Abstract
There is lacking research on risk factors and prediction models associated with Post-hemorrhagic hydrocephalus (PHH). Thus, this present study aimed to analyze the risk factors of PHH and establish a risk-scoring system through a large-scale study. A retrospective study of 382 patients with intracranial hemorrhage assessed age, history and diagnosis, Glasgow coma score (GCS), and fever time. After univariate and logistic regression analysis, a risk scoring system was established according to independent risk factors and evaluated using the area under the curve (AUC). Of the 382 patients, 133 (34.8%) had PHH, 43 (11.3%) received surgical treatment. Factor classification showed that age > 60 years old [odds ratio (OR): 0.347, II = 5 points], GCS < 5 (OR: 0.09, IV = 10 points), GCS 6‒8 (OR = 0.232, III = 6 points), fever time > 9 (OR: 0.202, III = 7 points), fever time 5–9 (OR: 0.341, II = 5 points), CSF-TP x time > 14,4000 group (OR: 0.267, IV = 6 points), and CSF-TP x time 9,601‒14,400 group (OR: 0.502, III = 3 points) were independent risk factors. The result of the receiver operating characteristic (ROC) prediction showed that AUC = 0.790 (0.744‒0.836). Low-risk (IV-VII), moderate (VIII-X), and high-risk group (XI-XIII) incidence of PHH were 11.76%, 50.55%, and 70.00% (p < 0.001), respectively. The coincidence rates in the validation cohort were 26.00%, 74.07%, and 100.0% (p < 0.001), respectively. AUC value was 0.860 (0.780‒0.941). The predictive model was conducive to determining the occurrence of PHH and facilitating early intervention.
Similar content being viewed by others
Introduction
Post-hemorrhagic hydrocephalus (PHH) is a common complication of adult intraventricular hemorrhage (IVH), intracerebral hemorrhage (ICH), subarachnoid hemorrhage (SAH), and traumatic brain injury (TBI). Systemic intraventricular hematocele may lead to cerebrospinal fluid circulation disorders, including obstruction and absorption dysfunction1,2, thereby inducing ventricular dilatation, which directly affects brain metabolism and function. Moreover, PHH is always associated with poor prognosis when patients are refractory to prompt treatment. The typical symptoms include neurological dysfunction, gait abnormalities, and urinary and fecal incontinence. A proportion of patients require surgery for the improvement of their symptoms3, otherwise, their conditions may worsen and eventually lead to death2. Currently, literature reports on hydrocephalus center on the risk factors or prediction models of a disease, whereas the pathogeneses of these diseases have common characteristics, which are gradually being recognized by the public. PHH is considered an important factor in the poor prognosis of ICH4, IVH5, SAH6, and TBI7. However, predictive models for PHH are lacking. The present, large-scale study retrospectively analyzed clinical data and risk factors and established a predictive scoring system to provide support for early clinical judgment and treatment of PHH.
Results
Patient characteristics in the derivation cohort
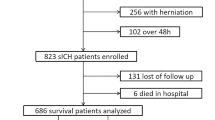
A total of 382 patients were included in the study based on the inclusion and exclusion criteria. Among them, 133 patients developed PHH (including 42 cases of ventricular-peritoneal (VP) shunt surgery, two cases of cyst drainage, and one case of death). The main reason why some patients did not receive hydrocephalus shunt is because their families did not give their consent to performing the operation after comprehensive consideration. There were no statistical differences between PHH and not post-hemorrhagic hydrocephalus (nPHH) in terms of sex and diabetes. Cerebrospinal fluid total protein (CSF-TP) changed over time, and was statistically significant in both PHH and nPHH. CSF-TP x time was not only a relevant factor selected in our study, but also a key factor in PHH; the present study therefore included it in the research sequence8. Univariate analysis showed that the occurrence of PHH was significantly correlated with patient’s ages, GCS on admission, Disease types, days (fever duration), hypertension, treatment methods, and CSF-TP (Table 1).
After multivariate logistic regression analysis, factors with poor predictive abilities were excluded. Ultimately, age, GCS, fever time, and CSF-TP x time were determined as important predictive factors (Table 2).
The prediction ability of derivation cohort was evaluated using ROC, and the results showed that AUC = 0.790, p < 0.001 (Fig. 1).
ROC curves for the scoring system in validation cohort .AUC = 0.790 (0.744–0.836), sensitivity = 0.767, specificity = 0.699. p < 0.001. Hosmer Lemeshow Test p = 0.964. ROC curves for the scoring system validation cohort. AUC = 0.860 (0.780–0.941), sensitivity = 0.868, specificity = 0.773, p < 0.001. Hosmer Lemeshow Test p = 0.244. ROC receiver operating characteristic, AUC area under the curve.
