Abstract
The aim of this study was to validate a motion-immune (MI) solution to dynamic CT myocardial perfusion measurement, in the presence of motion without image registration. The MI perfusion technique was retrospectively validated in six swine (37.3 ± 7.5 kg) with a motion-susceptible (MS) perfusion technique performed for comparison. In each swine, varying severities of stenoses were generated in the left anterior descending (LAD) coronary artery using a balloon under intracoronary adenosine stress, followed by contrast-enhanced imaging with 20 consecutive volume scans per stenosis. Two volume scans were then systematically selected from each acquisition for both MI and MS perfusion measurement, where the resulting LAD and left circumflex (LCx) measurements were compared to reference microsphere perfusion measurements using regression and diagnostic performance analysis. The MI (PMI) and microsphere (PMICRO) perfusion measurements were related through regression by PMI = 0.98 PMICRO + 0.03 (r = 0.97), while the MS (PMS) and microsphere (PMICRO) perfusion measurements were related by PMS = 0.62 PMICRO + 0.15 (r = 0.89). The accuracy of the MI and MS techniques in detecting functionally significant stenosis was 93% and 84%, respectively. The motion-immune (MI) perfusion technique provides accurate myocardial perfusion measurement in the presence of motion without image registration.
Similar content being viewed by others
Introduction
Coronary artery disease (CAD) is the leading cause of mortality in the United States. Fortunately, coronary computed tomography angiography (CTA) can accurately detect or rule out high-grade CAD and is non-inferior to functional testing in patients with low- to intermediate-risk of CAD1. That said, CTA cannot assess myocardial perfusion in such low- to intermediate-risk patients2, where ischemia-guided coronary revascularization is superior to angiography-guided revascularization3. Hence, functional testing such as SPECT and CMR are recommended, but such modalities remain limited as they only provide metrics of relative perfusion4,5. Of course, dynamic PET has the highest accuracy for diagnosis of myocardial ischemia6, yet, radiotracer availability and cost largely limit its routine application. Thus, the ability of dynamic CT to assess perfusion and ischemia in quantitative terms (mL/min/g) is of clinical merit7,8,9. More importantly, evidence supports the combined or tiered use of coronary CTA with dynamic CT perfusion for morphological and physiological assessment of CAD7,8,9,10,11,12. That said, routine use of dynamic CT perfusion remains limited by the added contrast and radiation dose required to measure perfusion. Specifically, dynamic CT techniques rely on ECG-gated scanning of the myocardium over 10 to 20 heart beats. While ECG-gating ensures the same cardiac phase is captured for each scan; unfortunately, beat-to-beat variation and imperfect breath-holding necessitates image registration between consecutive acquisitions. Nevertheless, registration itself inherently alters the myocardial CT number, incurring errors in quantitative perfusion measurement. Moreover, dynamic CT perfusion techniques underestimate perfusion as compared to quantitative PET, especially under hyperemic conditions13, where such inaccuracies are further exacerbated by cardiac and respiratory motion despite registration14. Hence, a better solution to dynamic CT perfusion measurement remains necessary.
Prior work in first-pass analysis (FPA) dynamic CT perfusion measurement has addressed some of these limitations via a new technique capable of accurate perfusion measurement with only two volume scans, where one volume scan may also double as a coronary CTA15,16,17,18. Yet, a remaining limitation of this motion-susceptible (MS) FPA technique is its reliance on image registration to minimize motion between the two volume scans of interest.
Hence, this study aimed to validate a motion-immune (MI) solution to FPA dynamic CT perfusion measurement using quantitative microsphere perfusion measurement as the reference standard. The central hypothesis was that accurate myocardial perfusion measurement is feasible with the MI perfusion technique in the presence of motion without image registration.
Methods
General methods
The study was performed on six male Yorkshire swine (37.3 ± 7.5 kg). It was approved by the Institutional Animal Care and Use Committee at UC Irvine (IACUC Protocol Number: AUP-22-015) and was carried out in accordance with all relevant regulations, as well as in compliance with the ARRIVE guidelines. In each animal, several different intermediate severity balloon stenoses were generated in the left anterior descending (LAD) coronary artery, after which dynamic imaging was performed. The data were then processed, in the absence of image registration, using the motion-immune (MI) perfusion technique as well as the previously validated motion-susceptible (MS) perfusion technique15,16, after which both techniques were compared to reference standard microsphere perfusion measurement. Of note, the raw data used were previously acquired to validate the MS perfusion technique with registration15, but the analysis and validation presented in this study are new and independent.
