Abstract
We hypothesized that thalamic volumes of patients with type 1 diabetes mellitus (DM) and nonpainful diabetic peripheral neuropathy (DPN) would be reduced relative to thalamic volumes of patients with type 1 DM and painful DPN. We calculated the standardized thalamic volumetric difference between these groups in a pilot sample to obtain a statistical power of 80% at a 5% significance level. Hence, we measured thalamic volumes from 15 patients with nonpainful DPN (10 women, mean age = 49 years, standard deviation [SD] = 11.5) and from 13 patients with painful DPN (8 women, mean age = 43 years, SD = 12.5) by using a manual segmentation approach. A volumetric difference of approximately 15% was found between the nonpainful (mean = 5072 mm3, SD = 528.1) and painful (mean = 5976 mm3, SD = 643.1) DPN groups (P < 0.001). Curiously, a volumetric difference between the left (mean = 5198 mm3, SD = 495.0) and the right (mean = 4946 mm3, SD = 590.6) thalamus was also found in patients with nonpainful DPN (P < 0.01), but not in patients with painful DPN (P = 0.97). Patients with nonpainful DPN have lower thalamic volumes than those with painful DPN, especially in the right thalamus.
Similar content being viewed by others
Introduction
Diabetes mellitus (DM) is still an incurable disease, for which novel therapeutic approaches are being developed to reduce the incidence of macrovascular and microvascular complications1. Diabetic peripheral neuropathy (DPN) is a common microvascular complication of DM, including a wide range of neuropathic involvement associated with significant morbidity2,3. The most common category of DPN is the sensorimotor type3, of which there are painful and nonpainful subtypes4.
Although, by definition, DPN corresponds to a condition involving the peripheral nervous system, magnetic resonance imaging (MRI) studies have demonstrated the occurrence of central nervous system (CNS) abnormalities, namely spinal cord volume loss (detected by measuring the cross-sectional area of the spinal cord)5 and gray matter volume loss in somatosensory regions of the brain6 (revealed by using voxel-based morphometry [VBM]7).
Previous studies have also found metabolic and perfusion abnormalities in the thalamus of patients with type 1 DM and DPN8,9. In particular, one of these studies showed lower relative cerebral blood volume and mean transit times in patients with nonpainful DPN than in patients with painful DPN using dynamic susceptibility contrast perfusion-weighted imaging9. With respect to thalamic volumetric abnormalities, it was recently established that patients with DPN have thalamic atrophy, irrespective of the concomitance of pain10. However, a higher degree of thalamic volume loss was found in patients with nonpainful DPN relative to patients with painful DPN, but such a difference was reported on the basis of a VBM analysis uncorrected for multiple comparisons6. It is, however, noteworthy to point out that the thalamus is a very difficult structure to segment using automatic segmentation methods based on signal intensity and contrast of T1-weighted images (T1-WI)11, as required for VBM analyses7. Therefore, we hypothesized that it could be possible to confirm a reduction in thalamic volumes of patients with type 1 DM and nonpainful DPN relative to those with painful DPN by using a manual segmentation approach12.
Materials and methods
All procedures involving human participants included in this study were in accordance with the ethical standards of the 2013 revision of the Helsinki Declaration and were approved by the local institutional ethics committee (Comissão de Ética para a Saúde do Hospital de São João). All patients provided written informed consent to be included.
Patients
The sample for this study comprised 28 patients with type 1 DM and DPN (18 women, mean age = 46 years, standard deviation [SD] = 12.2). Fifteen patients had nonpainful DPN (10 women, mean age = 49 years, SD = 11.5), and 13 patients had painful DPN (8 women, mean age = 43 years, SD = 12.5).
Patients included for this study fulfilled the American Diabetes Association criteria13. Their age was required to be > 18 and ≤ 70 years, and all of them had a duration of disease > 5 years.