Construction of the predictive scoring system
These factors were further classified according to Roman numerals. Age > 60 years, GCS < 5, GCS 6‒8, fever time > 9, fever time 5‒9, CSF-TP x time > 14,400 and 9601‒14,400 were identified as independent risk factors (Table 3).
After the logistic regression analysis, the distribution of regression coefficients was used to assign scores to each factor. A scoring system and the corresponding PHH probability were established according to the factor grade (Table 4).
Validation of the risk scoring system
In the following year, we validated the above scoring system. The validation cohort data included 82 patients with TBI, 38 of whom (46.34%) developed PHH. Using our risk stratification system, the PHH rates from the low-, medium-, and high-risk groups were 26.00%, 74.07%, and 100.00%, respectively. The observed incidence of PHH significantly increased as the risk score in the derivation and validation cohorts increased (p < 0.001, Supplementary material). To further evaluate the accuracy of PHH prediction, we plotted the ROC curve and calculated the AUC (Fig. 1). In the validation cohort, the AUC value of the model was 0.860 [95% confidence interval (CI), 0.780‒0.941], the sensitivity was 0.868, and the specificity was 0.773 (p < 0.001). The HL Test (p = 0.244) indicated that the risk scoring system was well differentiated and calibrated from the validation data.
Discussion
PHH was first introduced in 1967 by Murtagh and Lehman, originally referring to the progressive expansion of neonatal IVH complicated by the ventricular system. Later, the term was used to describe hydrocephalus after SAH, TBI, and IVH2,9. Reportedly, the mortality rate and poor prognosis are higher in patients with PHH than in those without PHH10,11. Based on the time of onset, hydrocephalus is categorized as acute (< 3 days), subacute (3‒14 days), and chronic (> 14 days). PHH mainly refers to chronic hydrocephalus, and the time of onset varies between two weeks to one month12. Studies have pointed out that cerebral hemorrhage is closely related to hydrocephalus13 and hydrocephalus may occur in about 9% of patients with cerebral hemorrhage. The results of long-term follow-up studies on cerebral hemorrhage have indicated that the mortality rate of chronic hydrocephalus is similar to the overall mortality rate of cerebral hemorrhage14, in which 51‒89% of IVH may develop into hydrocephalus1,2 and 50% of IVH secondary to cerebral hemorrhage could develop into hydrocephalus15. The incidence of hydrocephalus occurring after subarachnoid hemorrhage varies between 15 and 37%, of which approximately 17‒21% require permanent cerebrospinal fluid (CSF) shunt operations16. The incidence of hydrocephalus after TBI ranges from 10.84% to 29%17,18. In the clinic, we often encounter such phenomena: ICH accompanied by hematoma breaking into the ventricle; IVH extending into subarachnoid space and; SAH may accumulate in the ventricle or be accompanied by a hematoma. Based on the above reasons, the analysis of PHH including the above diseases is more meaningful than the analysis of single disease complicated with hydrocephalus from the macro perspective. CSF shunt devices are permanent and therefore prone to complications, including blockage of drainage tubes and intracranial infection. Despite the commonalities between PHH and the above-mentioned diseases, there is still a lack of related literature on risk factors and prediction models of PHH, although, few studies have focused on hydrocephalus in patients under coma. To reflect the clinical characteristics of PHH more accurately, data from coma patients were collected in our study.
Pathogenesis of PHH
The mass effect of blood clots and adhesion and obstruction mediated by intraventricular inflammation are the leading causes of hydrocephalus18,19,20. The animal model of PHH indicates that the main inducing factors of hydrocephalus are erythrocyte, hemoglobin, serum iron, and thrombin2, similar to the prior clinical observation results21. Complement 3 promotes microglia and phagocytes after red blood cell lysis, which is also a pathogenesis of PHH22. Numerous studies have revealed that the injury of ependymal cilia may lead to CSF disorder and hydrocephalus23,24 through damage models; intracerebral hemoglobin can induce the upregulation of the expression of lipocalin 2 (Lcn2), a protein related to iron treatment. Animal experiments have illustrated that the inhibition of Lcn2 is associated with a reduction in ventricular dilatation25. Transforming factor β1 (TGF-β1) is mainly released by platelets after hemorrhage, which can promote the synthesis of extracellular matrix proteins, further leading to subarachnoid fibrosis and eventually resulting in hydrocephalus by disrupting the flow of CSF6,26,27. Proteomic analysis of CSF showed that the expression levels of fibrinogen, carbonic anhydrase –I (CA-I), peroxidase-2(Prx-2), hemoglobin α and β chains, transferrin (TF), and N-terminal haptoglobin (HP) were significantly different from those of the control group28. In addition, interleukin (IL)-10, IL-6, IL-8, matrix metalloproteinase (MMP)-7, and MMP-9 levels were significantly elevated in the CSF protein analysis of PHH29,30. A new hypothesis on the cause of hydrocephalus suggests that part of the absorption and secretion of CSF occurs on the intraventricular membrane, and in some events, hydrocephalus is caused by changes in the osmotic pressure of the CSF31,32,33. In vivo studies in animals have shown that hydrocephalus is caused by an increase in the volume of CSF at high osmotic pressure and that this CSF is derived from interstitial fluid adjacent to blood vessels34. Krishnamurth et al. injected hypertonic dextran and fibroblast growth factor (FGF) into the ventricles of rats to simulate the increased protein content and osmotic pressure induced by blood–brain barrier rupture after IVH. These solutions increased the osmotic load and water inflow into the ventricle to normalize the osmotic gradient and successfully induced secondary hydrocephalus in a rat model25. Attention has been paid to the change in osmotic pressure caused by a large amount of protein accumulation in the CSF, which disrupts the osmotic balance of CSF circulation causing hydrocephalus35. Several studies have shown that early CSF drainage plus EVD can reduce the content of erythrocyte degradation products and CSF-TP, thereby reducing the incidence of CSF obstruction and hydrocephalus36,37,38. Given that the correlation between CSF-TP and hydrocephalus has been confirmed39, the present study included CSF-TP as the research object.