Motion-immune dynamic CT perfusion theory
Modelling the whole myocardium as one compartment, the average perfusion (PAVG) is approximately proportional to the first-pass rate of contrast mass entry into the compartment over time (dMC/dt: in grams of Iodine per minute), normalized by the average incoming aortic blood pool contrast concentration (CIN: in grams of Iodine per milliliter of blood) and total left ventricular tissue mass (MT: in grams), assuming no contrast mass outflow at the time of measurement15,16. Importantly, since the mass of Iodine is related to the enhancement of tissue and blood by the same physical constant which cancels in ratio, only the tissue and blood enhancement (in Hounsfield Units, HU) of two whole-heart volume scans acquired at the base and peak of the aortic enhancement (V1 and V2) are needed for perfusion measurement, where V1 is effectively a non-contrast volume scan while V2 is effectively a coronary CTA, as previously validated15,16. Differently, however, the integrated enhancement of V2 is determined by summating all myocardial enhancement values within V2. The integrated enhancement of V1 is then approximated by multiplying the average myocardial enhancement of V1 by the total number of myocardial voxels, n, within V2. Taken in difference, dMC/dt is calculated and normalized by the blood pool contrast concentration (CIN: the average aortic enhancement between V1 and V2) and total tissue mass (MT: the product of n, the voxel size, and the tissue density) to yield the average perfusion (PAVG), shown in Eq. (1.1). The average change in myocardial enhancement (ΔHUAVG) is then calculated as the average difference in voxel values between the V2 and V1 volume scans. Finally, the average enhancement of V1 is subtracted per-voxel from V2 to estimate the voxel-by-voxel differences in myocardial enhancement between V1 and V2 (ΔHU*). In combination, MI perfusion (PMI) in mL/min/g is derived, as described by Eq. (1.2). The assumptions are: (1) that the V2 and V1 myocardium are equivalent in volume, and (2) that the myocardial tissue density of V1 is homogenous since negligible contrast mass has entered the myocardium at the time of V1 acquisition.
Animal model
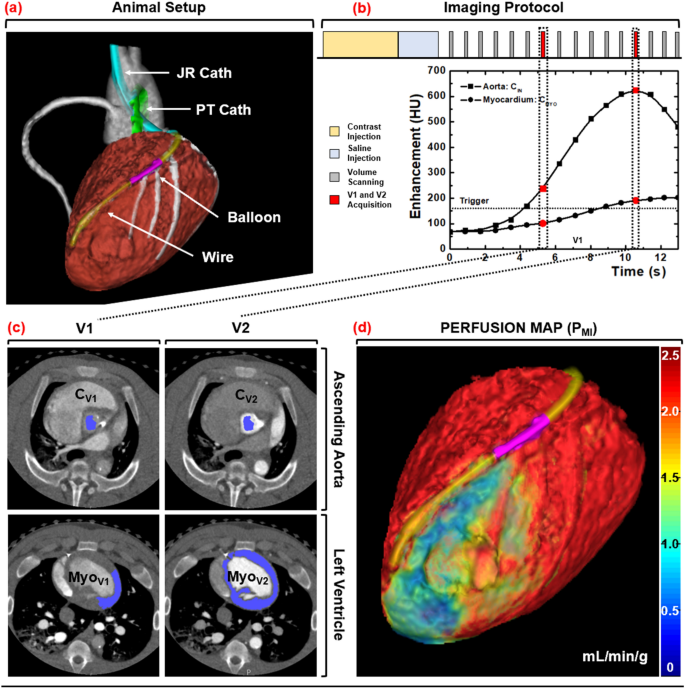
Induction of anesthesia was accomplished with Telazol (4.4 mg/kg), Ketamine (2.2 mg/kg), and Xylazine (2.2 mg/kg), followed by maintenance with 1.5–2.5% Isoflurane (Highland Medical Equipment and Baxter). Introducer sheaths (5–7 Fr, AVANTI®, Cordis Corporation) were then placed in both femoral arteries, one carotid artery, both femoral veins, and one jugular vein. Using the femoral arterial sheaths, a pigtail catheter was placed into the left ventricular blood pool for injection of microspheres, while a multipurpose catheter was placed into the abdominal aorta for withdrawal of reference blood. Using the carotid arterial sheath, a Judkins right catheter was placed, after which a fractional flow reserve wire (FFR) (PrimeWire, Volcano) was advanced down the LAD. A balloon was then passed into the LAD to generate stenoses with incrementally increasing FFR severities of 1.0, 0.9, 0.8, 0.7, and 0.6 at maximal intracoronary hyperemia (240 µg adenosine/min, Model 55-2222, Harvard Apparatus). Note that intracoronary hyperemia was used to minimize the hypotension and reflex tachycardia characteristic to intravenous adenosine, but limited stress perfusion measurement to the LAD alone. Next, the jugular vein sheath was set up for contrast injection, while the femoral vein sheaths were used for intravenous fluids and medications. Finally, the heart rate, end-tidal CO2, pulse oximetry, and arterial-line blood pressure were monitored continuously (SurgiVet, Smiths Medical) and logged every 15 min. Using these metrics, the intravenous fluid drip rate, anesthesia depth, and ventilation settings were adjusted accordingly to maintain the mean-arterial pressure greater than 65 mmHg and pulse oximetry greater than 92% for adequate myocardial perfusion and tissue oxygenation, respectively. The animal setup is shown in Fig. 1a.