To establish the probable occurrence of neuropathic features (e.g., sensation of pins and needles, ants crawling, paresthesia, numbness, cold freezing, allodynia in the lower limbs on a daily basis for at least 3 months, and decreased distal sensation or decreased/absent ankle reflexes), we used the expert panel recommendations published by Tesfaye et al.14. We used the Michigan Neuropathy Screening Instrument (MNSI)15,16 as a measure of DPN in all patients, which were required to have an MNSI score ≥ 3 to be included. To assess the concomitance of pain, we used the 11-point pain intensity numerical rating scale (PI-NRS)17 score. Patients with DPN without chronic pain symptoms (cf., with a PI-NRS score = 0) were classified as having nonpainful DPN. Patients with DPN and pain (i.e., with a PI-NRS score ≥ 1) were required to have a Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) pain scale18 score ≥ 12 or a Douleur Neuropathique 4 questionnaire (DN4)19 score ≥ 420,21 to be classified as having painful DPN.
Exclusion criteria comprised any evidence of CNS or psychiatric conditions, nondiabetic neuropathies, abuse of alcohol consumption, recurrent episodes (> 10) of severe hypoglycemia (i.e., glycemia < 60 mg/dl) during the year prior to baseline assessment, comorbidities precluding MRI scanning, MRI evidence of deep subcortical (lacunar) infarcts and focal thalamic lesions on T2-weighted images (T2-WI), as well as inability to provide written informed consent.
Magnetic resonance imaging protocol
MRI data were acquired using a scanner operating at 3 Tesla (Magnetom Trio, A Tim System, Siemens, Erlangen, Germany) and equipped with a 12-channel radiofrequency head coil. Axial two-dimensional (2D) inversion recovery (IR) turbo spin echo (TSE) T1-WI were acquired (echo time [TE] = 14 ms, repetition time [TR] = 4300 ms, flip angle = 60°, inversion time [TI] = 400 ms, field of view [FOV] = 240 mm, slice thickness = 2 mm, no interslice gap, number of slices = 44, acquisition matrix = 256 × 256, voxel resolution = 0.9 × 0.9 × 2.0 mm, scanning time = 7:29 min) to segment the thalamus. The choice of parameters (e.g., TI) was based on the optimization of a similar MRI sequence previously used to acquire input images to segment deep gray matter structures11.
Single-slab three-dimensional (3D) magnetization-prepared rapid gradient echo (MPRAGE) T1-WI (TE = 3 ms, TR = 2300 ms, flip angle = 9°, TI = 900 ms, FOV = 240 mm, slice thickness = 1.2 mm, number of slices = 160, acquisition matrix = 256 × 256, voxel resolution = 1 × 1 × 1.2 mm, scanning time = 9:14 min) were also acquired to assess measures of brain atrophy. Parameters used to acquire 3D MPRAGE T1-WI were specifically recommended for our scanner, according to the Alzheimer’s Disease Neuroimaging Initiative multicenter study22.
Image analysis
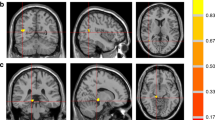
We applied a validated manual segmentation protocol12 using the MRIcron software (https://www.nitrc.org/projects/mricron) on bias-corrected 2D IR TSE T1-WI. Although the implementation of a manual segmentation procedure on axial T1-WI implicated a very slight adaptation of the original protocol, it is noteworthy to point out that the reliability analysis of such a protocol has shown that the corresponding intra-class correlation coefficient ranged from 0.95 to 0.98, and the inter-class correlation coefficient ranged from 0.92 to 0.9812. Figure 1 shows examples of the thalamic segmentation procedure implemented in the current study. To obtain accurate thalamic volumes, all images including the thalamus were cautiously selected. Care was taken to exclude the metathalamus, epithalamus, subthalamus, hypothalamus, and the choroidal plexuses from measurements. The segmentation procedure was carried out by two observers (A.J. B.-L. and J.J.R.), the more experienced of which (with approximately 2 decades of experience in diagnostic neuroradiology) supervising the other. We also used visual rating scales to assess medial temporal lobe atrophy (MTA)23 and global cortical atrophy (GCA)24 scores on 3D MPRAGE T1-WI in consensus by two observers (S.B. and A.J. B.-L.). At the time of each assessment, all observers were blinded to the clinical characteristics of the included patients.
