Abstract
We evaluated the effect of Acetyl-cholinesterase-inhibitors (AChEIs) on cognitive decline and overall survival in a large sample of older patients with late onset Alzheimer’s disease (LOAD), vascular dementia (VD) or Lewy body disease (LBD) from a real world setting. Patients with dementia enrolled between 2005 and 2020 by the "Alzheimer's Disease Research Centers" were analysed; the mean follow-up period was 7.9 years. A 1:1 propensity score matching was performed generating a cohort of 1.572 patients (786 treated [AChEIs +] and 786 not treated [AChEIs-] with AChEIs. The MMSE score was almost stable during the first 6 years of follow up in AChEIs + and then declined, while in AChEIs− it progressively declined so that at the end of follow-up (13.6 years) the average decrease in MMSE was 10.8 points in AChEIs- compared with 5.4 points in AChEIs + (p < 0.001). This trend was driven by LOAD (Δ-MMSE:−10.8 vs. −5.7 points; p < 0.001), although a similar effect was observed in VD (Δ-MMSE:−11.6 vs. −8.8; p < 0.001). No effect on cognitive status was found in LBD. At multivariate Cox regression analysis (adjusted for age, gender, dependency level and depression) a strong association between AChEIs therapy and lower all-cause mortality was observed (H.R.:0.59; 95%CI: 0.53–0.66); this was confirmed also in analyses separately conducted in LOAD, VD and LBD. Among older people with dementia, treatment with AChEIs was associated with a slower cognitive decline and with reduced mortality, after a mean follow-up of almost eight years. Our data support the effectiveness of AChEIs in older patients affected by these types of dementia.
Similar content being viewed by others
Introduction
Dementia is a major health problem in older populations, involving approximately 47 million worldwide1. In the U.S. the prevalence of dementia is about 15% in people over 68 years of age2, and Alzheimer’s disease (AD) represents the most frequent type, affecting 5.5 million people2; late-onset Alzheimer’s disease (LOAD) is the most common form of AD, with an onset over 65 years of age. Despite the recent and much-debated approval of Aducanumab by FDA for AD treatment, Acetyl-cholinesterase-inhibitors (AChEIs), such as donepezil, galantamine and rivastigmine, represent the first line pharmacological treatment options in patients with AD, with the aim of treating symptoms and slowing the natural course of the disease3,4,5. Although the efficacy of AChEIs has been evaluated in different randomized double-blind controlled trials (RCTs) conducted in subjects with AD6,7,8, as well as in patients affected by vascular dementia (VD)9,10, their efficacy in the real world setting has been questioned7,11. Doubtless, RCTs represents the “gold standard” method to evaluate the treatment outcomes12, but their results may be difficult to generalize in daily clinical practice due to the highly selected cohort included which only partially represents the real-life population13,14. Conversely, observational studies have the advantage of evaluating the effectiveness and safety of drugs in non-selected cohorts of patients. At present, only a few observational investigations have investigated how AChEIs treatment may influence cognitive decline in patients with LOAD or other forms of dementia15,16,17,18. However, definitive results were not obtained, especially concerning the effect of long-term treatment. Therefore, the aim of the present study was to evaluate the rate of cognitive decline, as well as the overall survival, in a large sample of patients affected by dementia, treated or not treated with AChEIs in a real-world setting.
Results
Characteristics of the study population
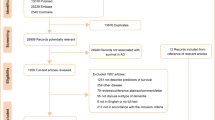
Overall, 3054 patients (1537 females, mean age 76.4 ± 5.6 years) met the inclusion criteria and were included into the study (Fig. 1). The baseline characteristics of the unmatched and matched groups and their relative medication use are presented in Table 1. In the entire cohort, LOAD resulted the first cause of dementia, followed by VD and LBD. As regards the AChEI + group, at baseline 62% of patients received donepezil, 24% rivastigmine and 14% galantamine. Before conducting the propensity score matching, AChEI− patients were older, more frequently males and required higher levels of assistance (all p < 0.001). Notably, the prevalence of diabetes and depression was higher in AChEI + patients (p < 0.001) (Table 1); moreover, these patients were more frequently treated with antidiabetic agents (p = 0.03), antidepressants (p < 0.001), and antipsychotics (p < 0.001), while received less frequently anxiolytic drugs (p = 0.01) compared with AChEI− patients. After the matching process, 786 patients were included into each group and, as expected, no more differences were observed. The mean follow-up period of the matched pairs was 7.9 ± 5.6 years [min: 2.3–max: 13.6 years].