Animal setup, imaging protocol, and image processing scheme. (a) Interventional setup displaying the Judkins Right (JR) and Pigtail (PT) catheters, coronary balloon, and pressure wire. (b) Dynamic CT imaging protocol, with V1 and V2 denoted in red. (c) Semi-automatic segmentation of the aortic blood pool and myocardium. (d) MI perfusion map derivation with a distal LAD defect displayed.
Imaging protocol
For each stenosis, hyperemia was induced and maintained throughout acquisition. For each acquisition, contrast (1 mL/kg, Isovue 370, Bracco Diagnostics) was injected (7 mL/s, Empower CTA, Acist Medical Systems) then chased by saline (0.5 mL/kg at 7 mL/s) with one microsphere color also injected. Dynamic whole-heart volume scanning was then performed at 100 kVp and 200 mA, where each volume scan was acquired as a full projection with a rotation time of 0.35 s and 320 × 0.5 mm collimation (Aquilion One, Canon Medical Systems). After each twenty-scan acquisition, a 15-min delay was observed. Then the stenosis severity was incrementally increased (as described above in 2.3), and the acquisition process was repeated, until all five of the available microsphere colors were used. The 32 cm diameter volumetric CT dose index (\({CTDI}_{vol}^{32}\)) and size-specific dose estimate (SSDE) were also recorded19. The acquisition protocol is shown in Fig. 1b.
Microsphere protocol
The 15.5 µm diameter NuFlow HydroCoat fluorescent microspheres (IMT Laboratories) were used as the reference standard for quantitative perfusion measurement in mL/min/g15. For each image acquisition, 2 mL of unique microsphere color was manually injected into the pigtail catheter then rapidly flushed into the left ventricular blood pool with 5 mL of saline, after which reference blood samples were withdrawn at 10 mL/min over two minutes using a syringe pump (GenieTouch; Kent Scientific). After all the microsphere colors had been injected, each animal was euthanized. Their hearts were surgically removed, and 10-g tissue plugs were extracted from the proximal and distal LAD, as well as from the left circumflex (LCx) perfusion territories. All tissue and blood samples were analyzed by IMT Laboratories.
Image processing
All volume scans were reconstructed with AIDR 3D (Canon Medical Systems) at 75% of the R-R interval with a voxel size of 0.43 × 0.43 × 0.50 mm. The V1 and V2 volume scans were then systematically selected for analysis15,16,17,18. For V1, volumetric region growing was used to measure the average enhancement of the aortic blood pool (Vitrea fX version 6.0, Vital Images). For V2, region growing was again used to measure the average enhancement of the aortic blood pool, while segmentation was used to extract and export the entire left ventricular myocardium. All data were then imported into custom in-house software for MI and MS perfusion calculation.
MI perfusion calculation
The integrated enhancement of V2 was first determined by summating all the myocardial enhancement values within the segmented left ventricular myocardium. Next, volumetric region growing was used to measure the average enhancement of the lateral wall of the V1 myocardium, which approximated the average enhancement of the total V1 myocardium. The integrated enhancement of V1 was then estimated by multiplying the average enhancement of the lateral wall of the V1 myocardium by the total voxel volume of the segmented myocardium from V2. The total difference in integrated enhancement between V2 and V1 (dMC/dt), was then normalized by the average incoming aortic blood pool contrast concentration (CIN) and left ventricular myocardium tissue mass (MT) to yield the average MI perfusion (PAVG_MI). Next, using the V2 segmentation, the average enhancement of the V1 myocardium was subtracted from each voxel of V2 (ΔHU*). Each voxel value was then normalized by the difference in average enhancement between the V2 and V1 myocardium (ΔHUAVG) to yield a perfusion ratio map. The average MI perfusion (PAVG_MI) was then multiplied by the perfusion ratio map (ΔHU*/ ΔHUAVG) to yield voxel-by-voxel MI perfusion measurements (PMI).