Examples of thalamic segmentation on bias corrected inversion recovery (IR) turbo spin echo (TSE) T1-weighted images (T1-WI) from one patient with nonpainful diabetic peripheral neuropathy (DPN) in the top row and one patient with painful DPN in the middle row. In the bottom row, an example of a similar segmentation procedure on a 46-year-old healthy subject is shown. This example is presented for visualization purposes only, and was randomly selected from a sample of a previous study using similar IR TSE T1-WI to segment deep gray matter structures11. Please note the apparent reduction of thalamic volume (especially the thinner cross-sectional thalamic distance) in the patient with nonpainful DPN, especially in the right thalamus, relative to the other examples.
Statistical analysis
Statistical analysis was carried out using IBM SPSS 26.0 (https://www.ibm.com/analytics/spss-statistics-software). Given that the continuous variables analyzed had an approximately normal distribution, we used the Student’s t-test to compare their means, namely the independent-samples Student’s t-test to compare thalamic volumes at the between-subject level (i.e., between groups), as well as the paired-samples Student’s t-test to compare volumes at the within-subject level (i.e., left–right differences). We used the Mann–Whitney U test to compare median scores between groups, the Pearson’s correlation coefficient (r) to test correlations between thalamic volumes and age, and the Spearman’s rank correlation coefficient (rs) to test correlations between thalamic volumes, brain atrophy scores, and neuropathy or pain severity scores. Statistical significance was considered when P values were < 0.05.
Statistical power estimates were previously carried out on the basis of the Altman’s nomogram25 to obtain a statistical power of at least 80% at a 5% significance level and to determine the appropriate total sample size. This was accomplished after calculating the standardized thalamic volumetric difference between the nonpainful and painful DPN groups (i.e., dividing the thalamic volumetric difference between these groups by the SD of the corresponding variable) in a pilot sample including 13 patients with DPN.
Ethics approval
As stated on the Materials and methods section of this manuscript, “all procedures involving human participants included in this study were in accordance with the ethical standards of the 2013 revision of the Helsinki Declaration and were approved by the local institutional ethics committee (Comissão de Ética para a Saúde do Hospital de São João)”.
Consent to participate
All subjects provided written informed consent to be included.
Consent for publication
There is consent for publication.
Results
Table 1 summarizes descriptive results for the most relevant variables in patients with nonpainful and painful DPN. No statistically significant difference was found between groups with respect to age (P = 0.19), education (P = 0.52), duration of disease (P = 0.64), glycated hemoglobin (P = 0.77), or body mass index (P = 0.99).
Patients with type 1 DM and DPN had a median and a mean MNSI score of 10 (SD = 3.2). Patients with nonpainful DPN had a median MNSI score of 8 and a mean of 9 (SD = 3.3), whereas patients with painful DPN had both a median and a mean MNSI score of 11 (SD = 2.5). The Mann–Whitney U test revealed a statistically significant difference of median MNSI scores between groups (P < 0.05). Patients with painful DPN had a median PI-NRS score of 6, a median LANSS score of 15, and a median DN4 score of 6. The corresponding means were 5 (SD = 2.5), 15 (SD = 5.1) and 6 (SD = 2.2), respectively.
The average left–right thalamic volume in patients with type 1 DM and DPN was 5492 mm3 (SD = 734.3). The corresponding volume in patients with nonpainful DPN was 5072 mm3 (SD = 528.1) and 5976 mm3 (SD = 643.1) in patients with painful DPN. Hence, the nonpainful DPN group had a thalamic volumetric reduction of approximately 15% (i.e., 904 mm3) relative to the painful DPN group (P < 0.001).
Curiously, a significant volumetric difference between the left (mean = 5198 mm3, SD = 495.0) and the right thalamus (mean = 4946 mm3, SD = 590.6) was found in patients with nonpainful DPN (P < 0.01), which contributed to an almost significant left–right volumetric difference in the entire sample of patients with type 1 DM and DPN (P = 0.08). However, no significant difference between the left and the right thalamus was found in patients with painful DPN (P = 0.97).
No statistically significant gender-related difference was found with respect to the average left–right thalamic volume in patients with type 1 DM and DPN (P = 0.79), either in patients with nonpainful DPN (P = 0.37) or in patients with painful DPN (P = 0.35).
Significant correlations were found between the left thalamic volume and age (r = − 0.55; P < 0.01), the right thalamic volume and age (r = − 0.43; P < 0.05), as well as between the average left–right thalamic volume and age (r = − 0.50; P < 0.01) of patients with DPN.
