelium17. Recent studies have suggested that HDL-C protects the endothelium by stimulating the production of endothelial anti-atherogenic nitric oxide. HDL-C promotes endothelial repair and also has antioxidant, anti-inflammatory, and antithrombotic effects18,19,20. HDL-C infusion inhibits neutrophil activation in patients with peripheral vascular disease21 and reduces pro-inflammatory cytokine (interleukin-6, chemokine ligand 2, and tumor necrosis factor α) production in mice22,23. In a recent study, statins reduced T cell immunoglobulin- and mucin-domain-containing molecule-3 expression on natural killer and natural killer T cells, associated with an elevated HDL-C/total cholesterol ratio and decreased total cholesterol and LDL levels24. Therefore, reduced HDL levels induce endothelial dysfunction and activate neutrophils and natural killer cells, suggesting that HDL is involved in the pathogenesis of BD.
Clustering of triglyceride, HDL-C, glucose, waist circumference, and blood pressure was seen in a pattern that emulates metabolic syndrome. Among them, low HDL-C has been found to be related to MI and stroke in patients with metabolic syndrome25. In addition, high HDL-C variability is associated with an increased risk for MI, stroke, end-stage renal disease, and diabetes7,8,9,10,11,26,27,28. It may cause plaque instability due to impaired cholesterol efflux from peripheral tissues and macrophages26,29. The result of our study suggest that HDL-C variability increases the risk for BD, similar to other diseases described above.
Our study had some limitations. Although we used a 5-year washout period before the index year, reverse causation cannot be ruled out because this was a retrospective study. There are no data on the effects of duration of HDL-C abnormalities on the risk for BD, and there was a short follow-up duration in our study. In addition, exclusion of participants who had less than three medical examinations may have introduced selection bias in our study. Because most patients with dyslipidemia are taking statins, the effect of statins on cholesterol variability should be considered. However, since statins have little long-term effect on HDL-C, it is considered that the effect on variability of HDL-C is also small7,8.
Despite these limitations, our study had several strengths. For one, we used data from a large nationwide database representative of the entire Korean population to determine the relationship between HDL-C variability and BD. To the best of our knowledge, this is the first study on the association between HDL-C levels and risk for BD. Low mean levels of HDL-C, and high variability, were independent predictors of BD. Physicians should be aware of the increased risk for BD in such patients. Treatment of BD should also focus on normalizing the HDL-C levels. Further studies on the effects of HDL-C variability on endothelial dysfunction are required to evaluate the mechanisms underlying the relationship between HDL-C and BD.
In conclusion, in order to lower the risk of BD, it is important to raise HDL-C (more than 58.7 mg/dL in men and more than 67.5 mg/dL in women) and keep this condition constant for lowering variability. Quitting smoking, oral estrogen replacement therapy, extensive aerobic exercise, and treatment with niacin, statins, or fibrates may help increase HDL-C30,31,32.
Methods
Study design and database
The Korean National Health Insurance System (NHIS) uses billing records of healthcare providers to collect data on age, sex, demographic variables, treatment records, general health examinations, lifestyle, and behavior of patients. Healthcare providers are advised to perform standardized medical examinations every 1 or 2 years. Enrollment in the NHIS is compulsory for all of the > 50 million Korean residents33,34.
Study population
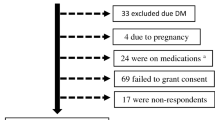
We collected data from 19,459,018 participants in the NHIS who underwent medical checkups between 2012 and 2013 (index year), and had been enrolled in NHIS for at least 5 years before the checkup (i.e., washout period: 2007–2011). We selected 5,632,394 participants who underwent a medical checkup in the index year and ≥ 2 checkups during the preceding 3 years. After exclusion of participants of age < 20 years (n = 435) or missing data (n = 34,810), data from 5,587,754 participants was included in this study (Fig. 3). Ethical approval was obtained from the Institutional Review Board of Uijeongbu St. Mary’s Hospital, Catholic University of Korea (UC18ZESI0094). Informed consent was not required as the data used for this study were anonymized. All methods were carried out in accordance with relevant guidelines and regulations. The need of the written informed consent has waived by the ethics committee of institutional review board of the Korean National Institute for Bioethics Policy and the Catholic University of Korea Institutional Review Board.