ChEIs treatment and cognitive decline
In the whole sample, the average MMSE score decreased in both groups over time (p for trend < 0.001). Specifically, the curve describing the descent of MMSE score in AChEI + dementia patients was almost stable during the first 6 years, while that of AChEI− patients progressively declined, so that the two curves began to diverge after 4 years of follow-up. In the following years, a greater reduction in MMSE score was observed in AChEI- patients; therefore, at the end of follow-up the average decrease in MMSE score in this group (Δ:−10.8 points) was much greater compared to that observed AChEI- patients (Δ:−5.4 points) (p < 0.001) (Fig. 2, Panel A).
This trend was substantially driven by the LOAD patients (Fig. 2, Panel B). Indeed, in this group the average MMSE score during the follow-up decreased in both groups (p for trend < 0.001), with a greater reduction at the end of follow-up in AChEI- patients (Δ:−10.8) compared to AChEI + (Δ:−5.4) (p < 0.001). The MMSE score in AChEI + was almost stable during the first 6 years, and the two curves began to diverge after 4 years of followup.
As reported in Supplementary Table 1, during the first 6 years of follow-up, most of LOAD AChEIs + patients displayed a stable/improved MMSE score; after that, MMSE progressively declined in all individuals.
The curves describing the descent of MMSE in patients with VD showed a different trend (Fig. 2, Panel C). The average MMSE score started to decrease immediately after baseline observation in both AChEI + and AChEI− patients; however, the two curves began to diverge after about 3 years, so that a greater cognitive decline was observed in AChEI− (Δ:−11.6) compared to AChEI + (Δ: −8.8) at the end of follow up (p < 0.001). In the VD group, most of patients treated with AChEIs displayed a stable/improved MMSE score only during the period of follow-up between 1.6 and 3.2 years; after that, MMSE substantially declined in all individuals.
As regards the few patients affected by LBD (n = 124 after matching), our analysis did not show any significant difference in the rate of cognitive decline based on presence or absence of the treatment with AChEIs (data not shown).
We performed additional analyses in order to examine the effect of AChEIs treatment on change over time in MMSE score. This was done by estimating the change in MMSE score over time according to AChEIs treatment (Supplementary Table 2). Overall, both groups of patients experienced a significant decline in MMSE score over the 8-year period (all p values < 0.001). However, the differences between the two groups became more pronounced as time progressed. In the fully adjusted random effect model, patients using AChEIs had an estimated average decline per year lower than those of patients not using AChEIs and the difference between the slopes (0.60 point per year) was statistically significant (p 0.002).
AChEIs treatment and overall survival
Kaplan–Meier curves comparing the overall survival in the whole sample (adjusted—after matching) between AChEI + and AChEI- patients (Fig. 3, Panel A), LOAD (Fig. 3, Panel B) and VD (Fig. 3, Panel C) demonstrated lower mortality rates in patients receiving the treatment (log-rank test, p < 0.0001 for all dementia and LOAD; p = 0.001 for subjects with VD).
At univariate Cox regression analysis, the treatment with AChEIs (HR: 0.61, 95%CI: 0.54–0.68; p < 0.0001), age (for 1 year increase, HR:1.04, 95%CI: 1.03–1.05, p < 0.0001), sex (males vs females, HR:0.64, 95%CI: 0.57–0.71, p < 0.0001), dependency level (HR:1.25, 95%CI: 1.20–1.30, p < 0.0001), and diagnosis of depression (HR: 1.32, 95% CI: 1.26–1.38, p < 0.0001) were independently associated with total mortality in all patients with dementia (unadjusted sample—before matching). At multivariate Cox regression analysis, after adjustment for age, gender, dependency level and depression and baseline MMSE, a strong association between AChEIs therapy and lower all-cause mortality was confirmed, also in sub-group analyses separately conducted in patients with LOAD and VD (Table 2). Similar significant results emerged in the few patients affected by LBD (Supplementary Table 3).