MS perfusion calculation
The segmented myocardium of V2 was applied as a binary mask to segment the myocardium of V1. The difference in integrated enhancement between V2 and V1 (dMC/dt) was then determined through image subtraction. After which, normalization by the average incoming aortic blood pool contrast concentration (CIN) and left ventricular myocardium tissue mass (MT) was performed to yield the average MS perfusion (PAVG_MS). Next, each voxel of the V1 myocardium was subtracted from each corresponding voxel of V2 (ΔHU) then normalized by the difference in average enhancement between the V2 and V1 myocardium (ΔHUAVG), yielding a perfusion ratio map. The average MS perfusion (PAVG_MS) was then multiplied by the perfusion ratio map (ΔHU/ ΔHUAVG) to yield voxel-by-voxel MS perfusion measurements (PMI).
Finally, virtual tissue plugs from the proximal LAD, distal LAD, and LCx perfusion territories were segmented, where these plugs were spatially matched to the physical tissue plugs using epicardial coronary landmarks. The per-voxel MI and MS perfusion values within each plug were then averaged and compared to corresponding microsphere perfusion measurements. The image processing steps are outlined in Fig. 1c,d.
Statistical analysis
As multiple measurements were made per animal, the intra-cluster correlation of measurement was first computed and found to be 0.12. Hence, there was minimal correlation between intra-animal measurements, i.e., all measurements were treated as independent. Overall, MI and MS measurements were compared to microsphere measurements with t-testing, regression, Bland–Altman, Pearson’s correlation coefficient (r), Lin’s concordance correlation coefficient (CCC), root-mean-square-error (RMSE: accuracy), and root-mean-square deviation (RMSD: precision). The diagnostic performance of the MI and MS techniques in identification of functionally significant stenoses, i.e., LAD microsphere perfusion less than 1.0 mL/min/g at hyperemia20, was also assessed via sensitivity, specificity, positive and negative predictive values, accuracy, and area under the receiver operator characteristic curve (AUC). P-values less than 0.05 indicate significant differences. Statistical software was used (SPSS, Version 22, IBM Corporation).
Results
General
The average body mass of the six male Yorkshire swine was 37.3 ± 7.5 kg, where the average left ventricular mass of the swine was 52.1 ± 12.7 g. During image acquisition, the average heart rate of the swine was 73 ± 5 beats per minute, while their average mean arterial blood pressure was 73 ± 10 mmHg. Following image acquisition and image processing, the average time delay between the V1 and V2 volume scans was 4.7 ± 1.2 s, corresponding to an average change in myocardial enhancement over that time of 25.0 ± 13.5 HU.
For the LAD coronary artery, the average MI perfusion was 2.60 ± 1.94 mL/min/g, the average MS perfusion was 1.79 ± 1.34 mL/min/g, and the average microsphere perfusion was 2.62 ± 1.94 mL/min/g, where the result of corresponding t-testing was p = 0.72 and p = 0.00, respectively. For the LCx coronary artery, the average MI perfusion was 0.85 ± 0.40 mL/min/g, the average MS perfusion was 0.67 ± 0.56 mL/min/g, and the average microsphere perfusion was 0.87 ± 0.34 mL/min/g, where the result of corresponding t-testing was p = 0.75 and p = 0.05, respectively. Notably, LAD perfusion measurements were performed during intracoronary hyperemia (stress flow), while LCx perfusion measurements were not (rest flow).
Accuracy and precision
The regression equation relating MI perfusion (PMI) to microsphere perfusion (PMICRO) was PMI = 0.98 PMICRO + 0.03, where the Pearson’s and Lin’s correlations were r = 0.97 and ρ = 0.97, respectively, while the RMSE and RMSD were 0.42 mL/min/g and 0.42 mL/min/g, respectively. Alternately, the regression equation relating MS perfusion (PMS) to microsphere perfusion (PMICRO) was PMS = 0.62 PMICRO + 0.15, where the Pearson’s and Lin’s correlations were r = 0.89 and ρ = 0.77, respectively, while the RMSE and RMSD were 1.07 mL/min/g and 0.58 mL/min/g, respectively. All regression analyses are listed in Table 1 and displayed in Fig. 2, with corresponding Bland–Altman analyses also shown.