Measurements and definitions
Body mass index (BMI) was calculated by dividing the weight (kg) by the square of height (m2), and obesity was defined as BMI ≥ 25 kg/m235. “Regular physical activity” was defined as ≥ 20 min of vigorous physical activity ≥ 3 times/week or ≥ 30 min of moderate-intensity physical activity ≥ 5 times/week.
Household income was dichotomized at 25% of monthly income36. Diabetes mellitus (DM) was recorded as being present if the fasting blood glucose level was ≥ 126 mg/dL or there was ≥ 1 claim/year for International Classification of Disease, tenth revision (ICD-10) codes E10–14 and ≥ 1 claim/year for antidiabetic drug prescriptions. Hypertension was recorded as being present if the blood pressure was ≥ 140/90 mm Hg or there was ≥ 1 claim/year for ICD-10 codes I10–15 and ≥ 1 claim/year for antihypertensive drug prescriptions. Dyslipidemia was recorded as being present if the total cholesterol was ≥ 240 mg/dL or there was ≥ 1 claim/year for ICD-10 code E78 and ≥ 1 claim/year for lipid-lowering drug prescriptions. Self-administered questionnaires were used to document social behaviors, including smoking, alcohol use, and physical activity.
Definition of HDL-C variability
HDL-C variability was calculated using HDL-C levels measured at two different health screenings. HDL-C variability was measured with the coefficient of variation (CV), variability independent of the mean (VIM), and average real variability (ARV). CV was calculated as (standard deviation [SD]/mean) × 100. VIM was calculated as (SD/meanβ) × 100, where β was the regression coefficient37,38. ARV was the average absolute difference between consecutive HDL-C levels39.
Definition of low mean HDL-C and high HDL-C variability
Mean HDL-C values differ between men and women; therefore, we used sex-specific cutoff values (Table 3). Participants with HDL-C levels in the lowest quartile (quartile 1) were included in the low-mean HDL-C group, and those with HDL-C levels in the remaining three quartiles (quartiles 2–4) were included in the high-mean HDL-C group. Participants with HDL-C variability in the highest quartile (quartile 4) were included in the high-variability group, and those with HDL-C variability in the remaining three quartiles (quartiles 1–3) were included in the low-variability group.
Study outcomes and follow-up
The end point of this study was a diagnosis of BD, defined as documentation of ICD-10 codes M35.2 or V139 (rare intractable diseases). The study participants were followed up from baseline to BD diagnosis or until December 31, 2016, whichever came earlier. The median follow-up duration was 4.22 (4.01–4.55) years.
Statistical analysis
Baseline demographics are presented as mean ± SD, median (interquartile range 25–75%), or n (%). Participants were grouped using HDL-C mean and CV quartiles. Hazard ratios (HRs) and 95% confidence interval (CI) values were calculated using the Cox proportional-hazards model. The HRs (95% CIs) of the low-mean HDL-C and high-variability groups were compared to those of the high-mean HDL-C and low-variability groups, respectively. Kaplan–Meier estimates were used to calculate the cumulative HRs for quartiles of HDL-C mean and variability as well as groups of combinations of mean and variability. These HRs were used in the Schoenfeld residuals test to evaluate the proportional hazards function. There was no significant departure from proportionality of hazards over time. A proportional-hazards model was applied after adjusting for age, sex, BMI, alcohol use, smoking, exercise, income, DM, and hypertension. We used stratified analysis and a likelihood-ratio test to evaluate potential modifier effects of age, sex, obesity, DM, hypertension, malignancy, and use of lipid-lowering agents. Statistical analyses were performed using SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA), and p-values ≤ 0.05 were considered to indicate significance.
Data availability
The data that support the findings of this study are available within the article.
References
Sakane, T., Takeno, M., Suzuki, N. & Inaba, G. Behcet’s disease. N. Engl. J. Med. 341, 1284–1291 (1999).