Discussion
We evaluated the effect of the treatment with AChEI on the rate of cognitive decline and on long-term overall survival in a large sample of patients from the National Alzheimer’s Coordinating Center Uniform Data Set (NACC UDS). Indeed, the impact of these drugs in patients affected by dementia in the “real world” has not been systematically evaluated and has been sometimes questioned7,11. In this regard, the National Institute for Health and Care Excellence (NICE, UK) in its first technology appraisal guidance 111 published in 2006 (and successively modified in 2011 after strong pressures by relatives/caregivers’ organizations), gave a negative opinion on the use of AChEI in patients with dementia. The question of the real effectiveness of AChEI is of great interest especially in view of the recent and discussed approval by FDA of Aducanumab for the treatment of AD.
A first data emerging from our study is that AChEIs were preferentially prescribed to “younger” and female patients with dementia, demonstrating a better performance in the basic and complex activities of daily living. This may depend on the fact that these drugs are approved for the treatment of the mild-moderate stages of AD only, but might also highlight an ageist attitude or a selection bias. For this reason, we matched the patients treated and not treated with AChEIs in order to obtain two comparable groups.
AChEIs treatment and cognitive decline
All patients with dementia treated with AChEIs showed a significant slower rate of cognitive decline (as measured by MMSE, although we noted some differences between LOAD, VD and LBD. In LOAD, AChEIs substantially stabilized the cognitive performance for a period of about 6 years after baseline, while in VD a significant slowing down of the cognitive function was observed only after about two years of treatment. It should be noted the faster rate of cognitive decline observed in VD compared with LOAD subjects, in good agreement with literature data19,20.
Currently, AChEIs are indicated only for the treatment of AD. On the contrary, their use in VD is considered “off label”21,22, although their efficacy has been suggested also in this type of dementia9,10 and it seems to be confirmed, also on long term period, by our study. Based on our results, patient with VD should be offered treatment with AChEIs also in the absence of comorbid AD, LBD or Parkinson’s disease dementia, as currently suggested by the National Health System (NHS, UK) and FDA (U.S.)21,22. On the other hand, AChEIs seem to be ineffective, as regards a possible effect on cognitive decline, in patients with LDB; however, we have to underline that the small size of the sample might have prevented the achievement of statistical significance. This result indirectly supports the conclusions of Rolinski et al. (Cochrane database review)23, who concluded that the effect of AChEIs in DLB remains unclear, rather than those of Matsunaga et al.24, who found a beneficial effect of AChEIs for LBD treatment.
In our LOAD cohort, the MMSE decline was slower compared to similar previous studies with AChEI25. However, we have to consider that: (a) the model of Mendiondo et al.26 reports a drop of 1.45 point per year in AD patients with MMSE score 24/30; since our patients started from an average MMSE over 26/30, we could expect an even smaller decline; (b) other studies from “real world” reported a slower MMSE decline compared to the results of Xu et al.27,28,29; (c) most important, previous studies mainly enrolled patients with moderate/severe AD, and this is associated with a faster MMSE decline26. Indeed, the baseline MMSE of our NAAC patients was much higher when compared with other cohorts, and we mainly included patients with mild form of dementia (79% had a MMSE ≥ 26/30.). This was probably due to: (a) early diagnosis of dementia among the NAAC clinics, due to different reasons (e.g. advanced diagnostic tools, increased awareness of population sensitized about cognitive decline); (b) exclusion of patients prematurely lost at follow-up (due to too short follow-up and partial lack of data) which means “accidental” exclusion of patients with more advanced form of dementia. Compared to those included in the final sample, patients excluded from the analysis had lower MMSE score and lower rate of AChEI treatment (51.5% vs. 70%), confirming that a higher number of patients with poor cognitive performance and not treated with ACheEI were excluded.
The evolution of cognitive decline and dependency before the treatment were not taken into account in our study; of consequence, other factors conducting the patient (or the physician) to accept AChEIs treatment were not considered (e.g. financial problem, last treatment failure, tolerance for side effects, personal believes, etc.). This is an important point in order to enforce the design and correct use of clinical registries for future confirmation studies. Indeed, although only RCTs could really obtain comparable groups, it is almost impossible to organize such a long RCTs, especially in older patients with dementia.
AChEIs treatment and overall survival
We also evaluated the effect of AChEIs on long-term survival in patients affected by dementia. Different parameters appeared to be independently associated with survival at multivariate analysis, including age, sex, levels of dependency and diagnosis of depression. These factors predicted survival not only in the whole sample but also in LOAD, VD and LDB groups, separately. With regards to the treatment with AChEIs, it was associated with a substantial reduction of total mortality in the entire cohort, with a 40% reduction observed in all patients, 33% in LOAD, 25% in VD, and 62% in LDB (all p < 0.001).
