Abstract
Anthropogenic carbon emissions are causing seawater pH to decline, yet the impact on marine calcifiers is uncertain. Scleractinian corals and coralline algae strongly elevate the pH of their calcifying fluid (CF) to promote calcification. Other organisms adopt less energetically demanding calcification approaches but restrict their habitat. Stylasterid corals occur widely (extending well below the carbonate saturation horizon) and precipitate both aragonite and high-Mg calcite, however, their mode of biocalcification and resilience to ocean acidification are unknown. Here we measure skeletal boron isotopes (δ11B), B/Ca, and U/Ca to provide the first assessment of pH and rate of seawater flushing of stylasterid CF. Remarkably, both aragonitic and high-Mg calcitic stylasterids have low δ11B values implying little modification of internal pH. Collectively, our results suggest stylasterids have low seawater exchange rates into the calcifying space or rely on organic molecule templating to facilitate calcification. Thus, despite occupying similar niches to Scleractinia, Stylasteridae exhibit highly contrasting biocalcification, calling into question their resilience to ocean acidification.
Similar content being viewed by others
Introduction
At current anthropogenic carbon emission rates, surface seawater pH is predicted to fall below 7.8, and carbonate saturation states (Ω = [Ca2+] × [CO32−]/K*sp) will greatly reduce before the end of this century1. Such large-magnitude and (geologically) rapid ocean acidification is expected to be a key stressor for marine calcifying organisms2, with many studies showing a reduction in stony coral health, abundance, and calcification rates under low seawater pH conditions (e.g.3). Yet without detailed knowledge of coral biocalcification mechanisms and adaptive strategies the full impact of ocean acidification on these marine calcifiers remains unclear. Particular concerns have been raised as to the vulnerability of deep-sea corals that remain poorly studied and occupy niches that are likely to be at the limits of their carbonate saturation tolerance already4,5.
Mineralogy plays an important role in dictating the susceptibility of carbonate to dissolution6,7. Calcite is a relatively dissolution-resistant form of CaCO3, typically adopted by unicellular foraminifera8. Aragonite is a more soluble polymorph, yet many organisms, including scleractinian corals, use this mineral9. As more magnesium is incorporated into calcite (up to ~ 140 mmol/mol Mg/Ca) its solubility increases towards that of aragonite7. Many organisms calcify using this intermediate solubility “high-Mg” calcite including Isidiid gorgonian “bamboo” corals10 and sea urchins11. As the Mg content of calcite increases further (> 140 mmol/mol Mg/Ca) the solubility of this high-Mg calcite falls below even that of aragonite. Organisms that adopt this more soluble mineral form to build their skeletons include crustose coralline algae12.
With a diverse array of carbonate minerals used by marine organisms, different calcification pathways are required to construct and maintain these skeletons. However, there have been comparatively few studies where microsensors or pH sensitive dyes have been placed within living biocalcifiers to monitor pH at the site of calcification as they require sophisticated laboratory culture setup and cannot be applied to coral specimens post-mortem (e.g.13,14,15). Skeletal geochemistry is therefore the go-to approach for understanding the mineralisation process, with the boron isotopic composition (δ11B; 11B/10B ratio relative to the standard NIST SRM 951 in ‰) of marine carbonates shown to be a powerful tool to assess pH at the site of calcification16,17.
Scleractinian corals and coralline algae adopt more dissolution-susceptible mineral forms, but calcify from fluids that are semi-restricted from ambient seawater and in which pH is biologically elevated to promote calcification. This “pH upregulation” varies by taxon, but in cold-water Scleractinia it is ~ 1 pH unit above ambient seawater16. This strong elevation of pH in the CF results in skeletal δ11B values > 8‰ higher than seawater borate values (Fig. 1; high ∆δ11B; where ∆δ11B = δ11Bcarbonate – δ11Bborate; Fig. 2)16,18,19,20,21,22. While photosynthetic coralline algae are restricted to the photic zone with its generally higher carbonate saturation states, the adaptation of pH upregulation has enabled azooxanthellate cold-water Scleractinia to live much deeper, even in undersaturated seawater conditions (Fig. 25). It has been suggested that biogenic aragonites that lack strong pH upregulation strategies (resulting in low ∆δ11B values) such as the benthic foraminifera Hoeglundina elegans have a more restricted habitat and only calcify when ambient seawater is oversaturated with respect to aragonite, in areas such as the shallow Bahamas platform (Fig. 223). Many studies have suggested that the increased buffering associated with pH upregulation will make Scleractinia resilient against moderate changes in ocean pH16,24, however others continue to highlight the potential vulnerability of these corals in the deep sea and question the energetic demands of this biocalcification strategy4,5,25.
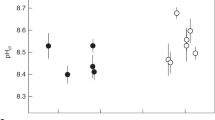
Coral δ11B plotted against δ11B of the borate ion in seawater (a function of pH; blue labels). Stylasterid corals (circles; this study) are compared to the well-characterised aragonitic scleractinian Desmophyllum dianthus16,30,31 that strongly upregulate pH at the site of calcification. D. dianthus replicates are averages and one shallow water fjord sample from McCulloch et al.16 was excluded because of high seawater pH variability at that site. Error bars denote the estimated uncertainty on seawater borate δ11B.
Skeletal carbonate δ11B offset from δ11B of borate in seawater (∆δ11B)—a measure of calcifying fluid pH upregulation—compared to (A) mineralogy and (B) carbonate ion saturation of seawater (Ω) habitat. Stylasterid coral ∆δ11B are compared to published δ11B values for aragonitic scleractinia16,30,31, high-Mg calcitic bamboo corals32, calcitic and aragonitic foraminifera35, and crustose coralline algae18,21,22. Mineral solubility from7.
