Abstract
Capacitive–resistive energy transfer therapy (CRet) is used to improve the rehabilitation of different injuries. This study aimed to evaluate and compare the changes in temperature and current flow during different CRet applications on upper and lower molars and incisors, with and without implants, on ten cryopreserved corpses. Temperatures were taken on molars and incisors with invasive devices and skin temperature was taken with a digital thermometer at the beginning and after treatments. Four interventions: 15 VA capacitive hypothermic (CAPH), 8 watts resistive (RES8), 20 watts resistive (RES20) and 75 VA capacitive (CAP75) were performed for 5 min each. All treatments in this study generated current flow (more than 0.00005 A/m2) and did not generate a significant temperature increase (p > 0.05). However, RES20 application slightly increased surface temperature on incisors without implants (p = 0.010), and molar with (p = 0.001) and without implant (p = 0.008). Also, CAP75 application increased surface temperature on molars with implant (p = 0.002) and upper incisor with implant (p = 0.001). In conclusion, RES8 and CAPH applications seem to be the best options to achieve current flow without an increase in temperature on molars and incisors with and without implants.
Similar content being viewed by others
Introduction
Dental implants are the most commonly used procedure to replace missing teeth1. A Dental implant consists of a piece of metal, usually titanium or titanium alloys, inserted and integrated into the bone, giving solid support for the final dental prosthesis. It is estimated that 12–18 million implants are inserted worldwide every year2. Furthermore, it is supposed to be one of the most common surgeries in the health field. Successfully integrated implants have a survival rate of more than 96.7% after 8 years3. Nowadays, the insertion of a dental implant requires a low-invasive surgical technique4. However, inflammation during the healing phase may be present, and the use of anti-inflammatory drugs (ibuprofen, indomethacin, diclofenac or celecoxib) are usually prescribed5.
Inflammation is a naturally occurring event following the early stages of tissue healing after an injury, or in this case, after a dental implant procedure. Ensuring a rapid and short inflammatory phase guarantees an early start of the proliferative phase. The invasion and proliferation of mesenchymal and endothelial (cells or tissue) will create the appropriate network for the dental implant osteointegration6. An excessive inflammatory reaction can cause the rejection of the implanted device7,8,9. This exacerbated inflammation can be multifactorial; some conditions or attitudes that conduce to rejection of implanted devices are: an inadequate surgical protocol, increased temperature of the bone bed, contamination of the surgical site and/or implanted device or an allergic adverse reaction7,8,9. After inflammation, the proliferative phase occurs, and new tissue grows to close the wound6. Any protocol or action towards increasing the speed of cell proliferation or migration results in favor of a successful osseointegration of dental implants. For this reason, capacitive-resistive energy transfer (CRet) can help increase the speed of cell proliferation and healing10. This technique is commonly used to treat muscular, bone, joint and tendinous lesions in the sports traumatology field11,12,13,14. CRet is a non-invasive electrothermic therapy classified as deep thermotherapy. Which consists of applying an electric current in the radiofrequency range of 300 kHz–1.2 MHz15. CRet therapy can be applied in two different treatment modes: capacitive and resistive. Capacitive mode is provided with an insulating ceramic layer and the energetic transmission generates heat in superficial tissue layers, with a selective action in tissues with low-impedance (water-rich)16. The resistive mode has no insulating ceramic layer; the radiofrequency energy passes directly through the body in the direction of the inactive electrode, generating heat in the deeper and more resistant tissues (with less water content)16.
The physiological effects of CRet therapy are generated by the application of an electromagnetic field, with a frequency of approximately 0.5 MHz, to the human body. The effects attributed to this technique include increased blood circulation, lymphatic effects, increased cell proliferation and, if desired, a thermal increase of deep and superficial structures15,17,18. Some of these reactions, such as increased blood perfusion, are related to an increase in temperature in the tissue. However, others, such as cell proliferation, appear to be more related to the flow of electric current17. It has been described that at 0.00005 A/m2 of current flow, the phenomenon of cell proliferation begins19.
Currently, there are five studies analyzing thermal changes and current flow in cadavers using CRet therapy15,16,18,20,21. These studies focus on the Achilles tendon region and the myotendinous junction of the gastrocnemius muscles15, on the capsular and intra-articular structures of the knee18, on the tendon and capsular structures of the glenohumeral joint20, on a clinical protocol for the elbow region21 and biceps femoris and quadriceps16. However, no study has been found that assesses changes in temperature and current flow in intraoral structures such as teeth or dental implants. The relevance of generating current flow and cell proliferation (cell proliferation stimulated by current flow) without excessively increasing or even decreasing the temperature could set a precedent for a more effective recovery of acute dental implant patients during their consequent inflammatory phase. It is unknown whether the presence of an implant can generate a higher temperature increase. For this reason, it seems interesting to study the differences in order to be able to perform safe treatments in living patients.