As shown by the Kaplan-Meyer curves, the effect of AChEIs on survival was observed after a period of about 2 years in the whole sample as well as in LOAD patients, while in VD patients AChEIs appeared to have an early effect on survival, soon after starting the treatment.
Our findings strengthen the results of other sporadic observational studies reporting lower mortality rates in patients with dementia treated with AChEIs. By analyzing data from the Swedish Dementia Registry (SveDem—mean follow-up: 17 months), Nordstrom et al. found a significant reduction of mortality (HR: 0.64) after multivariate analysis and matching30. Mueller et al. found a reduction of the risk of death by more than 20% in AD patients treated with AChEIs, after a median period of follow-up of 3 years31. More recently, Xu et al. compared a large sample of patients with Alzheimer’s dementia from the SveDem (mean follow-up: 5 years). These authors found that the use of AChEI was associated with a 27% lower risk of death (H.R.: 0.73) compared with non-users (30). However, previous studies evaluated a shorter follow-up, and no study had the opportunity of following the patients for a mean period of 7.9 years, as we did. Thus, our results are very similar in magnitude to those previously published. It is not known why AChEI treatment could increase survival in patients with dementia, and several mechanisms have been proposed including: (a) better compliance of patients actively treated with AChEI in life-style, diet, and management of their medical problems (even better caregiving); (b) AChEIs might reduce behavioral and psychological symptoms of dementia (BPSD), as suggested by some authors32, and this in turn would reduce the use of antipsychotic drugs and related mortality; (c) AChEIs may reduce peripheral cytokine production and increase vagal nerve activity30, and this has been associated with reduced cardiovascular mortality; (d) the slowdown of cognitive decline obtained by AChEIs therapy might contribute to the slowdown frailty progression thus reducing mortality rates. Finally, it might depend on “confounding by indication” bias, since healthier subjects are more often prescribed AChEI; however, after propensity score matching no differences emerged in the case–control cohorts.
Limitations
We must acknowledge some important limitations of the study. 1. Participants comprising the NACC dataset represent a convenience sample, including clinical-referrals and community-based U.S.; of consequence, the NACC sample are not representative of all the U.S. population, partially reducing the generalizability of our results. 2. We excluded patients with short follow-up (< 2 visits; most had incomplete data); in this way we excluded many patients with moderate-severe dementia, and this might have influenced the rate of MMSE decline. However, since we have excluded more not-treated patients compared to treated-patients, this should have no effect on the association between treatment and MMSE change. 3. Despite performing the propensity score matching, the effects of some residual confounding may have results in not firm conclusions. 4. Considering the general clinical stability of patients after the diagnosis of dementia, we cannot exclude that these patients were evaluated much earlier compared to the standard of care applied in other Countries. 5. The comorbidities observed may be different from those affecting patients with dementia in other regions of the world, partially limiting the generalizability of our findings. 6. We cannot exclude that some of AD patients had a rapidly progressive Alzheimer’s disease, which may have influenced the cognitive decline during the follow-up period; indeed, previous investigations have estimated this phenotype in about 17% of subjects with mild AD33,34.
Conclusions
Our results, obtained from the National Alzheimer’s Coordinating Center Uniform Data Set (NACC UDS), suggest that older people with dementia who are prescribed AChEIs have a slower decline in cognitive performance and a reduced mortality (by approximately 40%) after a follow-up of almost eight years. Basically, an early stabilization of cognitive performance, followed by a reduction in total mortality was observed in LOAD; in contrast, an early reduction in overall mortality and a subsequent slowdown in cognitive decline was observed in VD. Among subjects with LBD, the effect of AChEIs was negligible as regards cognition, but an independent effect of AChEIs on overall survival was observed. Our data support the effectiveness of AChEIs in older patients affected by these types of dementia.