Risk factors of PHH
Diringer et al. established a hydrocephalus score for the quantitative assessment of the hydrocephalus degree15. The significant mass effect caused by blood or hematoma in the ventricular system is the major cause of hydrocephalus, and the aggravation of hydrocephalus has been demonstrated to affect patient mortality directly43. The systolic blood pressure of patients with hydrocephalus on admission was significantly higher than that of patients without hydrocephalus, which was consistent with the results of the univariate analysis in this study. Multivariate analysis suggests that the degree of influence is limited; however, it is worth noting that controlling blood pressure reduces the increase of hematoma and the incidence of hydrocephalus40. The present study included cases of cerebral hemorrhage and SAH to explore the risk factors for PHH. The results revealed that hydrocephalus had no correlation with sex, age, bleeding location and type, and previous medical history. The risk factors included bleeding volume, intraventricular hemorrhage, and ventricular drainage. The incidence of hydrocephalus was approximately 50%, and the higher the IVH grade, the greater the risk41. A continuous study of 1,342 patients with cerebral hemorrhage observed that 26 had chronic hydrocephalus as a complication, and there was no statistical difference in general factors. The independent risk factors were ventricular dilatation, craniotomy, decompressive craniectomy (DC), and intracranial infection. In addition, the prognosis of patients with hydrocephalus is significantly worse than that of patients without hydrocephalus10. SAH retrospective analysis revealed that 36.3% of patients underwent shunt surgery after hydrocephalus, among which statistically significant factors included age, hypertension, Hunt-Hess grade, EVD operation, and intracranial infection42,43. Hunt-Hess grade and leukocytosis were independent risk factors for hydrocephalus. A retrospective analysis of 125 TBI cases revealed hydrocephalus in 116 patients. Statistically significant factors were related to poor prognoses, such as GCS < 8, SAH, subdural effusion, intracranial infection, annular cistern hour, coma time (> 2 months), and high fibrinogen level. Independent high-risk factors included the disappearance of the annular cistern, prolonged coma duration (> 2 months), increased plasma fibrinogen levels, and ventriculoperitoneal shunt implantation44. Alternatively, it was concluded that age, severe disability and low level of consciousness were independent risk factors for post-traumatic hydrocephalus (PTH)7,17. A national study observed that the risk of hydrocephalus in patients with TBI combined with SAH was significantly higher than that in the group without SAH, and the peak period was within 3 months after the onset of disease45. Due to the destruction of the anatomical and physiological integrity of the cranial cavity, the change in CSF dynamics after DC is usually considered the main cause of PTH in DC patients, and the size of craniotomy is related to hydrocephalus18,46,47. Continuous lumbar CSF drainage can greatly reduce the occurrence of PTH parathyroid hormone45.
Studies on risk factors have pointed out that craniotomy decompression and intracranial infection are independent predictors of chronic hydrocephalus7,48, among which larger craniotomy and decompressive craniectomy can reduce intracranial pressure but may concurrently reduce the absorption of CSF, thereby causing hydrocephalus49,50. Therefore, the present study excluded these two factors in the collection process when establishing the scoring system prediction model. Early prediction of PHH occurrence is conducive to assessing the patient's condition and improving prognosis.