Verity, D. H., Marr, J. E., Ohno, S., Wallace, G. R. & Stanford, M. R. Behcet’s disease, the Silk Road and HLA-B51: historical and geographical perspectives. Tissue Antigens 54, 213–220 (1999).
Lee, Y. B. et al. Incidence, prevalence, and mortality of Adamantiades-Behcet’s disease in Korea: a nationwide, population-based study (2006–2015). J. Eur. Acad. Dermatol. Venereol. 32, 999–1003 (2018).
de Chambrun, M. P., Wechsler, B., Geri, G., Cacoub, P. & Saadoun, D. New insights into the pathogenesis of Behcet’s disease. Autoimmun. Rev. 11, 687–698 (2012).
Rosenson, R. S. et al. Translation of high-density lipoprotein function into clinical practice: current prospects and future challenges. Circulation 128, 1256–1267 (2013).
Heinecke, J. HDL and cardiovascular-disease risk–time for a new approach?. N. Engl. J. Med. 364, 170–171 (2011).
Han, B. H., Han, K., Yoon, K. H., Kim, M. K. & Lee, S. H. Impact of mean and variability of high-density lipoprotein-cholesterol on the risk of myocardial infarction, stroke, and mortality in the general population. J. Am. Heart Assoc. 9, e015493 (2020).
Waters, D. D. et al. Visit-to-visit variability of lipid measurements as predictors of cardiovascular events. J. Clin. Lipidol. 12, 356–366 (2018).
Boey, E. et al. Visit-to-visit variability in LDL- and HDL-cholesterol is associated with adverse events after ST-segment elevation myocardial infarction: A 5-year follow-up study. Atherosclerosis 244, 86–92 (2016).
Kim, M. K. et al. Cholesterol variability and the risk of mortality, myocardial infarction, and stroke: a nationwide population-based study. Eur. Heart J. 38, 3560–3566 (2017).
Lee, S. H. et al. HDL-cholesterol, its variability, and the risk of diabetes: a nationwide population-based study. J. Clin. Endocrinol. Metab. 104, 5633–5641 (2019).
Mendoza-Pinto, C. et al. Etiopathogenesis of Behcet’s disease. Autoimmun. Rev. 9, 241–245 (2010).
Caliskan, M. et al. Endothelial functions are more severely impaired during active disease period in patients with Behcet’s disease. Clin. Rheumatol. 26, 1074–1078 (2007).
Yucel, C. et al. Evaluation of atherogenic laboratory markers in Behcet’s disease patients with vascular involvement. Arch. Med. Sci. 16, 531–537 (2020).
Soh, H. et al. Crohn’s disease and ulcerative colitis are associated with different lipid profile disorders: a nationwide population-based study. Aliment Pharmacol. Ther. 51, 446–456 (2020).
Tong, B., Liu, X., Xiao, J. & Su, G. Immunopathogenesis of Behcet’s Disease. Front. Immunol. 10, 665 (2019).
Jamwal, S. & Sharma, S. Vascular endothelium dysfunction: a conservative target in metabolic disorders. Inflamm. Res. 67, 391–405 (2018).
Besler, C., Heinrich, K., Riwanto, M., Lüscher, T. F. & Landmesser, U. High-density lipoprotein-mediated anti-atherosclerotic and endothelial-protective effects: a potential novel therapeutic target in cardiovascular disease. Curr. Pharm. Des. 16, 1480–1493 (2010).
Sorrentino, S. A. et al. Endothelial-vasoprotective effects of high-density lipoprotein are impaired in patients with type 2 diabetes mellitus but are improved after extended-release niacin therapy. Circulation 121, 110–122 (2010).
Charakida, M. et al. Vascular abnormalities, paraoxonase activity, and dysfunctional HDL in primary antiphospholipid syndrome. JAMA 302, 1210–1217 (2009).
Murphy, A. J. et al. Neutrophil activation is attenuated by high-density lipoprotein and apolipoprotein A-I in in vitro and in vivo models of inflammation. Arterioscler. Thromb. Vasc. Biol. 31, 1333–1341 (2011).