Stylasteridae (Class Hydrozoa: Order Anthoathecata) are the second most diverse group of hard corals in the global ocean26,27. The majority of stylasterid species can be found below 50 m water depth making this an important, but poorly studied, deep-water coral family26,27. These Hydrozoan corals differ significantly from scleractinian corals in their ability to precipitate skeletons of either aragonite, high-Mg calcite, or in some cases, both mineral polymorphs26. Compared to Scleractinia, skeletal δ18O and δ13C in stylasterid corals are close to equilibrium with seawater28, however a positive covariation of these δ18O and δ13C ratios suggests that there may be at least some biological modification of the internal seawater carbonate chemistry28. Contrary to expectation, the stylasterid corals that deviate least from equilibrium δ18O and δ13C values were found to be aragonitic specimens (~ − 3‰) implying that there is less modification of internal carbonate chemistry than high-Mg calcitic specimens, despite their greater mineralogical vulnerability to dissolution28. While this may represent a mineralogical control on skeletal stable isotope values, little is known about how stylasterids biomineralize and are able to thrive in undersaturated seawater conditions.
To address the gap in understanding of stylasterid coral biocalcification, we present the first skeletal δ11B, B/Ca, and U/Ca data obtained from stylasterid coral skeletons (Fig. 3). The samples in this study lived in seawater environments ranging from pH 7.86 to 8.04 (ΩArag. 0.9 to 2.0). They comprise two genera of stylasterid corals (Stylaster and Errina) that represent all three modes of stylasterid skeletal mineralogy: (i) solely aragonite, (ii) both aragonite and high-Mg calcite, and (iii) solely high-Mg calcite28,29. We use the same stylasterid specimens for which skeletal stable oxygen and carbon isotopes have been previously characterised28 for comparison with our carbonate system proxy results.
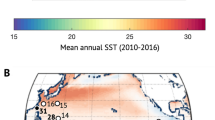
Location of Stylasteridae coral samples used in this study. Base map shows GLODAP v2 gridded seawater pH (total scale) at 700 m65. Map drawn using Ocean Data View software (Version 4.6.5; https://odv.awi.de/)72.
Results
Stylasterid boron isotopes
Replicate measurements of bulk stylasterid δ11B of the same individual were generally within 0.5‰ of each other (Fig. 1). Once δ11B replicates for each stylasterid specimen are averaged, mean δ11B values are typically within 1‰ of the estimated δ11B composition of borate in ambient seawater for each coral (Fig. 1). The only samples (excluding Stylaster ibericus) with mean δ11B values more than 1‰ higher than δ11Bborate were the high-Mg calcitic E. laterorifa (δ11B = δ11Bborate + ~ 1.5‰). We therefore find little appreciable difference in δ11B between high-Mg calcitic and aragonitic stylasterid corals that live in similar seawater pH conditions. The single Stylaster ibericus specimen in this study yielded anomalously high δ11B (and low B/Ca) compared to other aragonitic stylasterids. This is discussed in detail in the Supplementary Information; however, its inclusion here has little impact on our overall findings.
We perform linear regression of stylasterid δ11B measurements against δ11Bborate. The following relationships include all stylasterid δ11B measurements (regardless of mineralogy; Eq. 1), aragonitic stylasterids only (Eq. 2), and weighted regression of aragonitic stylasterids (Eq. 3), against δ11Bborate (SE):
The regression in Eq. (3) weights observations according to the inverse of the uncertainty on their mean skeletal δ11B measurements. For this, the range in δ11B of the two replicate analyses about the mean was used. In instances where this range was less than analytical uncertainty, a value of ± 0.16‰ was used instead. These equations open the possibility to utilise stylasterid corals as archives for past seawater pH reconstruction (Supplementary Information).
Stylasterid B/Ca and U/Ca
B/Ca replicate measurements made on individual pieces of stylasterid coral were typically within 7% for each specimen. U/Ca measurements were considerably more variable between replicates, on average differing by 14% for both aragonitic and calcitic specimens. Mean B/Ca and U/Ca values for high-Mg calcitic specimens were generally lower (< 330 and < 110 µmol/mol respectively) than aragonitic stylasterids (average 918 and 392 µmol/mol respectively) (Figs. 4 and 5). B/Ca and U/Ca results for the mixed mineralogy species E. gracilis lie on a mixing line between pure aragonitic specimens and high-Mg calcitic specimens, though falling closest to the pure aragonites, consistent with their 92 to 96 weight percent aragonite content28 (Figs. 4 and 5).
Carbonate B/Ca against δ11B. Stylasterid B/Ca and δ11B replicate results (this study) are compared to fibrous aragonite within scleractinia (D. dianthus )47, and crustose coralline algae (CCA18,21). Individual analyses from the same specimens are connected by solid trend lines (2 analyses or more). Dashed trendline shows the covariance between all aragonitic stylasterid measurements (p < 0.01). Blue arrow shows theoretical mixing line between solely aragonitic and solely high-Mg calcitic stylasterids.
Coral U/Ca against (A) δ18O and (B) δ11B (C) B/Ca. Stylasterid U/Ca, B/Ca, and δ11B data are from this study, paired with δ18O measurements in the same specimens28. Scleractinian D. dianthus data are from micro-sampled specimens by Chen et al.53 and Stewart et al.47. Individual analyses from the same specimens are connected by solid trend lines (2 analyses or more). Dashed trendlines show the covariance between all aragonitic stylasterid measurements (p < 0.01).
Where there were differences between B/Ca and δ11B duplicate measurements (i.e. > 0.2‰; n = 6; excluding S. ibericus), in all cases B/Ca was found to be negatively correlated with δ11B for both aragonitic and mixed mineralogy specimens (Fig. 4). U/Ca values in aragonitic stylasterids show little covariance with previous δ18O measurements in the same specimens (Fig. 5A; 28), but positive covariance with skeletal δ11B measurements (Fig. 5B), and slight negative covariance with B/Ca (Fig. 5C). These generalised trends hold both between specimens and between sample replicates (Figs. 4 and 5).