PHH is a common complication of hemorrhagic brain disease during neurosurgery. Because of the shared characteristics of these diseases, there are many studies on single disease factors, and the influencing factors are concentrated in hematoma thickness, bleeding distribution, and bleeding volume41,51. This study excluded the differences in disease types and established a prediction model of PHH based on hypertension, CSF-TP over time, GCS, and fever time. Moreover, our study developed a risk scoring system to predict the occurrence of PHH. A scoring system was established according to the factor grade and statistical distribution score, which is simple and practical. In addition, the AUC value was used to evaluate the predictive ability of the model. The predictive factor was observed to be an independent risk factor for PHH. The results show the following independent risk factors: age, GCS, fever time, and CSF-TP x time. Age > 60 years, GCS < 5, GCS 6‒8, fever time > 9, fever time 5‒9 and CSF-TP x time > 14,400 and 9601‒14,400 were independent risk factors (Table 3), which are consistent with the conclusions of related reports.
This study had certain limitations. First, the sample size obtained from a single center was limited, and patients who did not undergo craniotomy within 30 days, diagnosed with intracranial infection during the course of admission, and lacking lumbar puncture results were excluded. Second, the factors included in the scoring system were limited and may affect the accuracy of the scoring system. Third, there may be recall and selection biases, and it is not excluded that some factors are influencing factors of PHH. Fourth, although CSF-TP as clinical evidence can indirectly suggest changes in colloid osmotic pressure in cerebrospinal fluid. However, direct colloid osmotic pressure test results of cerebrospinal fluid are lacking. Fifth, information errors in evaluating patients may lead to the loss of some patients with hydrocephalus and a lack of long-term prognosis evaluation. However, early judgment and intervention are important in reducing the occurrence of PHH. Therefore, a scoring system was developed to increase the general applicability of the model for popularization and practicality in the clinic.
In conclusion, the pathogenesis of PHH is a complex clinical-pathological process. Numerous studies have been conducted regarding the related mechanisms, indicating that many factors are involved in its pathogenesis. Moreover, early prediction of PHH occurrence is conducive to assessing the patients’ condition and prognosis improvement. Furthermore, to the best of our knowledge, our study is the first to propose a risk scoring system for PHH. We observed that age, GCS, fever time, and CSF-TP x time were important risk factors for predicting PHH, which can positively contribute to the early detection and treatment of PHH by clinicians.
Methods
Case selection
Data regarding related cases (including ICH, IVH, SAH, and TBI) admitted to the Department of Neurosurgery of Jiangxi Provincial People's Hospital from January 2013 to January 2022 were collected retrospectively. We excluded patients without cerebrospinal fluid test results, decompressive craniectomy, and intracranial infection to explore the same characteristics of PHH. The objectives of this study were to analyze the risk factors for hemorrhagic brain diseases complicated by PHH and establish a comprehensive prediction model. This study was conducted in strict accordance with the 2013 Declaration of Helsinki guidelines. The cases were selected according to the following criteria:
Inclusion criteria
(1) A definitive diagnosis of hemorrhagic brain disease (TBI, SAH, IVH, ICH, etc.) confirmed by computed tomography (CT).
(2) Cerebrospinal fluid test results were available within 30 days of the duration of the disease.
(3) A traceable course of the disease for more than 2 weeks from admission.
Exclusion criteria
-
1.
A prior diagnosis of hematological diseases, brain tumors, or hydrocephalus.
-
2.
A diagnosis of intracranial infection during the course of the disease.
-
3.
Craniotomy and decompression of the bone flap were performed later on (not repaired within 30 days).
-
4.
Complications of the liver, kidney, gastrointestinal, respiratory, and cardiovascular systems, and other diseases that seriously affect safety evaluation.
-
5.
Complications with serious infectious diseases.
-
6.
Complications with coagulation dysfunction and patients on long-term anticoagulants
-
7.
Pregnant and lactating women.
Treatments
All patients received standardized nursing and neurosurgical treatments. Our department has a well-trained, professional team that strictly follows the state guidelines on neurosurgical treatment. After admission, the patients underwent cranial CT, routine blood and biochemical tests, other relevant examinations, and a physical examination by the neurosurgery specialist. The onset and medical history of the patients were precisely recorded.
Diagnosis of PHH
The diagnosis of PHH was mainly based on imaging results and clinical symptoms. Patients with hydrocephalus gradually developed characteristic symptoms of increased intracranial pressure (headache, nausea/vomiting, altered consciousness) and signs (such as low level of consciousness and papiloedema of the optic nerve).The specific criteria are as follows: (a) The Evans index was greater than 0.3; (b) An enlargement of the anterior horn, temporal horn, third ventricle of the lateral ventricle, and periventricular interstitial edema52; (c) Neurocognitive dysfunction in conscious patients (such as depression, difficulty in decision-making, memory or language disorder, physical activity disorders including walking instability and ataxia, and dysuria); (d) No improvement or worsening of consciousness in patients under coma (with images indicating hydrocephalus).