Methods
Population
Data have been obtained from the National Alzheimer’s Coordinating Center Uniform Data Set (NACC UDS), which is a nationwide repository for longitudinal data collected from approximately 34 current or previously NIA funded Alzheimer's Disease Research Centers (naccdata.org). In the present investigation, data collected between 2005 and June 2020 were analysed. Patients were included into the present study if: (I) they were aged ≥ 65 years at baseline; (II) they had a diagnosis of dementia, including LOAD, vascular dementia (VD) or Lewy body disease (LBD); (III) they had the baseline visit and at least two in-person visits with evaluation of MMSE with two years of follow-up; (IV) the MMSE score was ≥ 10/30 (mild to moderate dementia). Conversely, participants were excluded if: (I) they had less than three in person-visits and/or relative MMSE consecutive evaluations; (II) they were receiving memantine; (IV) the MMSE was < 10/30 (severe dementia—AChEI are not indicated). The baseline registration in NACC registry was initiated at the time of the dementia diagnosis when the treatment started. Comorbidities and functional status were evaluated at baseline (time 0) by the medical team of the clinic by means of medical history, medical records, medical examination, and blood chemistry tests.
Local ethics committees at each of the sites approved the study, and all participants provided written informed consent. All procedures were performed in accordance with the relevant guidelines and regulations.
Dementia’s definitions
Dementia was defined as meeting criteria for AD35 or VD or LBD36,37 defined as (1) objective cognitive impairment (i.e., performances falling greater than 1.5 standard deviations outside the age-adjusted normative mean) in at least 2 cognitive systems (memory, language, attention or executive functioning); and (2) cognitive impairment contributes directly to impaired activities of daily living.
Patients receiving available AChEIs (donepezil, rivastigmine or galantamine) were labelled as AChEIs recipients (ACheIs +). The ACheIs exposure was assumed to be constant.
Cognitive decline evaluation
The Mini Mental State Examination (MMSE), which represents the most widely used cognitive assessment tool in dementia38 has been administered during the outpatient visits to evaluate the rate of cognitive decline over time in AChEI + and non-recipients (AChEI−). As known, MMSE uses a 30-point score to assess orientation, short-term and delayed recall, calculations, language interpretation, naming, and praxis. Higher scores represent better cognitive performance.
Estimation of propensity scores
Before starting the analysis, 1965 patients were excluded (996 AD, 786 VD, 183 LBD) since they had a follow-up shorter than two years (most had incomplete data too) thus compromising the estimation of MMSE decline and mortality rates over time. These patients were older (mean age 82.1 ± 6.3 years), and 63.8% were males. MMSE score at baseline and after one year was available only in 40.4% (n. 787, mean MMSE 14.2/30) and 21.6% (n. 431, mean MMSE 11.3/30) of cases, respectively. Mortality data for these patients were available only for 6.5% of the cases. 51.5% of excluded patients assumed AChEI, compared to 70% of patients included into the study.
The population of interest included 3054 individuals with a mean MMSE score of 26.8/30; in more detail, 79% had a MMSE score ≥ 26, 16% had a MMSE between 20/30 and 26/30, and only 5% had a MMSE < 20/30. They were firstly divided into two groups, based on the presence or absence of a treatments with AChEI. Subsequently, due to differences in baseline covariates between AChEIs users and non-users, a 1:1 propensity score matching was performed. Specifically, each AChEI + patient was matched with an AChEI- patient with a similar propensity score, based on nearest-neighbour matching without replacement, using a caliper width equal to 0.1 of the SD of the logit of the propensity score, which indicate balance in covariates between groups39.
Based on this, a propensity score-matched cohort of 1.572 patients (786 AChEIs + and 786 AChEIs−) was generated, using a non-parsimonious multivariable logistic regression model to calculate, for each patient, a propensity score, using all covariates shown in Table 1, mean MMSE and mean follow-up length.
Compared with subjects included into the study after propensity score matching, the subjects excluded (n. 1482) were younger (72.8 ± 3.1 vs. 75.1 ± 2.8; p < 0.001), more frequently affected by VD (56.4% vs. 13.1%), less frequently affected by LOAD (38% vs. 78.9%), had a much lower average MMSE score (13.5 ± 3.1 vs. 27.6 ± 0.9; 45.1% of them ad a MMSE score < 10/30), and more frequently required assistance in basic activities of daily living (17.3% vs. 7.6%) (all p < 0.001).
Statistical analysis
Pearson Chi-square and Wilcoxon rank-sum tests for the pre-matched population, and McNemar’s test and paired sample t-test for the matched population were used to compare baseline characteristics between the two groups as appropriate.