Wang, Y. et al. Anti-inflammatory effects of HDL in mice with rheumatoid arthritis induced by collagen. Front. Immunol. 9, 1013 (2018).
Scanu, A. et al. HDL inhibit cytokine production in a mouse model of urate crystal-induced inflammation. Reumatismo 62(4), 266–272 (2011).
Zhang, N. et al. Statins reduce the expressions of Tim-3 on NK cells and NKT cells in atherosclerosis. Eur. J. Pharmacol. 821, 49–56 (2018).
Kolovou, G. D., Anagnostopoulou, K. K. & Cokkinos, D. V. Pathophysiology of dyslipidaemia in the metabolic syndrome. Postgrad. Med. J. 81, 358–366 (2005).
Lee, E. Y. et al. Effect of visit-to-visit LDL-, HDL-, and non-HDL-cholesterol variability on mortality and cardiovascular outcomes after percutaneous coronary intervention. Atherosclerosis 279, 1–9 (2018).
Koh, E. S. et al. Intra-individual variability in high density lipoprotein cholesterol and risk of end-stage renal disease: A nationwide population-based study. Atherosclerosis 286, 135–141 (2019).
Simpson, W. G. Biomarker variability and cardiovascular disease residual risk. Curr. Opin. Cardiol. 34, 413–417 (2019).
Navab, M., Reddy, S. T., Van Lenten, B. J. & Fogelman, A. M. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nat. Rev. Cardiol. 8, 222–232 (2011).
Kakafika, A., Athyros, V. G., Tziomalos, K., Karagiannis, A. & Mikhailidis, D. P. High density lipoprotein cholesterol and statin trials. Curr. Med. Chem. 15, 2265–2270 (2008).
Bermudez, V. et al. Pharmacologic management of isolated low high-density lipoprotein syndrome. Am. J. Ther. 15, 377–388 (2008).
Miller, M. Raising an isolated low HDL-C level: why, how, and when?. Cleve Clin. J. Med. 70, 553–560 (2003).
Ko, S. H. et al. Past and current status of adult type 2 diabetes mellitus management in Korea: a National Health Insurance Service Database analysis. Diabetes Metab. J. 42, 93–100 (2018).
Lee, Y. H. et al. Data analytic process of a nationwide population-based study using National Health Information Database established by National Health Insurance Service. Diabetes Metab. J. 40, 79–82 (2016).
Kim, M. K. et al. 2014 clinical practice guidelines for overweight and obesity in Korea. Endocrinol. Metab. (Seoul) 29, 405–409 (2014).
Song, S. O. et al. Background and data configuration process of a nationwide population-based study using the korean national health insurance system. Diabetes Metab. J. 38, 395–403 (2014).
Asayama, K. et al. Home blood pressure variability as cardiovascular risk factor in the population of Ohasama. Hypertension 61, 61–69 (2013).
Fukuda, K. et al. Day-by-day blood pressure variability and functional outcome after acute Ischemic stroke: Fukuoka Stroke Registry. Stroke 46, 1832–1839 (2015).
Mena, L. et al. A reliable index for the prognostic significance of blood pressure variability. J. Hypertens 23, 505–511 (2005).
Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) Grant funded by the Korean government (NRF-2018R1D1A1B07044100).
Author information
Authors and Affiliations
Contributions
Y.H.K., Y.B.L. and J.H.L. were responsible for the conception and design of the study. K.D.H. was responsible for acquisition of data. Y.H.K., H.J.K., J.W.P., K.D.H., Y.G.P., Y.B.L. and J.H.L. performed the data analysis. Y.H.K., H.J.K. and J.W.P. drafted the manuscript. Y.B.L. and J.H.L. contributed on revising the manuscript content. All authors participated in interpretation of the findings and all authors read and approved the final version of the manuscript. Y.B.L. and J.H.L. contributed equally to the work as corresponding authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, Y.H., Kim, H.J., Park, J.W. et al. Risk for Behçet’s disease gauged via high-density lipoprotein cholesterol: a nationwide population-based study in Korea. Sci Rep 12, 12735 (2022). https://doi.org/10.1038/s41598-022-17096-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-17096-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.