Data collection
Basic information, including age, sex, diagnosis, and Glasgow Coma Score (GCS), among others, were obtained from the inpatient information management system of the hospital. Medical history (such as history of hypertension, diabetes, illicit drug use, and personal history), treatment information (conservative treatment, external ventricular drain (EVD), embolization, and endoscopic treatment), fever time (the total time when the body temperature is ≥ 38.5 °C), cerebrospinal fluid biochemical results and time (from the onset of the disease to the obtainment of CSF-TP after lumbar puncture) were collected. Relevant studies suggest that inflammation and fever are related to hydrocephalus53,54. A large accumulation of various proteins in the cerebrospinal fluid will change osmotic pressure and cause hydrocephalus, which has been verified in animal models35,53, and the CSF-TP of hydrocephalus patients is higher than that of the normal group39. In our previous study, we observed for the first time that fever time and CSF-TP were two important factors in the statistical results8. The prognostic information of the selected patients was followed up within 6 months from their discharge. PHH diagnosis was based on the diagnosis when the patient was discharged from the hospital, re-examination of symptoms and examination results, re-admission examination, and treatment information. The collection of information and statistical analyses were carried out by professional personnel.
After a univariate analysis of all factors, those with p < 0.05 were selected for multivariate logistic regression analysis, and important risk factors were selected to establish the prediction model. The scoring system for the prediction model was obtained according to the distribution of the β regression coefficient values for each factor. The factor grade or the score grade and the corresponding prediction probability were established according to the above score, which is convenient for clinical use.
Statistical analysis
SPSS software (version 26.0, SPSS Inc., Chicago, Illinois, USA) was used for the statistical analysis. The statistical tests were bilateral. Categorical variables were expressed as count and percentage (%) and continuous variables were expressed as mean (x) ± standard deviation (SD). Univariate analysis was performed by t-test, Chi-square test, or U test. A p value < 0.05 was considered statistically significant. In the univariate analysis, variables related to PHH (p < 0.05) were included in the logistic regression model to determine the independent predictors of PHH. Factors with p < 0.05 were grouped into logistic regression analysis. The receiver operating characteristic (ROC) curve and area under the curve (AUC) were used to evaluate the discrimination ability of the scoring system, which was divided into good to excellent (AUC > 0.8), medium (AUC 0.7‒0.8), and low (AUC 0.6‒0.7). The Hosmer‒Lemeshow (HL) test was used to evaluate the calibration ability of the model.
Ethics approval
The study was approved by the biomedical Ethics Committee of Jiangxi Provincial People's Hospital (No: 2022-018) and the informed consent was waived by the biomedical Ethics Committee of Jiangxi Provincial People's Hospital. In accordance with the requirements of national legislation and institutions, written informed consent of patients or their families is not required for this study. All methods were carried out in accordance with relevant guidelines and regulations.
Data availability
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Abbreviations
- PHH:
-
Post-hemorrhagic hydrocephalus
- GCS:
-
Glasgow coma score
- AUC:
-
Area under curve
- CSF-TP:
-
Cerebrospinal fluid total protein
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- IVH:
-
Intraventricular hemorrhage
- ICH:
-
Intracerebral hemorrhage
- SAH:
-
Subarachnoid hemorrhage
- TBI:
-
Traumatic brain injury
- CT:
-
Computed tomography
- EVD:
-
External ventricular drain
- SD:
-
Sstandard deviation
- ROC:
-
Receiver operating characteristic
- HL:
-
Hosmer–Lemeshow
- VP:
-
Ventricular-peritoneal
- nPHH:
-
Not post-hemorrhagic hydrocephalus
- CSF:
-
Cerebrospinal fluid
- Lcn2:
-
Lipocalin 2
- TGF-β1:
-
Transforming factor-β1
- CA-I:
-
Carbonic anhydrase-I
- Prx-2:
-
Peroxidase-2
- TF:
-
Transferrin
- HP:
-
Haptoglobin
- IL:
-
Interleukin
- MMP:
-
Matrix metallo protein
- FGF:
-
Fibroblast growth factor
- DC:
-
Decompressive craniectomy
- PTH:
-
Post-traumatic hydrocephalus
References
Sun, T. et al. Shunting outcomes in post-hemorrhagic hydrocephalus: A protocol for systematic review and meta-analysis. Medicine 99, e21640. https://doi.org/10.1097/MD.0000000000021640 (2020).
Chen, Q. et al. Post-hemorrhagic hydrocephalus: Recent advances and new therapeutic insights. J. Neurol. Sci. 375, 220–230. https://doi.org/10.1016/j.jns.2017.01.072 (2017).