Based on the propensity score matching (PSM) method, the clinical baseline data of the two groups were balanced, and the regression model variables included age, sex, previous diabetes mellitus (DM), hypertension, stroke, smoking, alcohol consumption and systolic blood pressure (SBP), diastolic blood pressure (DBP) and heart rate of the patients after inclusion. The propensity score of each patient was calculated by the 1:1 nearest matching method, and caliper matching was employed to limit the logarithmic standard deviation of the propensity score to 0.10 to prevent the difference between each pair of matched individuals. Based on this, a propensity score-matched cohort of 1.572 patients (786 AChEIs + and 786 AChEIs−) was generated, using a non-parsimonious multivariable logistic regression model to calculate, for each patient, a propensity score, using as covariates: age at baseline (years), sex, years of formal education), dependence level, requirement of assistance for complex and basic activities, dementia type, comorbidities (atrial fibrillation, stroke/TIA, hypertension, diabetes, hypercholesterolemia, thyroid disease, depression in the last two years, urinary incontinence, anti-adrenergic agents, anxiolytic agents and anti-hypertensive drugs, antidepressants, antipsychotic agents, antidiabetic agents, lipid-lowering medications, baseline MMSE, and length of follow-up.
Difference in cognitive trajectories over time (MMSE score change) between patients using AChEIs and those not using AChEIs were estimated using mixed-effects repeated measures models of unstructured-variance–covariance matrix. To formally test the hypothesis of different MMSE slops over time the interaction term “time*AChEI treatment was fitted in the model. The model was adjusted for age, sex, education, number of medical follow-up visits and follow-up duration.
Multivariate Cox proportional hazards models were used to estimate the adjusted hazard ratios (HRs) and relative 95% confidence intervals (95%CI) for all-cause mortality in AChEIs + compared to AChEIs− in the unadjusted sample (before propensity score matching—whole sample, LOAD, VD, and LBD). Proportionality assumptions were examined through visual inspection of log–log survival curves, and analytical assessments using covariates-by-time interactions in the Cox model.
The association between AChEIs administration and all-cause mortality during the follow-up period was graphically evaluated using the Kaplan–Meier method. Subgroup analyses were also performed to examine the association between AChEIs administration and mortality among patients with LOAD and vascular dementia. Statistical significance was defined as p < 0.05. Statistical analyses were performed using SPSS package version 20.0 (SPSS, Chicago, IL, USA).
References
Mattiuzzi, C. & Lippi, G. Worldwide disease epidemiology in the older persons. Eur. Geriatr. Med. 11, 147–153. https://doi.org/10.1007/s41999-019-00265-2 (2020).
Arvanitakis, Z., Shah, R. C. & Bennett, D. A. Diagnosit and management of dementia: Review. JAMA 322, 15891599 (2019).
Takeda, A. et al. A systematic review of the clinical effectiveness of donepezil, rivastigmine and galantamine on cognition, quality of life and adverse events in Alzheimer’s disease. Int. J. Geriatr. Psychiatry. 21, 17–28. https://doi.org/10.1002/gps.1402 (2006).
Marucci, G. et al. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology https://doi.org/10.1016/j.neuropharm.2020.108352 (2020).
Wilkinson, D. G., Francis, P. T., Schwam, E. & Payne-Parrish, J. Cholinesterase inhibitors used in the treatment of Alzheimer’s disease: the relationship between pharmacological effects and clinical efficacy. Drugs Aging. 21, 453–478. https://doi.org/10.2165/00002512-200421070-00004 (2004).
Birks, J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD005593 (2006).
Knight, R., Khondoker, M., Magill, N., Stewart, R. & Landau, S. A systematic review and meta-analysis of the effectiveness of acetylcholinesterase inhibitors and memantine in treating the cognitive symptoms of dementia. Dement. Geriatr. Cogn. Disord. 45, 131–151. https://doi.org/10.1159/000486546 (2018).
Dou, K. X. et al. Comparative safety and effectiveness of cholinesterase inhibitors and memantine for Alzheimer’s disease: A network meta-analysis of 41 randomized controlled trials. Alzheimers Res. Ther. 10, 126. https://doi.org/10.1186/s13195-018-0457-9 (2018).
Erkinjuntti, T. et al. Efficacy of galantamine in probable vascular dementia and Alzheimer’s disease combined with cerebrovascular disease: A randomised trial. Lancet 359, 1283–1290. https://doi.org/10.1016/S0140-6736(02)08267-3 (2002).