Discussion
The most important result in this study is the low skeletal δ11B measured in all stylasterid corals (Fig. 1) with no apparent offset from ambient seawater δ11Bborate (low ∆δ11B; Fig. 2). These results are in stark contrast to high ∆δ11B values recorded in scleractinian corals16,30,31 and coralline algae18,21,22 known to strongly modify pH at the site of calcification to promote mineral growth (Fig. 2). Instead, the low ∆δ11B values we record in stylasterid corals are more similar to bamboo corals32, sea urchins11,33, bivalves33,34, and unicellular foraminifera (e.g. Cibicidoides wuellerstorfi or H. elegans)35 (Fig. 2). However, many of these organisms are composed of dissolution-resistant mineral forms (e.g. calcite). Stylasterids on the other hand, while not the first aragonitic taxon to exhibit low skeletal δ11B (e.g. H. elegans), are the first to show this feature whilst living at depths close to and below the carbonate saturation horizon36. Assuming that stylasterid δ11B reflects the pH of the CF according to the abiotic relationship between the borate ion and pH (Eq. (4); Methods; e.g.16,17), these δ11B results suggest no modification of internal seawater pH in stylasterids. Furthermore, application of a physiochemical biocalcification model based on scleractinian corals by DeCarlo et al.37 (Methods), the high B/Ca values in our aragonitic stylasterids imply low [CO32−] in the CF (average 110 µmol/kg excluding S. ibericus) similar to external seawater (average 90 µmol/kg). Low [CO32−] in the CF would in turn translate to low CF ΩAragonite (average 1.5 excluding S. ibericus; 4 corals have ΩAragonite < 1). This contrasts with similar modelling of δ11B and B/Ca data in scleractinian corals which suggest CF ΩAragonite is typically more than 5 times that of ambient seawater38. Although our estimates for stylasterid CF ΩAragonite represent minimum values (i.e. no transport of Ca2+ to the CF), it remains hard to explain how stylasterids commence calcification at all at such low saturation states. Like stylasterid corals, the δ11B of many foraminifera record the δ11B of borate and thus external seawater pH35,39, however microsensor studies show the pH of foraminiferal CF is actually slightly elevated (+ 0.5 pH units)40. We therefore explore alternative mechanisms that may facilitate mineralisation at low Ω and/or decouple the boron skeletal chemistry from calcification processes including: (i) bicarbonate active transport41, (ii) high boric acid to seawater flushing rates25, and (iii) organic molecule templating42.
Despite cold-water scleractinian corals strongly upregulating pH, unusually low δ11B values (8.5‰) have been observed in their centres of calcification (or early mineralisation zones), particularly in Lophelia pertusa41,43,44. This disconnect between the internal pH and skeletal δ11B has been explained by bicarbonate (HCO3−) active transport to the site of calcification41. The use of HCO3− as a source of DIC rather than CO2 (from respiration and diffusion) promotes mineralisation as it releases fewer protons during conversion to CO32−. The bicarbonate active transport model suggests that seawater borate is co-transported to the site of calcification, resulting in low skeletal δ11B values closer to seawater borate41. While this mechanism could explain low δ11B values in stylasterid corals, studies of the scleractinian coral Lophelia pertusa suggest that these centres of calcification are also associated with low B/Ca compared to the bulk coral (− 40%)41,44. This contrasts with the extremely high B/Ca values we observe in our aragonitic stylasterid corals compared to Scleractinia, suggesting an alternative mechanism is driving their boron skeletal chemistry.
Another approach to reconciling discrepancies between pH derived from microsensors and geochemistry comes from coral modelling work by Gagnon et al.25. In this model, internal scleractinian pH is held at a high, but constant, value of 8.9 (resulting in their characteristic high ∆δ11B) and low skeletal δ11B values are instead explained by higher rates of boric acid diffusion (DB) relative to seawater exchange in the CF (kz; where k is the rate of seawater exchange and z is the volume to surface area ratio of the CF); where DB/kz scales linearly with seawater dissolved inorganic carbon ([DIC]). High DB/kz may therefore explain the low δ11B values in stylasterid corals, with low seawater flushing rates allowing them to occupy hostile growth environments (e.g. ΩAragonite as low as 0.5 (depths > 500 m) in the high latitude North Pacific45). To explore this hypothesis further, we use U/Ca and B/Ca ratios, with their sensitivity to the carbonate chemistry of seawater to test the potential role of DB/kz in stylasterids.
A positive correlation between carbonate δ11B and B/Ca is readily explained because at high pH more of the boron is in the borate ionic form and readily incorporated into the carbonate lattice46. This phenomenon has been observed in scleractinian corals47 and crustose coralline algae18,21 (Fig. 4). However, we find the opposite—a negative correlation between δ11B and B/Ca in sample replicates of stylasterid corals and particularly high B/Ca ratios in aragonitic specimens which cannot be explained by this effect. Although boron is preferentially incorporated into an aragonite lattice over calcite 48, this cannot account for the near double skeletal boron concentration in aragonitic stylasterids compared to scleractinian corals with the same mineralogy. While this may imply low concentrations of [DIC] and/or [CO32−] (e.g. DeCarlo et al.37 model (Methods)), an alternative explanation for high B/Ca ratios and the negative correlation between δ11B and B/Ca in stylasterid corals is high DB/kz. Higher rates of boric acid diffusion relative to seawater exchange would simultaneously drive δ11B low whilst increasing [B] in the CF, thus driving B/Ca high (i.e. high [B]/[DIC]49).