No statistically significant correlations were found between thalamic volumes of patients with DPN and MNSI scores, nor between thalamic volumes of patients with painful DPN and PI-NRS, LANSS or DN4 scores. Finally, no statistically significant differences were found between patients with nonpainful or painful DPN with respect to MTA (P = 0.89) or GCA (P = 0.41), nor significant correlations were found between thalamic volumes and MTA or GCA.
Discussion
As predicted, our results showed a very significant thalamic volumetric reduction (of approximately 15%) in patients with type 1 DM and nonpainful DPN relative to those with painful DPN. In other words, patients with nonpainful DPN have increased thalamic volume loss relative to patients with painful DPN, as previously anticipated6. Curiously, we additionally found a reduction of approximately 5% in the right thalamic volume relative to the left thalamic volume in patients with nonpainful DPN.
As expected, this study confirmed the occurrence of a strong and significant correlation between lower thalamic volumes and increasing age, which corroborates the results of previous studies indicating thalamic volume loss in usual ageing, irrespective of gender26,27. Given that no significant difference was found between the mean age of patients with nonpainful DPN and those with painful DPN (i.e., both groups were reasonably matched for age), the possible effect of aging as a confounder seems to be negligible in the current study.
Our study showed that the average left–right thalamic volume was 5492 mm3 in patients with type 1 DM and DPN, 5072 mm3 in patients with nonpainful DPN, and 5976 mm3 in patients with painful DPN. Taking as a reference the results of a previous study reporting thalamic volumes in healthy subjects by using a similar segmentation method12, our data suggest that thalamic volumes in patients with type 1 DM and nonpainful DPN are substantially lower than those of healthy subjects (Fig. 1). This is consistent with results of a recently published study10. Nevertheless, the corresponding proportion of volume reduction should be confirmed in future studies including healthy control subjects. It is, however, noteworthy to point out that thalamic volumes calculated on the basis of automatic segmentation methods or stereology have always indicated values > 7300 mm3 in healthy young adults28,29.
The mechanism by means of which the thalamus can be affected in patients with DPN is unknown. Most likely, it can be secondary to loss of sensory input resulting from peripheral nerve damage because strategic parts of the thalamus are key structures in processing sensory stimuli. Alternatively, it has been postulated that a primary involvement of the CNS—leading to neuronal death or dysfunction—might also occur in the so-called diabetic ‘peripheral’ neuropathy. This finding speaks to the aforementioned assumption that thalamic volumes can indeed be reduced in the advanced stage of DPN and is entirely consistent with the reduction of N-acetyl aspartate—a marker of neuro-axonal death or dysfunction—found in previous proton magnetic resonance spectroscopy studies8,10,30.
According to the neuroanatomical views presented in recent editions of Gray’s Anatomy, “the ventral posterior (VP) nucleus is the principal thalamic relay for the somatosensory pathways. It is thought to consist of two major divisions, the ventral posterolateral (VPl) and the ventral posteromedial (VPm) nuclei. The VPl nucleus receives the medial lemniscal and spinothalamic pathways, and the VPm nucleus receives the trigeminothalamic pathway”. The combination of the aforementioned nuclei is often referred to as the “ventrobasal complex”, which is (along with several other thalamic nuclei) the most relevant target for nociceptive afferents. However, given that small thalamoperforating arteries virtually perfuse all thalamic nuclei, it is plausible that the underlying microvascular complication of DM is widespread and, therefore, not just restricted to the abovementioned nociceptive thalamic nuclei. Nonetheless, in the setting of nonpainful DPN, a selective vulnerability to microvascular pathology of such nuclei could well be demonstrated using a thalamic parcellation method based on diffusion tensor imaging31.
The curious finding of an additional degree of volume loss in the right thalamus of patients with nonpainful DPN is consistent with findings of a VBM meta-analysis generally revealing significantly lower right thalamic gray matter density in type 1 DM32, as well as with findings indicating that reduced gray matter density in the right thalamus is strongly associated with pain characteristics33. Likewise, further right brain hemispheric structures, such as the right insula, the right ventromedial prefrontal cortex, the right nucleus accumbens, and the right amygdala, seem to be involved in the so-called “chronic complex regional pain syndrome34”. Accordingly, there is compelling evidence that somatosensory information is preferably processed in the right hemisphere of the brain, especially in some parts of the thalamus35,36. In other words, the possible loss of nociceptive neurons in the right thalamus might explain why we found predominant right thalamic atrophy in the nonpainful DPN group.