Kavirajan, H. & Schneider, L. S. Efficacy and adverse effects of cholinesterase inhibitors and memantine in vascular dementia: A meta-analysis of randomised controlled trials. Lancet Neurol. 6, 782–792. https://doi.org/10.1016/S1474-4422(07)70195-3 (2007).
Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics (Review). Mol. Med. Rep. 20, 1479–1487. https://doi.org/10.3892/mmr.2019.10374 (2019).
Kennedy-Martin, T., Curtis, S., Faries, D., Robinson, S. & Johnston, J. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials 16, 495. https://doi.org/10.1186/s13063-015-1023-4 (2015).
Cherubini, A., Del Signore, S., Ouslander, J., Semla, T. & Michel, J. P. Fighting against age discrimination in clinical trials. J. Am. Geriatr. Soc. 58, 1791–1796 (2010).
Peres-Zepeda MU, Cherubini A., Garcia Pena C, Zengarini E., Gutierrez Robledo LM. Clinical trials on aging research. In Aging research- methodological issues. Garcia Pena C, Gutierrez Robledo LM, Peres-Zepeda MU, (Springer) 115–127 (2018).
Nelson, P. T. et al. Acetylcholinesterase inhibitor treatment is associated with relatively slow cognitive decline in patients with Alzheimer’s disease and AD + DLB. J. Alzheimers Dis. 16, 29–34. https://doi.org/10.3233/JAD-2009-0926 (2009).
Calabria, M., Geroldi, C., Lussignoli, G., Sabbatini, F. & Zanetti, O. Efficacy of acetyl-cholinesterase-inhibitor (ACHEI) treatment in Alzheimer’s disease: A 21-month follow-up ‘“real world”’ study. Arch. Gerontol. Geriatr. 49, e6–e11. https://doi.org/10.1016/j.archger.2008.07.006 (2009).
Santoro, A. et al. Effects of donepezil, galantamine and rivastigmine in 938 Italian patients with Alzheimer’s disease: A prospective, observational study. CNS Drugs 24, 163–176. https://doi.org/10.2165/11310960-000000000-00000 (2010).
Vaci, N. et al. Real-world effectiveness, its predictors and onset of action of cholinesterase inhibitors and memantine in dementia: Retrospective health record study. Br. J. Psychiatry https://doi.org/10.1192/bjp.2020.136 (2020).
Gill, D. P. et al. Differences in rate of functional decline across three dementia types. Alzheimer’s Dementia. 9, S63–S71 (2013).
Pilon, M. H. et al. Differences in rate of cognitive decline and caregiver burden between alzheimer’s disease and vascular dementia: A retrospective study. Neurology (ECronicon). 2, 278–286 (2016).
Kumar A, Sharma S. Donepezil. [Updated 2020 Aug 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513257/
National Institute for Health and Care Excellence (UK). Dementia: Assessment, management and support for people living with dementia and their carers. London: National Institute for Health and Care Excellence (UK); 2018 Jun. (NICE Guideline, No. 97.) 11, Cholinesterase inhibitors and memantine for dementia. Available from: https://www.ncbi.nlm.nih.gov/books/NBK536484/
Rolinski, M., Fox, C., Maidment, I. & McShane, R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane Database Syst. Rev. (3), CD006504 (2012).
Matsunaga, S., Kishi, T., Yasue, I. & Iwata, N. Cholinesterase inhibitors for lewy body disorders: A meta-analysis. Int. J. Neuropsychopharmacol. 19, pyv086 (2016).
Hong, Xu. et al. Long term effects of cholinesterase inhibitors on cognitive decline and mortality. Neurology https://doi.org/10.1212/WNL.0000000000011832 (2021).
Mendiondo, M. S., Wesson Ashford, J., Kryscio, R. J. & Schmitt, F. A. Modelling mini mental state examination changes in alzheimer’s disease. Statist. Med. 19, 1607–1616 (2000).
Nelson, P. T. et al. Acetylcholinesterase inhibitor treatment is associated with relatively slow cognitive decline in patients with alzheimer’s disease and AD + DLB. J Alzheimers Dis. 16(1), 29–34. https://doi.org/10.3233/JAD-2009-0926 (2009).
Wattmo, C., Wallin, Å. K., Londos, E. & Minthon, L. Predictors of long-term cognitive outcome in Alzheimer’s disease. Alzheimer’s Res. Ther. 3, 23 (2011).