The objective was to evaluate and compare the changes in temperature and current flow of different CRet applications on upper and lower molars and incisors with and without implants by performing invasive measurements on cadaveric specimens.
The null hypothesis was that there are no significant temperature differences in the application of CRet therapy in cadaveric specimens with and without implants and that there is no current flow in cadaveric specimens.
The alternative hypothesis was that there are significant temperature differences in the application of CRet therapy in cadaveric samples with and without implants and that there is current flow in the cadaveric samples.
Results
The current flow was stable during the applications. The incisor region without implants showed values of 0.12 A ± 0.1 (CAPH), 0.23 A ± 0.2 (CAP75), 0.24 A ± 0.1 (RES8) and 0.36 A ± 0.2 (RES20). In the incisor region with implants, we found values of 0.24 A ± 0.14 (CAPH), 0.29 A ± 0.22 (CAP75), 0.27 A ± 0.09 (RES8) and 0.47 A ± 0.15 (RES20).
In the molar region without implants, we found values of 0.09 A ± 0.1 (CAPH), 0.18 A ± 0.19 (CAP75), 0.18 A ± 0.15 (RES8) and 0.27 A ± 0.24 (RES20). In the molar region with implants, values of 0.13 A ± 0.02 (CAPH), 0.26 A ± 0.03 (CAP75), 0.31 A ± 0.05 (RES8) and 0.47 A ± 0.08 (RES20) were found.
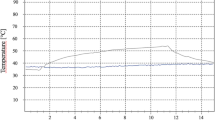
The temperature recorded during the different applications at the beginning and at the end of the treatment, both in the superficial region and in the upper and lower molar/incisor region, is shown in Table 1 for the incisors and in Table 2 for the molars.
In the between-group analysis of the incisors, statistically significant differences were found in the superficial region temperature of the RES20 application (p = 0.014), the upper incisors temperature of the CAP75 application (p = 0.013) and RES20 (p = 0.045), in the lower incisor’s temperature of the CAPH application (p = 0.010). In the within-group analysis of the incisors, statistically significant differences were found in the RES20 application in the superficial region temperature (p = 0.010) and in the upper incisor’s temperature (p = 0.006) in the non-implant group with an increase in temperature of 1.81° and 2.40° respectively.
In the between-group analysis of molars only, statistically significant differences were found in the lower molar temperature of the CAPH application (p = 0.028). In the within-group analysis of the molars, statistically significant differences were found in the superficial region temperature of the CAP75 and RES20 applications. In the CAP75 application, the group with implants (p = 0.002) and without implants (p = 0.033) showed a superficial temperature increase of 6.41° and 4.70°, respectively. In the RES20 application, the group with implants (p < 0.001) and without implants (p = 0.008) showed a superficial temperature increase of 4.41° and 3.32°, respectively.
Discussion
Based on the results obtained, the alternative hypothesis, that there are significant temperature differences in the application of CRet therapy in the cadaveric samples with and without implants and that there is current flow in the cadaveric samples, can be accepted. Therefore, the null hypothesis, that there are no significant temperature differences in the application of CRet therapy in cadaveric specimens with and without implants and that there is no current flow in cadaveric specimens, can be rejected. However, in two applications (RES8 and CAPH) no significant temperature differences were observed between the implant and non-implant groups.
The highest significant temperature rise produced in the upper and lower molar and incisor applications was only 2.4° of temperature at the surface level. In addition, the living tissue would likely experience an even lower temperature rise since, in these subjects, the blood actively circulates through the body dissipating heat to adjacent areas22. This process, called thermoregulation, prevents unwanted hyperthermia in nearby tissues, as well as excessive heat during treatment22.