It is currently uncertain whether thalamic volume loss can be considered a marker of DPN severity. Although it is conceivable to assume that patients with any sort of thalamic dysfunction are at a more advanced stage of DPN, “no biomarker or clear consensus on the clinical definition of either painful or nonpainful diabetic neuropathy exists4”. As a matter of fact, we have found both statistically significant lower thalamic volumes and median MNSI scores in patients with type 1 DM and nonpainful DPN relative to patients with painful DPN. Given that the MNSI is considered to be a valid measure of DPN in type 1 DM, the assumption that reduced thalamic volumes can be regarded as a possible marker of DPN severity is at odds with the difference found for the median MNSI score between groups. Further studies are, therefore, warranted to elucidate this apparent jigsaw.
Patients with DM are also at increased risk of developing brain atrophy and cognitive impairment37. Nevertheless, there was no evidence of substantial GCA or MTA in our sample38.
The fact that we have used a manual segmentation protocol for the thalamus on IR TSE T1-WI probably represents an advantage of this study, given that IR T1-weighted sequences provide images with higher contrast to represent subcortical structures39 than other types of T1-WI, such as 3D MPRAGE or spoiled gradient recalled (SPGR) T1-WI. In fact, 3D MPRAGE and SPGR T1-WI are considered to be modalities of choice when detailed anatomical or multiplanar reconstruction is required. Most of the current automatic volumetric methodological approaches rely on their usage to generate input images for brain segmentation7,40. Nonetheless, their potential drawback for segmenting the thalamus is a lack of contrast secondary to the higher myelin content in its lateral part—compared to the cortical gray matter and basal ganglia—which diminishes the ability to differentiate the thalamus from the adjacent posterior limb of the internal capsule11.
Apart from not including healthy control subjects nor patients without DPN, an additional limitation of the current study is the limited sample size. However, a previous estimation of statistical power was made, which makes it suitable to demonstrate the main difference of interest. Moreover, the exclusion of patients with DPN with lacunar infarcts and focal thalamic lesions on T2-WI—to avoid the concomitance of ischemic small vessel disease as a confounder in terms of image contrast—further contributed to reducing the number of available eligible patients.
Another limitation of this study was its cross-sectional design. In the future, longitudinal studies might demonstrate to what extent thalamic volume loss is a marker of DPN progression and severity. Further multimodal neuroimaging studies, including metabolic and perfusion-weighted MRI sequences (e.g., arterial spin labeling), are also warranted.
In conclusion, our study showed that patients with type 1 DM and nonpainful DPN have lower thalamic volumes than patients with painful DPN, especially on the right side.
Data availability
Imaging data are stored in a local repository but can be made available upon request using the e-mail address of the author for correspondence.
Code availability
The software applied is open source.
References
Katsarou, A. et al. Type 1 diabetes mellitus. Nat. Rev. Dis. Primers 3, 17016. https://doi.org/10.1038/nrdp.2017.16 (2017).
Tesfaye, S. et al. Vascular risk factors and diabetic neuropathy. N. Engl. J. Med. 352, 341–350. https://doi.org/10.1056/NEJMoa032782 (2005).
Izenberg, A., Perkins, B. A. & Bril, V. Diabetic neuropathies. Semin. Neurol. 35, 424–430. https://doi.org/10.1055/s-0035-1558972 (2015).
Gylfadottir, S. S. et al. Painful and non-painful diabetic polyneuropathy: Clinical characteristics and diagnostic issues. J. Diabetes Investig. 10, 1148–1157. https://doi.org/10.1111/jdi.13105 (2019).
Eaton, S. E. et al. Spinal-cord involvement in diabetic peripheral neuropathy. Lancet 358, 35–36. https://doi.org/10.1016/S0140-6736(00)05268-5 (2001).
Selvarajah, D. et al. Magnetic resonance neuroimaging study of brain structural differences in diabetic peripheral neuropathy. Diabetes Care 37, 1681–1688. https://doi.org/10.2337/dc13-2610 (2014).