The pH and/or [CO32−] dependency of uranyl ion complexation in seawater gives a strong theoretical basis for the sensitivity of coral U/Ca to the carbonate system50,51. The master variable however is not clear and many potential drivers of coral U/Ca have been suggested including: inverse relationships with (i) calcifying fluid [CO32−]50,52,53, (ii) pH53,54, and (iii) [DIC]55, as well as (iv) positive correlation with the rate of seawater replenishment of the coral calcifying space (kz)55. Evidence for the pH effect on U/Ca comes from positive covariance between δ18O and U/Ca53 (Fig. 5A) and negative covariance between δ11B and U/Ca47 (Fig. 5B) measured in replicate samples of scleractinian corals that strongly upregulate pH at the site of calcification. Thus while covariation of δ18O and δ13C ratios suggests perhaps some modification of internal pH28, the absence of correlation between δ18O and U/Ca (either between stylasterid coral specimens or between replicates) suggests that any pH upregulation in stylasterids is small enough not to impact U/Ca (Fig. 5A). Stylasterid coral results further contrast with scleractinian corals by exhibiting a positive correlation between δ11B and U/Ca between sample replicates (Fig. 5B). This is a counterintuitive result in the context of the pH and [CO32−] effects on coral U/Ca ratios (e.g.53), however if DB/kz were the main driver of stylasterid coral U/Ca (e.g.55) then the positive covariance between δ11B and U/Ca can be reconciled (Fig. 5B) along with low U/Ca values that negatively correlate with B/Ca (Fig. 5C). Thus, high DB/kz can explain both the boron and uranium skeletal chemistry of stylasterids, with any small degree of biologically induced pH increase at the site of calcification (required to facilitate boric acid diffusion) potentially masked by low seawater flushing rates.
The mechanisms discussed so far have largely focussed on physiochemical processes inside a coral where mineralisation (ion-by-ion attachment) is dictated by Ω of the CF and where the elemental skeletal chemistry is driven by growth rate effects. Recent studies however highlight the important role that coral organic molecules may play in biocalcification (e.g. review by Drake et al.42). Acid-rich proteins in corals can spontaneously catalyse the formation of amorphous CaCO356. Under this biologically mediated framework, the calcifying space requiring manipulation is just nanometers in size, comprising vesicles that both bind Ca2+ and transport the amorphous CaCO3 to the site of deposition42. This mechanism yields elemental skeletal chemistry which is more dependent on coral physiology42. The contention that stylasterid mineralogy (aragonite or high-Mg calcite) is more dictated by genetics rather than environmental parameters (e.g. seawater ΩAragonite)29 does speak for a strong biological control on calcification. Thus, while aragonite may be vulnerable to dissolution7, it is potentially the more thermodynamically favourable mineral to form when mediated by acid-rich proteins in corals from modern seawater56. A reliance on organic matrix templates for mineralisation may therefore explain the paradoxically low skeletal δ11B values in stylasterids given their depth habitat. The presence of seawater at the site of calcification would also result in low skeletal δ11B values directly linked to ambient seawater pH. A strong biological control on calcification would therefore not preclude the use of stylasterid δ11B for use as a tracer for past seawater pH (e.g. Eq. (1); Supplementary information).
The energetic burden of calcifying via biologically-controlled organic molecule templating is considered low, such that it may confer resilience to organisms against future changes in ocean pH56. However, many of the low pH and undersaturated habitats in which stylasterid corals appear to thrive are on seamounts45. These submarine features, particularly those at high-latitude, are often areas of localised current variability (suitable for larval dispersion), high primary production, and high organic flux to the seafloor (food supply), resulting in biodiversity hot-spots57. A ready source of metabolic energy is important in many marine calcifiers (e.g.14), therefore it is possible that stylasterid corals compensate for low saturation conditions with additional metabolic energy sourced from an abundant food supply in these otherwise seemingly inhospitable environments. Some resilience to low pH waters may also come from their relatively thick layer of organic tissue that has been shown to shield the skeletons of other corals from undersaturated seawater58.
Conclusions
Stylasterid corals are of great ecological importance, occupying niches that other corals do not, thus providing vital habitat for other benthic organisms26,59. Understanding and mitigating the impacts of climate change on these corals is therefore key to their conservation. The low skeletal δ11B values that we record in all stylasterid corals, regardless of their mineralogy, closely resemble seawater δ11Bborate suggesting that any biological upregulation of pH at the site of calcification in stylasterids is small. This style of biocalcification contrasts greatly with other aragonitic deep-water biocalcifiers (e.g. Scleractinia) and may be a result of an entirely different evolutionary history. For instance, many benthic marine fauna are thought to have originated in shallow waters and evolved to fill deep-water niches, whereas stylasterids are thought to have undergone an opposing, offshore to onshore, habitat expansion60. We argue that the low δ11B and generally high B/Ca and low U/Ca values we record in aragonitic stylasterids are best explained by (i) lower seawater exchange rates and/or higher rates of boric acid diffusion into the CF compared to scleractinian corals or (ii) a reliance on organic matrix templating for calcification.
Without a strong internal pH upregulating mechanism, physiochemical models of biocalcification would predict that stylasterids precipitating aragonite will be more vulnerable to future declines in seawater pH than those that have adopted the more dissolution-resistant high-Mg calcitic mineral form. However, if their calcification is strongly biologically mediated, this strategy may prove advantageous over other taxa which rely on ion pumping to modify their calcifying fluid chemistry (e.g. scleractinian corals). Determining the energetic burden of their calcification strategy will therefore be key to understanding the resilience of stylasterids to future change.