As with any implantable device in hard tissues, dental implants need to be osseointegrated in the bone to be functional23. Osseointegration is an ordered cascade of events that lead to the integration of an implantable device into a hard-living tissue. Osseointegration follows the sequence of tissue healing, being among the first steps in the inflammatory phase. In a physiological environment, the inflammatory phase begins 10 min after the injury and takes hours to days to end24; a shorter time is considered better for the healing process. After the injury, platelets arrive, and first events occur, such as degranulation and histamine derived vasodilatation, leading to increased blood flow and decreased stream velocity. Limiting the inflammatory phase, we ensure to move as quickly as possible to the proliferative phase. A recent publication studying the effects of CRet on cell proliferation and migration showed that 6 h after the application, migration and proliferation were increased. In this same study, results after 12 h of the CRet application showed a slight decrease in the phosphorylation of p38 proteins and a slight increase of other proteins related to a MAPK pathway, possibly indicating that the CRet treatments might have an anti-inflammatory effect (as well as a proliferative induction/effect)10. Achieving cellular proliferation could be indicated in inflammatory pathologies that need tissue regeneration, especially during the first 2 weeks25,26.
The evolution from the inflammatory to the proliferative phase encompasses the formation of a new collagenous extracellular matrix and angiogenesis. The duration of this phase lasts from a few days to a few weeks. Fibroblasts will start to proliferate via integrins and migrate using an amoeboid movement from the healthy surrounding tissue into the wound, where the blood clot is forming, and inflammatory cells are present27. The proliferative phase also includes the formation of new blood vessels in the wound site, which is a mandatory prerequisite for tissue healing27.
In this way, CRet therapy could be a strategy to increase cell proliferation without increasing the temperature (which would be negative in an inflammatory phase of the tissues)19. This improvement in cell proliferation would result in less pain for patients and possible better consolidation of the implant in a shorter time15,17,18. However, this hypothesis should be verified in future studies with living patients.
This study has several limitations, which are described below. First, it is a study performed on cadavers in which there is no thermoregulation or active blood circulation. In living subjects, the body's thermoregulatory effect dissipates heat, probably producing more significant temperature increases in the cadaveric samples than those expected in living subjects22. Another limitation is found in the properties of the tissues. Another limitation is that cadaveric studies are performed with a small sample size, and despite being cryopreserved cadavers, the properties of the tissues may vary slightly from those of living subjects. Despite these limitations, the authors consider that the use of donor bodies has made it possible to know how different CRet applications affect the temperature and current flow values in the molar and incisor region and to know their effects and applicability before applying them to real patients.
In conclusion, all applications used in this study generated current flow and did not generate significant temperature increases in the tooth region with or without implants. However, the RES20 and CAP75 applications generated a thermal increase in some conditions. So, RES8 and CAPH applications seem to be the best options to achieve current flow without temperature increase in molars and incisors with and without implants. These basic science results may be the precedent for using RES8 and CAPH applications in living patients.
Methods
Study design
A cross-sectional study was conducted to determine the effect of electrical resistive/capacitive energy transfer of the T-Plus (Wintecare SA, Chiasso, Switzerland) device on temperature and current in the intraoral region (incisors and molars with and without implants) in cadaveric samples. The body donation program of the Faculty of Medicine and Health Sciences of the Universitat Internacional de Catalunya provided all the samples. This study was approved by the ethical committee “Comitè d’Ètica de Recerca (CER) of Universitat Internacional de Catalunya” with reference number CBAS-2019-17 approved on April 4, 2022.
Cadaveric sample
The sample consisted of 10 complete corpses (5 with implants and 5 without implants), cryopreserved and fresh. Measurements were taken on the right and left molars and the right and left incisors on each corpse. This generated a total sample of 10 molars with implants, 10 incisors with implants, 10 molars without implants and 10 incisors without implants. The cadavers were stored at 3 °C and kept at room temperature18 for 36 h before the study16. The mean age of the cadavers was 67.7 [± 6.0] years. None of the cadaveric specimens used for this study had evidence of trauma or surgical scars in the craniomandibular region.
Intervention
The power range of a T-Plus (Wintecare SA, Chiasso, Switzerland) (Fig. 1) device used in this study varies from 1 to 300 watts in resistive mode and from 1 to 450 VA in capacitive mode15. The power was determined by the protocol used depending on the region to be treated. In the mandibular region, the aim was not to generate an undesired increase in temperature, knowing that an increase in temperature could lead to an increase in inflammation and thus a rejection of the dental implant4.
In this study, low power applications were used, not exceeding the 0.3 A limit15. Applications were performed with a 15 VA capacitive hypothermic electrode (CAPH), 8 watts resistive (RES8), 20 watts resistive (RES20) and 75 VA capacitive (CAP75).
Four interventions were performed (CAPH, RES8, RES20 and CAP75) for 5 min each. The base plate was placed in the scapular area of the specimen contralateral to the side to be treated. The mobile electrode was placed in the lateral region of the jaws for treatment on the molars and the anterior area for the incisors (Fig. 2). Dynamic movements like those used with real patients were performed with constant pressure. The treatments were performed by a physiotherapist (HGC) with more than 10 years of experience in the use of T-Plus.