Karen, S., Tim, W., Karoline, K., Oliver, S. & Zuzana, S. T. W. Rate of cognitive decline in alzheimer’s disease stratified by age. J. Alzheimers Dis. 69(4), 1153–1160 (2019).
Nordström, P., Religa, D., Wimo, A., Winblad, B. & Eriksdotter, M. The use of cholinesterase inhibitors and the risk of myocardial infarction and death: a nationwide cohort study in subjects with Alzheimer’s disease. Eur. Heart J. 34, 2585–2591. https://doi.org/10.1093/eurheartj/eht182 (2013).
Mueller, C., Perera, G., Hayes, R. D., Shetty, H. & Stewart, R. Associations of acetylcholinesterase inhibitor treatment with reduced mortality in Alzheimer’s disease: A retrospective survival analysis. Age Ageing. 47, 88–94. https://doi.org/10.1093/ageing/afx098 (2018).
Tan, E. C. K. et al. Do Acetylcholinesterase inhibitors prevent or delay psychotropic prescribing in people with dementia? Analyses of the Swedish dementia registry. Am. J. Geriatric Psychiatry 28(1), 108–117 (2020).
Ba, M. et al. Alzheimer’s disease neuroimaging initiative. The prevalence and biomarkers’ characteristic of rapidly progressive Alzheimer’s disease from the Alzheimer’s Disease Neuroimaging Initiative database. Alzheimers Dement. (N Y) 3, 107–113. https://doi.org/10.1016/j.trci.2016.12.005 (2017).
Schmidt, C. et al. Rapidly progressive Alzheimer disease. Arch. Neurol. 68, 1124–1130. https://doi.org/10.1001/archneurol.2011.189 (2011).
McKhann, G. et al. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–944. https://doi.org/10.1212/wnl.34.7.939 (1984).
McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): Report of the Consortium on DLB International Workshop. J Alzheimers Dis. 9, 417-423 (2006); https://doi.org/10.3233/jad-2006-9s347.
Román, G. C. et al. Vascular dementia: Diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 43, 250–260. https://doi.org/10.1212/wnl.43.2.250 (1993).
Pezzotti P, Scalmana S, Mastromattei A, Di Lallo D; Progetto Alzheimer Working Group. The accuracy of the MMSE in detecting cognitive impairment when administered by general practitioners: a prospective observational study. BMC Fam. Pract. 9, 29 (2008); https://doi.org/10.1186/1471-2296-9-29.
Austin, P. C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar. Behav. Res. 46, 399–424 (2011).
Funding
The NACC database is funded by NIA/NIH Grant U01 AG016976. + NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI Robert Vassar, PhD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG005131 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD). National Alzheimer’s Coordinating Center Uniform Data Set, Grant/Award Number: U01 AG016976I.
Author information
Authors and Affiliations
Contributions
M.Z. and G.Z. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: G.Z.; M.Z.; Acquisition, analysis, or interpretation of data: M.Z., G.Z., S.V., A.C.; L.F.; Drafting of the manuscript: M.Z., G.Z. Critical revision of the manuscript for important intellectual content: G.Z., S.V.; S.F.; A.C. Statistical analysis: M.Z., G.Z. Supervision: G.Z. All authors had access to all the study data and read, contributed to, and approved the submission of the manuscript for final publication.
Corresponding author
Ethics declarations
Competing interests
AC reports personal fees from Nestle, personal fees from Bristol Myers Squibb and payment /honoraria from MSD, outside the submitted work; SV reports Consulting fees from Angelini SPA and Payment/honoraria from Abbot Vifor, outside from the submitted work. MZ, GZ and LF have no conflicts of interest to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zuin, M., Cherubini, A., Volpato, S. et al. Acetyl-cholinesterase-inhibitors slow cognitive decline and decrease overall mortality in older patients with dementia. Sci Rep 12, 12214 (2022). https://doi.org/10.1038/s41598-022-16476-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-16476-w
This article is cited by
-
Perioperative point-of-care-testing of plasmacholinesterases identifies older patients at risk for postoperative delirium: an observational prospective cohort study
BMC Geriatrics (2024)
-
A systematic review and meta-analysis of the effects of long-term antibiotic use on cognitive outcomes
Scientific Reports (2024)
-
Acetyl-cholinesterase-inhibitors reconsidered. A narrative review of post-marketing studies on Alzheimer’s disease
Aging Clinical and Experimental Research (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.