Accuracy and diagnostic performance. Regression analysis comparing (a) MI and (b) MS perfusion measurement to reference standard microsphere perfusion measurement. Bland–Altman analysis comparing (c) MI and (d) MS to reference standard microsphere perfusion measurement. AUC of the ROC for the (e) MI and (f) MS techniques in detection of functionally significant LAD stenosis, defined as stress perfusion less than 1.0 mL/min/g.
Diagnostic performance
The sensitivity, specificity, and accuracy of the MI technique were 80%, 98%, and 93%, respectively, with an AUC of the ROC of 0.99. Whereas the sensitivity, specificity, and accuracy of the MS technique were 93%, 80%, and 84%, respectively, with an AUC of the ROC of 0.95. All diagnostic performance analyses are shown in Table 2 with each ROC displayed in Fig. 2. Example MI and MS perfusion maps are also shown in Fig. 3. Finally, the CT dose index was 10.8 mGy while the size-specific dose estimate was 17.8 mGy.
Motion-immune (MI) versus motion-susceptible (MS) perfusion mapping. For a single acquisition in the presence of motion without registration, MI perfusion mapping (left panel) produced accurate hyperemic perfusion measurements in the LAD territory, i.e., the anterior left ventricular wall, while corresponding MS perfusion mapping (right panel) underestimated perfusion measurements in the LAD territory, as well as globally. Images are displayed as axial views. The color bar indicates quantitative perfusion in milliliters per minute per gram of myocardium.
Discussion
Indication of results
MI perfusion measurement agreed well with microsphere perfusion measurement, with near unity regression slope, excellent Pearson’s and Lin’s correlation, and minimal RMSE and RMSD. MI perfusion measurement also identified functionally significant stenoses with high accuracy, while only requiring two volume scans per measurement. Hence, accurate myocardial ischemia assessment, in the presence of motion without image registration, is feasible at a low dose (less than 3 mSv15,16,17,18).
Comparison to previous reports
Notably, the MS perfusion technique was previously validated versus microsphere perfusion, using fully co-registered, motion-corrected data. As a result of registration, the RMSE, RMSD, and diagnostic performance were all improved as compared to the present MS technique without registration. Hence, image registration does improve the accuracy of dynamic CT perfusion measurement in the presence of motion between image acquisitions. However, the MI perfusion technique, in the absence of registration, demonstrated a lower RMSE and RMSD with better diagnostic performance than the MS perfusion technique both with15 and without registration; highlighting the inherent limitations of image registration. In particular, registration cannot accurately reproduce the true quantitative CT-number or position of a target following deformation, whereby the degree of inaccuracy increases as the magnitude of motion and required deformation increases21,22. As dynamic CT perfusion techniques rely on the change in myocardial CT number over many cardiac cycles, any process that modifies the true CT number, i.e., registration, motion, partial volume averaging, beam hardening, etc., will reduce the accuracy of perfusion measurement (via underestimation) leading to higher false positive rates, reduced specificity, and increased positive predictive value14. Of course, static CT perfusion techniques are less impacted, only displaying gantry rotational-motion induced blurring, but dynamic techniques perform superiorly in detection of intermediate severity CAD, especially under stress conditions23,24. Yet, even dynamic techniques, such as the maximum slope model, underestimate perfusion, since they rely on small tissue volumes-of-interest (VOI) for measurement, where these VOIs are subject to contrast mass entry and exit over the measurement time, especially at hyperemia. Whereas the MI technique defines the entire myocardium as a single large VOI, where measurements are made prior to hyperemic transit; thus, solving the problem of perfusion underestimation. Hence, the MI technique represents a “best-of-both-worlds” solution as it provides the diagnostic advantages of dynamic CT perfusion, while mathematically eliminating the negative impacts of motion and registration.