Methods
Stylasterid samples
Stylasterid samples were collected for this study from the Labrador Sea, (RRS Discovery DY081), Northeast Atlantic (RRS James Cook JC136), Equatorial Atlantic (RRS James Cook JC094), Drake Passage (R/V Nathaniel B. Palmer 1103 and 0805; RRS James Clark Ross JR15005), Galapagos Archipelago (Alucia Cruise AL1508) and (Supplementary Information). With the exception of two individuals (which appear to have “pristine” preservation based on visual inspection), all samples in this study were collected alive, with associated organic tissue, thus ensuring paired hydrographic data are representative. Stylasterid samples are grouped by mineralogy (i) solely aragonite (E. antarctica, E. boschmai, E. altispina, S. robustus, S. densicaulis, S. erubescens, S. ibericus, S. marenzelli; Mg/Ca ~ 2.5 mmol/mol), (ii) both aragonite and high-Mg calcite (E. gracilis; Mg/Ca ~ 12 mmol/mol), and (iii) solely high-Mg calcite (E. laterorifa; Mg/Ca ~ 90 mmol/mol)28,29,61. The Li/Mg and Sr/Ca ratios of these same specimens were previously measured by Stewart et al.61. Because E. laterorifa Mg/Ca values are less than 140 µmol/mol, the high-Mg calcitic corals in this study are considered theoretically more resistant to dissolution than pure aragonitic counterparts based solely on their mineralogy7.
Matching hydrographic data
To characterise ambient seawater conditions at each site we use the same paired hydrographic data to that of Stewart et al.61. Alkalinity and [DIC] measurements were used in conjunction with temperature, salinity, and nutrient data to calculate full carbonate system parameters at each location including pH (total scale); Seacarb package in R62). This calculation used carbonate dissociation constants from Lueker et al.63 and the boron to salinity ratios of Lee et al.64. Where possible seawater hydrographic data were taken from co-located remotely operated vehicle or CTD (conductivity, temperature, and depth) profiles taken during the respective research cruise (10 samples from cruises DY081, JC094, NBP1103, and NBP0805). Where paired shipboard data were unavailable results from nearby bottle data in the GLODAP v2 database were used65. Hydrographic stations were typically within ~ 150 km of coral sampling sites and 30 m depth of the samples. The maximum offset between corals and water data was at the Galapagos site (530 km distance and 89 m water depth) owing to the poor regional sampling coverage. To assess uncertainty on pH estimates caused by both true in-situ variability and distance between coral and hydrographic stations, a further two nearby bottle seawater measurements were selected from the combined GLODAP v2 and research cruise databases (Supplementary Information). For most sites this variability was within ± 0.04 pH units (2σ) with a maximum variation of ± 0.09 pH units. At sites where pH was more variable the most proximal bottle data is still preferred over averaging all three pH estimates.
δ11B of borate in seawater (δ11Bborate)
Both the concentration and δ11B of the borate ion (δ11Bborate) increase as a function of seawater pH46,66,67. The incorporation of this charged ion into marine calcium carbonates as they precipitate therefore forms the premise of the boron isotope pH-proxy66. Here we calculate δ11Bborate of ambient seawater experienced by each coral using the seawater pH estimates above and the simplified equation (Eq. 4) rearranged from Zeebe and Wolf-Gladrow67:
where δ11Bsw (39.61‰) is the δ11B of seawater68, αB (1.027) is the fractionation factor between boric acid and the borate ion69, and pKB* is the dissociation constant of the two boron species calculated using site specific temperature, salinity, and pressure estimates (8.83 at 3 °C, 35 psu, and 700 m respectively, typical of sites in this study). Factoring for variation in temperature and salinity on pKB*, estimates of seawater pH uncertainty outlined above correspond to uncertainties on δ11Bborate that are typically better than ± 0.15‰ (2 SD; maximum ± 0.57‰).
Estimation of internal ΩAragonite
Inorganic aragonite precipitation experiments show that B/Ca ratios are most strongly correlated with either (i) [B]/[DIC] or (ii) [B(OH)4−]/[CO32–] of the CF49. Previous biocalcification models of scleractinian corals have therefore used the theoretical relationship between coral B/Ca and [CO32–] of the CF to obtain a second carbonate system parameter using Eqs. (5) and Eq. (6)37,38,49:
where DB/Ca is the partition coefficient of boron into aragonite from seawater, BT is the total boron concentration in seawater (432.5 µmol/kg at salinity 35 psu68) and [H+]CF is calculated using coral δ11B derived pH estimates of the CF. We apply this model to aragonitic stylasterid δ11B and B/Ca data and convert [CO32−]CF to ΩAragonite assuming the [Ca2+]CF is equal to that of ambient seawater (10.3 mmol/kg). Uncertainty on [CO32−]CF is calculated by 1000 Monte Carlo iterations that incorporate analytical uncertainty on δ11B and B/Ca measurements and seawater temperature uncertainty based on proximal hydrographic data.
Coral sample preparation
Coral samples for this study were air dried following collection. The majority of organic matter was removed through both physical scraping and treatment in dilute NaClO for 12 h. Cross sectional discs (~ 2 mm thickness) were cut using a rotary cutting tool from the central trunk (or widest branch) of stylasterid samples. Microstructures within deep-sea corals are known to exhibit contrasting boron concentration47 and isotopic composition43. Similarly, the δ18O composition of growing tips of stylasterid branches has been shown to be further from seawater equilibrium than bulk samples28, hence apical tips were avoided and large samples were cut (~ 50 mg; i.e. × 10 mass required for analysis) to diminish the influence of a single microstructural component. To confirm this, corals were also sampled and analysed in duplicate to characterise variability between solid pieces cut from the same coral.
Coral fragments were finely crushed using a pestle and mortar before 5 to 10 mg of the homogenous powder was taken. Residual organic matter was removed using warm 1% H2O2 (80 °C; buffered in NH4OH) and a weak acid leach (0.0005 M HNO3) before powders were dissolved in distilled 0.5 M HNO3.
Analytical techniques
All analyses were performed at the University of Bristol. An aliquot of the dissolved sample was analysed by ICP-MS using well-characterised, matrix-matched, synthetic standard solutions to give B/Ca and U/Ca ratios. Samples and standards were introduced in 0.5 M HNO3 and a 0.5 M HNO3 and 0.3 M HF acid wash solution was utilised between samples/standards to aid B wash out31. Repeat analysis of NIST RM 8301 (Coral) yielded mean B/Ca and U/Ca ratios of 527 ± 2% and 822 ± 1% µmol/mol (RSD; n = 35) which are within analytical uncertainty of the interlaboratory consensus value for this reference material70.