Ashburner, J. & Friston, K. J. Voxel-based morphometry—the methods. Neuroimage 11, 805–821. https://doi.org/10.1006/nimg.2000.0582 (2000).
Selvarajah, D. et al. Thalamic neuronal dysfunction and chronic sensorimotor distal symmetrical polyneuropathy in patients with type 1 diabetes mellitus. Diabetologia 51, 2088–2092. https://doi.org/10.1007/s00125-008-1139-0 (2008).
Selvarajah, D., Wilkinson, I. D., Gandhi, R., Griffiths, P. D. & Tesfaye, S. Microvascular perfusion abnormalities of the thalamus in painful but not painless diabetic polyneuropathy: A clue to the pathogenesis of pain in type 1 diabetes. Diabetes Care 34, 718–720. https://doi.org/10.2337/dc10-1550 (2011).
Hansen, T. M. et al. Reduced thalamic volume and metabolites in type 1 diabetes with polyneuropathy. Exp. Clin. Endocrinol. Diabetes https://doi.org/10.1055/a-1347-2579 (2021).
Brandão, S. & Bastos-Leite, A. J. Magnetisation-prepared rapid gradient-echo versus inversion recovery turbo spin-echo T1-weighted images for segmentation of deep grey matter structures at 3 T. Clin. Radiol. 71, 1304–1308. https://doi.org/10.1016/j.crad.2016.09.009 (2016).
Power, B. D. et al. Validation of a protocol for manual segmentation of the thalamus on magnetic resonance imaging scans. Psychiatry Res. Neuroimaging 232, 98–105. https://doi.org/10.1016/j.pscychresns.2015.02.001 (2015).
American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care 39, S13-22. https://doi.org/10.2337/dc16-S005 (2016).
Tesfaye, S. et al. Diabetic neuropathies: Update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 33, 2285–2293. https://doi.org/10.2337/dc10-1303 (2010).
Herman, W. H. et al. Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in Type 1 diabetes: Results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet. Med. 29, 937–944. https://doi.org/10.1111/j.1464-5491.2012.03644.x (2012).
Feldman, E. L. et al. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 17, 1281–1289 (1994).
Farrar, J. T., Young, J. P. Jr., LaMoreaux, L., Werth, J. L. & Poole, R. M. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 94, 149–158 (2001).
Bennett, M. The LANSS Pain Scale: The Leeds assessment of neuropathic symptoms and signs. Pain 92, 147–157 (2001).
Bouhassira, D. et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain 114, 29–36. https://doi.org/10.1016/j.pain.2004.12.010 (2005).
Spallone, V. et al. Validation of DN4 as a screening tool for neuropathic pain in painful diabetic polyneuropathy. Diabet. Med. 29, 578–585. https://doi.org/10.1111/j.1464-5491.2011.03500.x (2012).
Themistocleous, A. C. et al. The pain in neuropathy study (PiNS): A cross-sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy. Pain 157, 1132–1145. https://doi.org/10.1097/j.pain.0000000000000491 (2016).
Mueller, S. G. et al. The Alzheimer’s disease neuroimaging initiative. Neuroimaging Clin. N. Am. 15, 869–877. https://doi.org/10.1016/j.nic.2005.09.008 (2005).
Scheltens, P. et al. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: Diagnostic value and neuropsychological correlates. J. Neurol. Neurosurg. Psychiatry 55, 967–972 (1992).
Pasquier, F. et al. Inter- and intraobserver reproducibility of cerebral atrophy assessment on MRI scans with hemispheric infarcts. Eur. Neurol. 36, 268–272. https://doi.org/10.1159/000117270 (1996).
Altman, D. G. Practical Statistics for Medical Research (Chapman & Hall, 1991).
Van Der Werf, Y. D. et al. Thalamic volume predicts performance on tests of cognitive speed and decreases in healthy aging. A magnetic resonance imaging-based volumetric analysis. Cogn. Brain Res. 11, 377–385 (2001).
Sullivan, E. V., Rosenbloom, M., Serventi, K. L. & Pfefferbaum, A. Effects of age and sex on volumes of the thalamus, pons, and cortex. Neurobiol. Aging 25, 185–192 (2004).