Limitations
While referred to as “motion-immune,” it is important to note that the MI perfusion technique does not eliminate gantry motion blurring, which is inherent to cardiac CT image acquisition. Nevertheless, for motion between consecutive acquisitions caused by beat-to-beat variation (as in this study) or by imperfect breath-holding (to be studied in future work), the mathematics of the MI technique effectively eliminate the impact of such motion; thus, bypassing the need for and errors inherent to image registration. Additionally, the MI perfusion theory assumes that the unenhanced myocardial tissue density is relatively uniform within V1, i.e., that the average myocardial enhancement approximates the per-voxel enhancement with minimal variance. While this assumption holds true for patients without a prior history of myocardial infarction, chronically infarcted myocardium is known to be hypoattenuating secondary to reduced capillary density with increased adiposity25, where spotty myocardial calcifications may also be present (rarely)26. Hence, in the case of scar, both rest and stress perfusion may be underestimated, but the relation between coronary flow reserve and stress perfusion, i.e., coronary flow capacity, can be used to accurately discriminate scar from ischemia27.
Dynamic scanning was intentionally performed over many cardiac cycles to retrospectively develop and validate the two-volume MI technique. Hence, prospective validation remains necessary, and will require specialized acquisition timing of V1 and V2 at the base and peak of the aortic enhancement. Fortunately, a diluted test bolus protocol can be used, but requires extra contrast and radiation dose28. Alternatively, dynamic bolus tracking combined with a peak timing relation enables acquisition of V2 at approximately the aortic peak (within ± 2 cardiac cycles) while maintaining perfusion measurement accuracy29. In both cases, future clinical implementation will also require incorporation of breath-holding, to be initiated directly after contrast injection but before acquisition of V1, with exhalation after acquisition of V2. Meaning, V1 and V2 should be acquired during the same breath hold. Fortunately, such breath-holding is easily prompted on most clinical CT scanners and should not pose an issue to translation of the MI technique.
Additionally, prior work assessed perfusion in the LAD and LCx territories of the left ventricle along with the RCA territory of the right ventricle15, while this study only assessed perfusion in the LAD and LCx territories within the left ventricle. Specifically, image registration of V1 and V2 enables maximum intensity projection (MIP) image generation, where the resulting biventricular opacification can be used for segmentation of the main wall of the right ventricular for RCA perfusion measurements15. Without registration, however, only left ventricular segmentation can be performed. That said, if a triphasic injection protocol were to be employed, left and right ventricular opacification can be achieved within the V2 volume scan alone30, enabling biventricular perfusion measurement. Moreover, regarding the perfusion territories assessed, spatially matched virtual tissue plugs were used primarily for perfusion validation. In practice, however, minimum-cost-path perfusion territory or sub-territory assignment will be used for automatic delineation of the coronary perfusion territories, as previously validated31.
Finally, this study did not evaluate the impact of different registration algorithms on perfusion measurement accuracy. However, our prior CT perfusion results that employed image registration15 were discussed above. Additionally, as the raw data were previously acquired15, the limitations attributed to study design—central contrast injection, intracoronary adenosine, coronary balloon stenosis, small effective chest diameter, and acquisition timing—were all already addressed in detail and did not significantly impact the results of this work15,16,17,18,29.
Conclusion
The motion-immune FPA perfusion technique provides a solution to dynamic CT perfusion measurement in the presence of motion without image registration. By eliminating image registration and using only two volume scans for dynamic CT perfusion measurement, the motion-immune perfusion technique can improve the quantitative accuracy of CT-based CAD risk assessment while also reducing the radiation dose.
Data availability
Available upon request to the Corresponding Author, Sabee Molloi.
Abbreviations
- CAD:
-
Coronary artery disease
- CCC:
-
Lin’s concordance correlation coefficient
- \({CTDI}_{vol}^{32}\) :
-
32 Cm diameter volumetric CT dose index
- FFR:
-
Fractional flow reserve
- FPA:
-
First-pass analysis
- HU:
-
Hounsfield unit
- MI:
-
Motion-immune FPA perfusion technique
- MS:
-
Motion-susceptible FPA perfusion technique
- SSDE:
-
Size-specific dose estimate
- V1:
-
Volume scan 1
- V2:
-
Volume scan 2
References
Douglas, P. S. et al. Outcomes of anatomical versus functional testing for coronary artery disease. N. Engl. J. Med. 372, 1291–1300. https://doi.org/10.1056/NEJMoa1415516 (2015).
George, R. T. et al. Computed tomography myocardial perfusion imaging with 320-row detector computed tomography accurately detects myocardial ischemia in patients with obstructive coronary artery disease. Circ. Cardiovasc. Imaging 5, 333–340. https://doi.org/10.1161/CIRCIMAGING.111.969303 (2012).