An aliquot of the dissolved sample containing ~ 25 ng B was separated from the carbonate matrix using 20 μl micro-columns containing Amberlite IRA 743 boron-specific anionic exchange resin71. The δ11B of purified boron samples were measured by Multi-Collector ICP-MS against NIST SRM 95135,71. In this case, all samples, blanks, and standard solutions were introduced to the instrument in a 0.5 M HNO3 and 0.3 M HF acid matrix again to ensure optimal B wash out31. Full procedural uncertainty was assessed using repeat measurement of NIST RM 8301 (Coral) yielding an average δ11B value of 24.27 ± 0.16‰ (n = 25; 2 s) which again was within uncertainty of interlaboratory consensus values70. Total procedural blanks (n = 6) were small (less than 39 ng of boron) and were thus < 0.2% of the boron loaded onto columns for samples.
Data availability
All new boron isotope (δ11B), B/Ca, and U/Ca data from stylasterid corals in this study are included in Supplementary Information. These tables also include coral species and location information and matched proximal hydrographic data.
References
Stocker, T. F. et al. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (eds T.F. Stocker et al.) Ch. TS, 33–115 (Cambridge University Press, 2013).
Doney, S. C., Fabry, V. J., Feely, R. A. & Kleypas, J. A. Ocean acidification: The other CO2 problem. Ann. Rev. Mar. Sci. 1, 169–192 (2009).
Albright, R. et al. Carbon dioxide addition to coral reef waters suppresses net community calcification. Nature 555, 516–519 (2018).
Chen, C.-T.A. et al. Deep oceans may acidify faster than anticipated due to global warming. Nat. Clim. Chang. 7, 890–894 (2017).
Guinotte, J. M. et al. Will human-induced changes in seawater chemistry alter the distribution of deep-sea scleractinian corals?. Front. Ecol. Environ. 4, 141–146 (2006).
Figuerola, B. et al. A review and meta-analysis of potential impacts of ocean acidification on marine calcifiers from the southern ocean. Front. Mar. Sci. 8, 584445 (2021).
Ries, J. B. Skeletal mineralogy in a high-CO2 world. J. Exp. Mar. Biol. Ecol. 403, 54–64 (2011).
Blackmon, P. D. & Todd, R. Mineralogy of some foraminifera as related to their classification and ecology. J. Paleontol. 33, 1–15 (1959).
Oliver, W. A. Jr. The relationship of the scleractinian corals to the rugose corals. Paleobiology 6, 146–160 (1980).
Sinclair, D. J. et al. Reproducibility of trace element profiles in a specimen of the deep-water bamboo coral Keratoisis sp. Geochim. Cosmochim. Acta 75, 5101–5121 (2011).
Liu, Y.-W., Sutton, J. N., Ries, J. B. & Eagle, R. A. Regulation of calcification site pH is a polyphyletic but not always governing response to ocean acidification. Sci. Adv. 6, eaax1314 (2020).
Cornwall, C. E. et al. Understanding coralline algal responses to ocean acidification: Meta-analysis and synthesis. Glob. Change Biol. 28, 362–374 (2022).
Al-Horani, F. A., Al-Moghrabi, S. M. & de Beer, D. Microsensor study of photosynthesis and calcification in the scleractinian coral, Galaxea fascicularis: Active internal carbon cycle. J. Exp. Mar. Biol. Ecol. 288, 1–15 (2003).
Al-Horani, F. A., Al-Moghrabi, S. M. & de Beer, D. The mechanism of calcification and its relation to photosynthesis and respiration in the scleractinian coral Galaxea fascicularis. Mar. Biol. 142, 419–426 (2003).
Le Goff, C. et al. In vivo pH measurement at the site of calcification in an octocoral. Sci. Rep. 7, 11210 (2017).
McCulloch, M. et al. Resilience of cold-water scleractinian corals to ocean acidification: Boron isotopic systematics of pH and saturation state up-regulation. Geochim. Cosmochim. Acta 87, 21–34 (2012).
Gilbert, P. U. P. A. et al. Biomineralization: Integrating mechanism and evolutionary history. Sci. Adv. 8, eabl9653 (2022).
Donald, H. K., Ries, J. B., Stewart, J. A., Fowell, S. E. & Foster, G. L. Boron isotope sensitivity to seawater pH change in a species of Neogoniolithon coralline red alga. Geochim. Cosmochim. Acta 217, 240–253 (2017).
Krief, S. et al. Physiological and isotopic responses of scleractinian corals to ocean acidification. Geochim. Cosmochim. Acta 74, 4988–5001 (2010).
Hönisch, B. et al. Assessing scleractinian corals as recorders for paleo-pH: Empirical calibration and vital effects. Geochim. Cosmochim. Acta 68, 3675–3685 (2004).
Anagnostou, E., Williams, B., Westfield, I., Foster, G. L. & Ries, J. B. Calibration of the pH-δ11B and temperature-Mg/Li proxies in the long-lived high-latitude crustose coralline red alga Clathromorphum compactum via controlled laboratory experiments. Geochim. Cosmochim. Acta 254, 142–155 (2019).
Cornwall, C. E., Comeau, S. & McCulloch, M. T. Coralline algae elevate pH at the site of calcification under ocean acidification. Glob. Change Biol. 23, 4245–4256 (2017).
Rosenthal, Y., Lear, C. H., Oppo, D. W. & Linsley, B. K. Temperature and carbonate ion effects on Mg/Ca and Sr/Ca ratios in benthic foraminifera: Aragonitic species Hoeglundina elegans. Paleoceanography 21, PA1007 (2006).