Keller, S. S. et al. Volume estimation of the thalamus using freesurfer and stereology: Consistency between methods. Neuroinformatics 10, 341–350. https://doi.org/10.1007/s12021-012-9147-0 (2012).
Velasco-Annis, C., Akhondi-Asl, A., Stamm, A. & Warfield, S. K. Reproducibility of brain MRI segmentation algorithms: Empirical comparison of Local MAP PSTAPLE, FreeSurfer, and FSL-FIRST. J. Neuroimaging 28, 162–172. https://doi.org/10.1111/jon.12483 (2018).
Wilkinson, I. D. et al. Magnetic resonance imaging of the central nervous system in diabetic neuropathy. Curr. Diab. Rep. 13, 509–516. https://doi.org/10.1007/s11892-013-0394-8 (2013).
Fan, X., Thompson, M., Bogovic, J. A., Bazin, P. L. & Prince, J. L. A novel contrast for DTI visualization for thalamus delineation. Proc. SPIE Int. Soc. Opt. Eng. 7625, 25. https://doi.org/10.1117/12.844473 (2010).
Moulton, C. D., Costafreda, S. G., Horton, P., Ismail, K. & Fu, C. H. Meta-analyses of structural regional cerebral effects in type 1 and type 2 diabetes. Brain Imaging Behav. 9, 651–662. https://doi.org/10.1007/s11682-014-9348-2 (2015).
Apkarian, A. V. et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J. Neurosci. 24, 10410–10415. https://doi.org/10.1523/JNEUROSCI.2541-04.2004 (2004).
Geha, P. Y. et al. The brain in chronic CRPS pain: Abnormal gray-white matter interactions in emotional and autonomic regions. Neuron 60, 570–581. https://doi.org/10.1016/j.neuron.2008.08.022 (2008).
Coghill, R. C., Gilron, I. & Iadarola, M. J. Hemispheric lateralization of somatosensory processing. J. Neurophysiol. 85, 2602–2612. https://doi.org/10.1152/jn.2001.85.6.2602 (2001).
Ji, G. & Neugebauer, V. Hemispheric lateralization of pain processing by amygdala neurons. J. Neurophysiol. 102, 2253–2264. https://doi.org/10.1152/jn.00166.2009 (2009).
Wessels, A. M. et al. Cognitive performance in type 1 diabetes patients is associated with cerebral white matter volume. Diabetologia 50, 1763–1769. https://doi.org/10.1007/s00125-007-0714-0 (2007).
Ferreira, D. et al. Practical cut-offs for visual rating scales of medial temporal, frontal and posterior atrophy in Alzheimer’s disease and mild cognitive impairment. J. Intern Med. 278, 277–290. https://doi.org/10.1111/joim.12358 (2015).
Reich, C. A., Hudgins, P. A., Sheppard, S. K., Starr, P. A. & Bakay, R. A. A high-resolution fast spin-echo inversion-recovery sequence for preoperative localization of the internal globus pallidus. Am. J. Neuroradiol. 21, 928–931 (2000).
Cardoso, M. J. et al. LoAd: A locally adaptive cortical segmentation algorithm. Neuroimage 56, 1386–1397. https://doi.org/10.1016/j.neuroimage.2011.02.013 (2011).
Acknowledgements
The authors are indebted to Sérgio Ferreira for assistance with the layout of the illustration.
Author information
Authors and Affiliations
Contributions
Guarantor of integrity of the entire study: A.J.B.L. Study concepts and design: J.L.N. and A.J.B.L. Literature research: J.L.N., J.J.R., L.M.F., D.C., M.B., S.B., A.J.B.L. Clinical studies: J.L.N., J.J.R., L.M.F., D.C., M.B., S.B., A.J.B.L. Data analysis: J.L.N., J.J.R., L.M.F., S.B., A.J.B.L. Manuscript preparation: A.J.B.-L. Manuscript editing: J.L.N., S.B. and A.J.B.L.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Novo, J.L., Ruas, J.J., Ferreira, L.M. et al. Thalamic volumetric abnormalities in type 1 diabetes mellitus and ‘peripheral’ neuropathy. Sci Rep 12, 13053 (2022). https://doi.org/10.1038/s41598-022-16699-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-16699-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.