Tonino, P. A. L. et al. Angiographic versus functional severity of coronary artery stenoses in the FAME study: Fractional flow reserve versus angiography in multivessel evaluation. J. Am. Coll. Cardiol. 55, 2816–2821. https://doi.org/10.1016/j.jacc.2009.11.096 (2010).
Rieber, J. et al. Cardiac magnetic resonance perfusion imaging for the functional assessment of coronary artery disease: A comparison with coronary angiography and fractional flow reserve. Eur. Heart J. 27, 1465–1471. https://doi.org/10.1093/eurheartj/ehl039 (2006).
Doukky, R. et al. Impact of appropriate use on the prognostic value of single-photon emission computed tomography myocardial perfusion imaging. Circulation 128, 1634–1643. https://doi.org/10.1161/circulationaha.113.002744 (2013).
Danad, I. et al. Comparison of coronary CT angiography, SPECT, PET, and hybrid imaging for diagnosis of ischemic heart disease determined by fractional flow reserve. JAMA Cardiol. 2, 1100–1107. https://doi.org/10.1001/jamacardio.2017.2471 (2017).
Branch, K. R. et al. Myocardial computed tomography perfusion. Cardiovasc. Diagn. Ther. 7, 452–462. https://doi.org/10.21037/cdt.2017.06.11 (2017).
Seitun, S. et al. CT myocardial perfusion imaging: A new frontier in cardiac imaging. Biomed. Res. Int. 2018, 7295460. https://doi.org/10.1155/2018/7295460 (2018).
Nieman, K. & Balla, S. Dynamic CT myocardial perfusion imaging. J. Cardiovasc. Comput. Tomogr. 14, 303–306. https://doi.org/10.1016/j.jcct.2019.09.003 (2020).
Lubbers, M. et al. Comprehensive cardiac CT with myocardial perfusion imaging versus functional testing in suspected coronary artery disease: The multicenter randomized CRESCENT-II trial. JACC Cardiovasc. Imaging 11, 1625–1636. https://doi.org/10.1016/j.jcmg.2017.10.010 (2018).
Pontone, G. et al. Dynamic stress computed tomography perfusion with a whole-heart coverage scanner in addition to coronary computed tomography angiography and fractional flow reserve computed tomography derived. JACC Cardiovasc. Imaging 12, 2460–2471 (2019).
Rochitte, C. E. et al. Computed tomography angiography and perfusion to assess coronary artery stenosis causing perfusion defects by single photon emission computed tomography: the CORE320 study. Eur. Heart J. 35, 1120–1130. https://doi.org/10.1093/eurheartj/eht488 (2013).
Rossi, A. et al. Dynamic computed tomography myocardial perfusion imaging. Circ. Cardiovasc. Imaging 10, e005505. https://doi.org/10.1161/CIRCIMAGING.116.005505 (2017).
Cademartiri, F. et al. Myocardial blood flow quantification for evaluation of coronary artery disease by computed tomography. Cardiovasc. Diagn. Ther. 7, 129–150. https://doi.org/10.21037/cdt.2017.03.22 (2017).
Hubbard, L. et al. Comprehensive assessment of coronary artery disease by using first-pass analysis dynamic CT perfusion: Validation in a swine model. Radiology 286, 93–102. https://doi.org/10.1148/radiol.2017162821 (2018).
Hubbard, L. et al. Functional assessment of coronary artery disease using whole-heart dynamic computed tomographic perfusion. Circ. Cardiovasc. Imaging 9, 1–8. https://doi.org/10.1161/circimaging.116.005325 (2016).
Hubbard, L. et al. Low-Radiation-dose stress myocardial perfusion measurement using first-pass analysis dynamic computed tomography: A preliminary investigation in a swine model. Invest. Radiol. 54, 774–780. https://doi.org/10.1097/rli.0000000000000613 (2019).
Hubbard, L., Malkasian, S., Zhao, Y., Abbona, P. & Molloi, S. Combining perfusion and angiography with a low-dose cardiac CT technique: A preliminary investigation in a swine model. Int. J. Cardiovasc. Imaging https://doi.org/10.1007/s10554-020-02130-x (2021).
Boone, J. et al. Size-specific dose estimates (SSDE) in pediatric and adult body CT examinations: report of AAPM task group 204. College Park, MD: American Association of Physicists in Medicine (2011).
Johnson, N. P., Gould, K. L., Di Carli, M. F. & Taqueti, V. R. Invasive FFR and noninvasive CFR in the evaluation of ischemia: What is the future?. J. Am. Coll. Cardiol. 67, 2772–2788. https://doi.org/10.1016/j.jacc.2016.03.584 (2016).