Gori, A. et al. Physiological response of the cold-water coral Desmophyllum dianthus to thermal stress and ocean acidification. PeerJ 4, e1606 (2016).
Gagnon, A. C., Gothmann, A. M., Branson, O., Rae, J. W. B. & Stewart, J. A. Controls on boron isotopes in a cold-water coral and the cost of resilience to ocean acidification. Earth Planet. Sci. Lett. 554, 116662 (2021).
Cairns, S. D. Global diversity of the Stylasteridae (Cnidaria: Hydrozoa: Athecatae). PLoS ONE 6, e21670 (2011).
Cairns, S. D. Deep-water corals: An overview with special reference to diversity and distribution of deep-water scleractinian corals. Bull. Mar. Sci. 81, 311–322 (2007).
Samperiz, A. et al. Stylasterid corals: A new paleotemperature archive. Earth Planet. Sci. Lett. 545, 116407 (2020).
Cairns, S. D. & Macintyre, I. G. Phylogenetic implications of calcium carbonate mineralogy in the Stylasteridae (Cnidaria: Hydrozoa). Palaios, 96–107 (1992).
Anagnostou, E., Huang, K. F., You, C. F., Sikes, E. L. & Sherrell, R. M. Evaluation of boron isotope ratio as a pH proxy in the deep sea coral Desmophyllum dianthus: Evidence of physiological pH adjustment. Earth Planet. Sci. Lett. 349–350, 251–260 (2012).
Rae, J. W. B. et al. CO2 storage and release in the deep Southern Ocean on millennial to centennial timescales. Nature 562, 569–573 (2018).
Farmer, J. R., Hönisch, B., Robinson, L. F. & Hill, T. M. Effects of seawater-pH and biomineralization on the boron isotopic composition of deep-sea bamboo corals. Geochim. Cosmochim. Acta 155, 86–106 (2015).
Sutton, J. N. et al. δ11B as monitor of calcification site pH in divergent marine calcifying organisms. Biogeosciences 15, 1447–1467 (2018).
Heinemann, A. et al. Conditions of Mytilus edulis extracellular body fluids and shell composition in a pH-treatment experiment: Acid-base status, trace elements and δ11B. Geochem. Geophys. Geosyst. 13, 1–17 (2012).
Rae, J. W. B., Foster, G. L., Schmidt, D. N. & Elliott, T. Boron isotopes and B/Ca in benthic foraminifera: Proxies for the deep ocean carbonate system. Earth Planet. Sci. Lett. 302, 403–413 (2011).
Auscavitch, S. R. et al. Distribution of deep-water scleractinian and stylasterid corals across abiotic environmental gradients on three seamounts in the Anegada Passage. PeerJ 8, e9523 (2020).
DeCarlo, T. M., Holcomb, M. & McCulloch, M. T. Reviews and syntheses: Revisiting the boron systematics of aragonite and their application to coral calcification. Biogeosciences 15, 2819–2834 (2018).
McCulloch, M. T., D’Olivo, J. P., Falter, J., Holcomb, M. & Trotter, J. A. Coral calcification in a changing world and the interactive dynamics of pH and DIC upregulation. Nat. Commun. 8, 15686 (2017).
Henehan, M. J. et al. Calibration of the boron isotope proxy in the planktonic foraminifera Globigerinoides ruber for use in palaeo-CO2 reconstruction. Earth Planet. Sci. Lett. 364, 111–122 (2013).
Rink, S., Kühl, M., Bijma, J. & Spero, H. Microsensor studies of photosynthesis and respiration in the symbiotic foraminifer Orbulina universa. Mar. Biol. 131, 583–595 (1998).
Fietzke, J. & Wall, M. Distinct fine-scale variations in calcification control revealed by high-resolution 2D boron laser images in the cold-water coral Lophelia pertusa. Sci. Adv. 8, eabj4172 (2022).
Drake, J. L. et al. How corals made rocks through the ages. Glob. Change Biol. 26, 31–53 (2020).
Blamart, D. et al. Correlation of boron isotopic composition with ultrastructure in the deep-sea coral Lophelia pertusa: Implications for biomineralization and paleo-pH. Geochem. Geophys. Geosyst. 8, Q12001 (2007).
Jurikova, H. et al. Boron isotope composition of the cold-water coral Lophelia pertusa along the Norwegian margin: Zooming into a potential pH-proxy by combining bulk and high-resolution approaches. Chem. Geol. 513, 143–152 (2019).
NOAA Deep Sea Coral Research & Technology Program. NOAA National Database for Deep-Sea Corals and Sponges (version 20201021-0), https://deepseacoraldata.noaa.gov/ (2017).
Dickson, A. G. Thermodynamics of the dissociation of boric acid in synthetic seawater from 273.15 to 318.15 K. Deep Sea Res. Part A Oceanogr. Res. Pap. 37, 755–766 (1990).
Stewart, J. A., Anagnostou, E. & Foster, G. L. An improved boron isotope pH proxy calibration for the deep-sea coral Desmophyllum dianthus through sub-sampling of fibrous aragonite. Chem. Geol. 447, 148–160 (2016).
Mavromatis, V., Montouillout, V., Noireaux, J., Gaillardet, J. & Schott, J. Characterization of boron incorporation and speciation in calcite and aragonite from co-precipitation experiments under controlled pH, temperature and precipitation rate. Geochim. Cosmochim. Acta 150, 299–313 (2015).
Holcomb, M., DeCarlo, T. M., Gaetani, G. A. & McCulloch, M. Factors affecting B/Ca ratios in synthetic aragonite. Chem. Geol. 437, 67–76 (2016).
DeCarlo, T. M., Gaetani, G. A., Holcomb, M. & Cohen, A. L. Experimental determination of factors controlling U/Ca of aragonite precipitated from seawater: Implications for interpreting coral skeleton. Geochim. Cosmochim. Acta 162, 151–165 (2015).