Wang, Z., Wang, K., Qian, Z., Zeng, L. & Gao, L. Lumboperitoneal and ventriculoperitoneal shunt surgery for posthemorrhagic communicating hydrocephalus: A comparison. World Neurosurg. 127, e638–e643. https://doi.org/10.1016/j.wneu.2019.03.235 (2019).
Xi, G., Strahle, J., Hua, Y. & Keep, R. F. Progress in translational research on intracerebral hemorrhage: Is there an end in sight?. Prog. Neurobiol. 115, 45–63. https://doi.org/10.1016/j.pneurobio.2013.09.007 (2014).
Garton, T. et al. Intraventricular hemorrhage: The role of blood components in secondary injury and hydrocephalus. Transl. Stroke Res. 7, 447–451. https://doi.org/10.1007/s12975-016-0480-8 (2016).
Dong, C. et al. Icariside II attenuates chronic hydrocephalus in an experimental subarachnoid hemorrhage rat model. J. Pharm. Pharm. Sci. 21, 318–325. https://doi.org/10.18433/jpps29811 (2018).
Chen, H. et al. Predicting posttraumatic hydrocephalus: derivation and validation of a risk scoring system based on clinical characteristics. Metab Brain Dis 32, 1427–1435. https://doi.org/10.1007/s11011-017-0008-2 (2017).
Wang, Z. et al. Risk factor of posthemorrhagic hydrocephalus: Cerebrospinal fluid total protein. Front. Surg. 9, 692383. https://doi.org/10.3389/fsurg.2022.692383 (2022).
Kaestner, S. & Dimitriou, I. TGF beta1 and TGF beta2 and their role in posthemorrhagic hydrocephalus following SAH and IVH. J. Neurol. Surg. A 74, 279–284. https://doi.org/10.1055/s-0033-1342929 (2013).
Hu, R. et al. Long-term outcomes and risk factors related to hydrocephalus after intracerebral hemorrhage. Transl. Stroke Res. 12, 31–38. https://doi.org/10.1007/s12975-020-00823-y (2021).
Sprigg, N. et al. Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage (TICH-2): An international randomised, placebo-controlled, phase 3 superiority trial. Lancet 391, 2107–2115. https://doi.org/10.1016/S0140-6736(18)31033-X (2018).
Tian, H. L. et al. Risk factors related to hydrocephalus after traumatic subarachnoid hemorrhage. Surg. Neurol. 69, 241–246. https://doi.org/10.1016/j.surneu.2007.02.032 (2008) (discussion 246).
Balami, J. S. & Buchan, A. M. Complications of intracerebral haemorrhage. Lancet Neurol. 11, 101–118. https://doi.org/10.1016/S1474-4422(11)70264-2 (2012).
Hanley, D. F. Intraventricular hemorrhage: severity factor and treatment target in spontaneous intracerebral hemorrhage. Stroke 40, 1533–1538. https://doi.org/10.1161/STROKEAHA.108.535419 (2009).
Diringer, M. N., Edwards, D. F. & Zazulia, A. R. Hydrocephalus: a previously unrecognized predictor of poor outcome from supratentorial intracerebral hemorrhage. Stroke 29, 1352–1357. https://doi.org/10.1161/01.str.29.7.1352 (1998).
Sanusi, T. D., McLarnon, M. & Abouharb, A. Risk factors of chronic shunt dependent hydrocephalus following aneurysmal subarachnoid haemorrhage. Clin. Neurol. Neurosurg. 198, 106095. https://doi.org/10.1016/j.clineuro.2020.106095 (2020).
Kammersgaard, L. P., Linnemann, M. & Tibaek, M. Hydrocephalus following severe traumatic brain injury in adults. Incidence, timing, and clinical predictors during rehabilitation. NeuroRehabilitation 33, 473–480. https://doi.org/10.3233/NRE-130980 (2013).
De Bonis, P. & Anile, C. Post-traumatic hydrocephalus: the Cinderella of Neurotrauma. Expert Rev Neurother 20, 643–646. https://doi.org/10.1080/14737175.2020.1779059 (2020).
Kanat, A. Pathophysiology of acute hydrocephalus after subarachnoid hemorrhage. World Neurosurg. 82, e386-387. https://doi.org/10.1016/j.wneu.2013.08.007 (2014).
Xiong, G. et al. Traumatic brain injury-induced ependymal ciliary loss decreases cerebral spinal fluid flow. J Neurotrauma 31, 1396–1404. https://doi.org/10.1089/neu.2013.3110 (2014).
Garton, T. P. et al. Hemoglobin-induced neuronal degeneration in the hippocampus after neonatal intraventricular hemorrhage. Brain Res. 1635, 86–94. https://doi.org/10.1016/j.brainres.2015.12.060 (2016).