Ali, I., Alsbou, N., Jaskowiak, J. & Ahmad, S. Quantitative evaluation of the performance of different deformable image registration algorithms in helical, axial, and cone-beam CT images using a mobile phantom. J. Appl. Clin. Med. Phys. 19, 62–73. https://doi.org/10.1002/acm2.12246 (2018).
Huang, Y. et al. A quantitative evaluation of deformable image registration based on MV cone beam CT images: Impact of deformation magnitudes and image modalities. Phys. Med. 71, 82–87. https://doi.org/10.1016/j.ejmp.2020.02.016 (2020).
Schwarz, F. et al. Myocardial CT perfusion imaging in a large animal model: Comparison of dynamic versus single-phase acquisitions. JACC Cardiovasc. Imaging 6, 1229–1238. https://doi.org/10.1016/j.jcmg.2013.05.018 (2013).
Danad, I., Szymonifka, J., Schulman-Marcus, J. & Min, J. K. Static and dynamic assessment of myocardial perfusion by computed tomography. Eur. Heart J. Cardiovasc. Imaging 17, 836–844. https://doi.org/10.1093/ehjci/jew044 (2016).
Madaj, P. & Budoff, M. J. Risk stratification of non-contrast CT beyond the coronary calcium scan. J. Cardiovasc. Comput. Tomogr. 6, 301–307. https://doi.org/10.1016/j.jcct.2012.02.008 (2012).
Nance, J. W. Jr., Crane, G. M., Halushka, M. K., Fishman, E. K. & Zimmerman, S. L. Myocardial calcifications: Pathophysiology, etiologies, differential diagnoses, and imaging findings. J. Cardiovasc. Comput. Tomogr. 9, 58–67. https://doi.org/10.1016/j.jcct.2014.10.004 (2015).
Johnson, N. P. & Gould, K. L. Integrating noninvasive absolute flow, coronary flow reserve, and ischemic thresholds into a comprehensive map of physiological severity. JACC Cardiovasc. Imaging 5, 430–440. https://doi.org/10.1016/j.jcmg.2011.12.014 (2012).
Hubbard, L., Malkasian, S., Zhao, Y., Abbona, P. & Molloi, S. Contrast-to-noise ratio optimization in coronary computed tomography angiography: Validation in a swine model. Acad. Radiol. 2, 1–11 (2018).
Hubbard, L., Malkasian, S., Zhao, Y., Abbona, P. & Molloi, S. Timing optimization of low-dose first-pass analysis dynamic CT myocardial perfusion measurment: Validation in a swine model. Eur. Radiol. Exp. 3(16), 1–9 (2019).
Scholtz, J.-E. & Ghoshhajra, B. Advances in cardiac CT contrast injection and acquisition protocols. Cardiovasc. Diagn. Ther. 7, 439–451. https://doi.org/10.21037/cdt.2017.06.07 (2017).
Malkasian, S., Hubbard, L., Dertli, B., Kwon, J. & Molloi, S. Quantification of vessel-specific coronary perfusion territories using minimum-cost path assignment and computed tomography angiography: Validation in a swine model. J. Cardiovasc. Comput. Tomogr. 12, 425–435 (2018).
Acknowledgements
This work was supported, in part, by the Department of Radiological Sciences at the University of California, Irvine, by the American Heart Association under award numbers 17CPRE33650059 and 19TPA34910176, and by the National Heart Lung and Blood Institute of the National Institutes of Health under award number 1F30HL13728801A1. Please also note that this work was presented previously as an oral abstract at the 16th Annual Scientific Meeting of the Society of Cardiovascular Computed Tomography (JCCT, Volume 15, Issue 4, Supplement, July-August 2021, Page S29, Abstract 265).
Author information
Authors and Affiliations
Contributions
All data was acquired by all the authors. All data was analyzed by author L.H. The manuscript was then drafted by L.H. and revised by all the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hubbard, L., Malkasian, S. & Molloi, S. Dynamic CT myocardial perfusion without image registration. Sci Rep 12, 12608 (2022). https://doi.org/10.1038/s41598-022-16573-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-16573-w
This article is cited by
-
Evaluation and timing optimization of CT perfusion first pass analysis in comparison to maximum slope model in pancreatic adenocarcinoma
Scientific Reports (2023)
-
Dynamic myocardial CT perfusion imaging—state of the art
European Radiology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.