Reeder, R. J., Nugent, M., Lamble, G. M., Tait, C. D. & Morris, D. E. Uranyl Incorporation into Calcite and Aragonite: XAFS and Luminescence Studies. Environ. Sci. Technol. 34, 638–644 (2000).
Anagnostou, E. et al. Seawater nutrient and carbonate ion concentrations recorded as P/Ca, Ba/Ca, and U/Ca in the deep-sea coral Desmophyllum dianthus. Geochim. Cosmochim. Acta 75, 2529–2543 (2011).
Chen, S., Littley, E. F. M., Rae, J. W. B., Charles, C. D. & Adkins, J. F. Uranium distribution and incorporation mechanism in deep-sea corals: Implications for seawater [CO32–] proxies. Front. Earth Sci. 9, 159 (2021).
Inoue, M., Suwa, R., Suzuki, A., Sakai, K. & Kawahata, H. Effects of seawater pH on growth and skeletal U/Ca ratios of Acropora digitifera coral polyps. Geophys. Res. Lett. 38, L12809 (2011).
Gothmann, A. M. & Gagnon, A. C. The primary controls on U/Ca and minor element proxies in a cold-water coral cultured under decoupled carbonate chemistry conditions. Geochim. Cosmochim. Acta 315, 38–60 (2021).
Mass, T. et al. Cloning and characterization of four novel coral acid-rich proteins that precipitate carbonates in vitro. Curr. Biol. 23, 1126–1131 (2013).
Rogers, A. D. Advances in Marine Biology, Vol. 79 (ed Sheppard, C.) 137–224 (Academic Press, 2018).
Rodolfo-Metalpa, R. et al. Coral and mollusc resistance to ocean acidification adversely affected by warming. Nat. Clim. Chang. 1, 308–312 (2011).
Hoarau, L. et al. Unexplored refugia with high cover of scleractinian Leptoseris spp. and hydrocorals Stylaster flabelliformis at lower mesophotic depths (75–100 m) on lava flows at Reunion Island (Southwestern Indian Ocean). Diversity 13, 141 (2021).
Lindner, A., Cairns, S. D. & Cunningham, C. W. From offshore to onshore: Multiple origins of shallow-water corals from deep-sea ancestors. PLoS ONE 3, e2429 (2008).
Stewart, J. A. et al. Refining trace metal temperature proxies in cold-water scleractinian and stylasterid corals. Earth Planet. Sci. Lett. 545, 116412 (2020).
Seacarb: Seawater Carbonate Chemistry. R package version 3.0.6 .http://CRAN.R-project.org/package=seacarb (2015).
Lueker, T. J., Dickson, A. G. & Keeling, C. D. Ocean pCO2 calculated from dissolved inorganic carbon, alkalinity, and equations for K1 and K2: Validation based on laboratory measurements of CO2 in gas and seawater at equilibrium. Mar. Chem. 70, 105–119 (2000).
Lee, K. et al. The universal ratio of boron to chlorinity for the North Pacific and North Atlantic oceans. Geochim. Cosmochim. Acta 74, 1801–1811 (2010).
Olsen, A. et al. The Global Ocean Data Analysis Project version 2 (GLODAPv2)—An internally consistent data product for the world ocean. Earth Syst. Sci. Data 8, 297–323 (2016).
Hemming, N. G. & Hanson, G. N. Boron isotopic composition and concentration in modern marine carbonates. Geochim. Cosmochim. Acta 56, 537–543 (1992).
Zeebe, R. E. & Wolf-Gladrow, D. A. CO2 in Seawater: Equilibrium, Kinetics, Isotopes Vol. 65 (Elsevier, 2001).
Foster, G. L., Pogge von Strandmann, P. A. E. & Rae, J. W. B. Boron and magnesium isotopic composition of seawater. Geochem. Geophys. Geosyst. 11, Q08015 (2010).
Klochko, K., Kaufman, A. J., Yao, W., Byrne, R. H. & Tossell, J. A. Experimental measurement of boron isotope fractionation in seawater. Earth Planet. Sci. Lett. 248, 276–285 (2006).
Stewart, J. A. et al. NIST RM 8301 boron isotopes in marine carbonate (simulated coral and foraminifera solutions): Inter-laboratory δ11B and trace element ratio value assignment. Geostand. Geoanal. Res. 45, 77–96 (2020).
Foster, G. L. Seawater pH, pCO2 and [CO32−] variations in the Caribbean Sea over the last 130 kyr: A boron isotope and B/Ca study of planktic foraminifera. Earth Planet. Sci. Lett. 271, 254–266 (2008).
Schlitzer, R. Ocean Data View, Version 4.6.5 http://odv.awi.de, (2021).
Acknowledgements
We acknowledge the crew and researchers on board the research vessels that obtained the samples for this study. Cruise AL1508 was supported by The Dalio Explore Fund. We also acknowledge the Galápagos National Park directorate for permission to map and collect submarine rock and biological samples (PC-44-15), and the Charles Darwin Foundation for facilitating scientific collaboration in the Galápagos. We thank C. Coath, and C. Taylor for their help with laboratory work and A. Gagnon for helpful discussion while preparing this manuscript. Funding was provided by an Antarctic Bursary awarded to J.A.S., ERC and NERC grants awarded to L.F.R. (NE/S001743/1; NE/R005117/1; NE/N003861/1).
Author information
Authors and Affiliations
Contributions
J.A.S. and I.S. collected the geochemical data. L.F.R. acquired the bulk of research funding and collected samples. J.A.S., I.S., J.K., and L.F.R. contributed to the interpretation of these results and the construction of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stewart, J.A., Strawson, I., Kershaw, J. et al. Stylasterid corals build aragonite skeletons in undersaturated water despite low pH at the site of calcification. Sci Rep 12, 13105 (2022). https://doi.org/10.1038/s41598-022-16787-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-16787-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.