Yang, S. et al. The role of complement C3 in intracerebral hemorrhage-induced brain injury. J. Cereb. Blood Flow Metab. 26, 1490–1495. https://doi.org/10.1038/sj.jcbfm.9600305 (2006).
Wang, L. et al. Recombinant human erythropoietin improves the neurofunctional recovery of rats following traumatic brain injury via an increase in circulating endothelial progenitor cells. Transl. Stroke Res. 6, 50–59. https://doi.org/10.1007/s12975-014-0362-x (2015).
Hirst, R. A., Rutman, A. & O’Callaghan, C. Hydrogen peroxide at a concentration used during neurosurgery disrupts ciliary function and causes extensive damage to the ciliated ependyma of the brain. Childs Nerv. Syst. 25, 559–561. https://doi.org/10.1007/s00381-008-0768-4 (2009).
Bu, Y. et al. Mechanisms of hydrocephalus after intraventricular haemorrhage in adults. Stroke Vasc. Neurol. 1, 23–27. https://doi.org/10.1136/svn-2015-000003 (2016).
Jimenez, A. J. et al. Increased levels of tumour necrosis factor alpha (TNFalpha) but not transforming growth factor-beta 1 (TGFbeta1) are associated with the severity of congenital hydrocephalus in the hyh mouse. Neuropathol. Appl. Neurobiol. 40, 911–932. https://doi.org/10.1111/nan.12115 (2014).
Li, T. et al. Thrombin-induced TGF-beta1 pathway: A cause of communicating hydrocephalus post subarachnoid hemorrhage. Int. J. Mol. Med. 31, 660–666. https://doi.org/10.3892/ijmm.2013.1253 (2013).
Connor, D. E. Jr. et al. Variations in the cerebrospinal fluid proteome following traumatic brain injury and subarachnoid hemorrhage. Pathophysiology 24, 169–183. https://doi.org/10.1016/j.pathophys.2017.04.003 (2017).
Harris, C. A., Morales, D. M., Arshad, R., McAllister, J. P. 2nd. & Limbrick, D. D. Jr. Cerebrospinal fluid biomarkers of neuroinflammation in children with hydrocephalus and shunt malfunction. Fluids Barriers CNS 18, 4. https://doi.org/10.1186/s12987-021-00237-4 (2021).
Gris, T. et al. Innate immunity activation in the early brain injury period following subarachnoid hemorrhage. J. Neuroinflamm. 16, 253. https://doi.org/10.1186/s12974-019-1629-7 (2019).
Higashi, K. & Maza, T. The effect of osmotic pressure on the production of cerebrospinal fluid. Neurol. Med. Chir. 16, 5–13. https://doi.org/10.2176/nmc.16pt1.5 (1976).
Bulat, M. & Klarica, M. Recent insights into a new hydrodynamics of the cerebrospinal fluid. Brain Res. Rev. 65, 99–112. https://doi.org/10.1016/j.brainresrev.2010.08.002 (2011).
Oreskovic, D. & Klarica, M. The formation of cerebrospinal fluid: Nearly a hundred years of interpretations and misinterpretations. Brain Res. Rev. 64, 241–262. https://doi.org/10.1016/j.brainresrev.2010.04.006 (2010).
Klarica, M., Mise, B., Vladic, A., Rados, M. & Oreskovic, D. “Compensated hyperosmolarity” of cerebrospinal fluid and the development of hydrocephalus. Neuroscience 248, 278–289. https://doi.org/10.1016/j.neuroscience.2013.06.022 (2013).
Oreskovic, D. & Klarica, M. Development of hydrocephalus and classical hypothesis of cerebrospinal fluid hydrodynamics: Facts and illusions. Prog. Neurobiol. 94, 238–258. https://doi.org/10.1016/j.pneurobio.2011.05.005 (2011).
Kuo, L. T. & Huang, A. P. The pathogenesis of hydrocephalus following aneurysmal subarachnoid hemorrhage. Int. J. Mol. Sci. 22, 5050. https://doi.org/10.3390/ijms22095050 (2021).
Qian, C. et al. Effect of the drainage of cerebrospinal fluid in patients with aneurismal subarachnoid hemorrhage: A meta-analysis. Medicine 95, e5140. https://doi.org/10.1097/MD.0000000000005140 (2016).
Basaldella, L. et al. External ventricular drainage alone versus endoscopic surgery for severe intraventricular hemorrhage: A comparative retrospective analysis on outcome and shunt dependency. Neurosurg. Focus 32, E4. https://doi.org/10.3171/2012.1.FOCUS11349 (2012).
Lenski, M. et al. Role of cerebrospinal fluid markers for predicting shunt-dependent hydrocephalus in patients with subarachnoid hemorrhage and external ventricular drain placement. World Neurosurg. 121, e535–e542. https://doi.org/10.1016/j.wneu.2018.09.159 (2019).
Qureshi, A. I., Foster, L. D., Lobanova, I., Huang, W. & Suarez, J. I. Intensive blood pressure lowering in patients with moderate to severe grade acute cerebral hemorrhage: Post hoc analysis of antihypertensive treatment of acute cerebral hemorrhage (ATACH)-2 trial. Cerebrovasc. Dis. 49, 244–252. https://doi.org/10.1159/000506358 (2020).
AlShardan, M. M., Mubasher, M., Orz, Y. & AlYamany, M. Factors that predict hydrocephalus following intraventricular hemorrhage. Br. J. Neurosurg. 29, 225–228. https://doi.org/10.3109/02688697.2014.960365 (2015).
Sheehan, J. P., Polin, R. S., Sheehan, J. M., Baskaya, M. K. & Kassell, N. F. Factors associated with hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery 45, 1120–1127. https://doi.org/10.1097/00006123-199911000-00021 (1999) (discussion 1127-1128).
Brisman, J. L. & Berenstein, A. Factors related to hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery 54, 1031. https://doi.org/10.1227/01.neu.0000117123.32806.f9 (2004).
Sun, S., Zhou, H., Ding, Z. Z. & Shi, H. Risk factors associated with the outcome of post-traumatic hydrocephalus. Scand. J. Surg. 108, 265–270. https://doi.org/10.1177/1457496918812210 (2019).
Jiao, Q. F. et al. Influencing factors for posttraumatic hydrocephalus in patients suffering from severe traumatic brain injuries. Chin. J. Traumatol. 10, 159–162 (2007).
Fotakopoulos, G., Tsianaka, E., Siasios, G., Vagkopoulos, K. & Fountas, K. Posttraumatic hydrocephalus after decompressive craniectomy in 126 patients with severe traumatic brain injury. J. Neurol. Surg. A 77, 88–92. https://doi.org/10.1055/s-0035-1558411 (2016).
Ding, J., Guo, Y. & Tian, H. The influence of decompressive craniectomy on the development of hydrocephalus: A review. Arq. Neuropsiquiatr. 72, 715–720. https://doi.org/10.1590/0004-282x20140106 (2014).
Czosnyka, M. et al. Post-traumatic hydrocephalus: Influence of craniectomy on the CSF circulation. J. Neurol. Neurosurg. Psychiatry 68, 246–248. https://doi.org/10.1136/jnnp.68.2.246a (2000).
Shapiro, K., Fried, A., Takei, F. & Kohn, I. Effect of the skull and dura on neural axis pressure-volume relationships and CSF hydrodynamics. J. Neurosurg. 63, 76–81. https://doi.org/10.3171/jns.1985.63.1.0076 (1985).
Honeybul, S. & Ho, K. M. Incidence and risk factors for post-traumatic hydrocephalus following decompressive craniectomy for intractable intracranial hypertension and evacuation of mass lesions. J. Neurotrauma 29, 1872–1878. https://doi.org/10.1089/neu.2012.2356 (2012).
Strahle, J. et al. Mechanisms of hydrocephalus after neonatal and adult intraventricular hemorrhage. Transl. Stroke Res. 3, 25–38. https://doi.org/10.1007/s12975-012-0182-9 (2012).
Langner, S. et al. Diagnosis and differential diagnosis of hydrocephalus in adults. Rofo 189, 728–739. https://doi.org/10.1055/s-0043-108550 (2017).
Wessell, A. P. et al. A sustained systemic inflammatory response syndrome is associated with shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage. J. Neurosurg. 130, 1984–1991. https://doi.org/10.3171/2018.1.JNS172925 (2018).
Adil, S. M. et al. Healthcare economics of hydrocephalus after aneurysmal subarachnoid hemorrhage in the United States. Transl. Stroke Res. 10, 650–663. https://doi.org/10.1007/s12975-019-00697-9 (2019).
Acknowledgements
In particular, I would like to thank supervisor Ruen Liu for her great help in the project design and revision process, and all the participating authors for their positive contributions.
Funding
This study was funded by Beijing Municipal Science and Technology Commission (Z181100001518005).
Author information
Authors and Affiliations
Contributions
R.L. and Z.W. carried out the paper design and verification together. Z.W. and B.X. wrote the main manuscript text and prepared Figs. 1and Tables 1‒4. B.Y., J.Z., M.W. and C.W. helped collect data and revise manuscripts. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Z., Xi, B., Yu, B. et al. Prediction of adult post-hemorrhagic hydrocephalus: a risk score based on clinical data. Sci Rep 12, 12213 (2022). https://doi.org/10.1038/s41598-022-16577-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-16577-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.