Experimental procedure
Each cadaver was placed in supine position with partially opened mouth.
A dentist (CSJ) with 10 years of experience performed the extraction of teeth from the cadavers and the placement of implants in the implicated specimens (Bone level tapered implants, 4.1 mm × 13 mm (diameter × length); Roxolid, Basel, Switzerland) in the incisors and molars. In the case of the samples without implants, only tooth extraction was performed to measure the implant's exact point.
The order of the treatment protocols and the treatment of the cadavers were randomized prior to the study. An external researcher carried out this randomization using the “random.org” software. Before applying each treatment it was ensured that the basal temperature of each corpse returned to the initial values before applying the next treatment.
Before starting the measurements it was ensured that all the instrumentation used had a calibration certificate. Invasive temperature devices “Hart Scientific PT25 5628-15 (Fluke, Everett, Washington, USA)” were used to measure the temperature (°C) of the molars and incisors. One of the gauges was placed on the lower molar/incisor and the other on the upper molar/incisor of the same side to be treated. In the case of the implant group, this gauge was placed in contact with the implant. In the case of subjects without implants, the temperature gauge was placed right in the tooth socket. A “Thermocomed (AB Medica Group, Barcelona, Spain)” digital thermometer was used to measure the superficial temperature of the skin in the mandibular region.
The T-Plus’s return electrode (base plate) was placed on the scapular area of the cadavers. The treatment was performed with the mobile electrode of the T-Plus in the previously explained treatment region according to each application for 5 min. Initial surface and deep tissue temperatures were measured. These measurements were recorded before starting the application and immediately after finishing (at the end of the 5-min treatment). In addition, temperature changes during each minute of treatment were recorded as a temperature control measure. Before the treatment, impedance was always recorded (Multimeter Fluke 8846A) to ensure that the values marked by the T-Plus Wintecare device were correct. In addition, the actual current flow for each application was calculated using the average voltage divided by the initial impedance.
Statistical analysis
Statistical analysis was performed with SPSS (Version 22.0; IBM, Armonk, NY, USA). Normal distribution was calculated with the Shapiro–Wilk test (p > 0.05). The mean and standard deviation of the superficial temperature and of the temperatures taken with the invasive devices were calculated depending on each application. For within-group analysis, the repeated samples ANOVA (2 × 4) test with Bonferroni’s post-hoc was used. For between-group analysis, the one-factor ANOVA test was used. The value of p < 0.05 was considered statistically significant.
Ethics approval
The Comité d´Ètica de Recerca from Universitat Internacional de Catalunya approved the study (CBAS-2019-17) approved on April 4, 2022. The investigation conformed with the principles outlined in the Declaration of Helsinki. The informed consent from “body donors” was obtained before the death and any personal data was hidden.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CRet:
-
Capacitive–resistive electric transfer
- CAPH:
-
15 VA capacitive hypothermic electrode
- RES8:
-
8 Watts resistive
- RES20:
-
20 Watts resistive
- CAP75:
-
75 VA capacitive
References
Esposito, M., Grusovin, M. G., Willings, M., Coulthard, P. & Worthington, H. V. Interventions for replacing missing teeth: Different times for loading dental implants. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD003878.pub5 (2013).
Klinge, B. et al. Dental implant quality register—A possible tool to further improve implant treatment and outcome. Clin. Oral Implants Res. 29, 145–151. https://doi.org/10.1111/clr.13268 (2018).
Buser, D. et al. Long-term evaluation of non-submerged ITI implants. Part 1: 8-year life table analysis of a prospective multi-center study with 2359 implants. Clin. Oral Implants Res. 8, 161–172. https://doi.org/10.1034/J.1600-0501.1997.080302.X (1997).
Buser, D., Mericske-Stern, R., Dula, K. & Lang, N. P. Clinical experience with one-stage, non-submerged dental implants. Adv. Dent. Res. 13, 153–161. https://doi.org/10.1177/08959374990130010501 (1999).
Luo, J. D., Miller, C., Jirjis, T., Nasir, M. & Sharma, D. The effect of non-steroidal anti-inflammatory drugs on the osteogenic activity in osseointegration: A systematic review. Int. J. Implant Dent. https://doi.org/10.1186/S40729-018-0141-7 (2018).
Terheyden, H., Lang, N. P., Bierbaum, S. & Stadlinger, B. Osseointegration–communication of cells. Clin. Oral Implants Res. 23, 1127–1135. https://doi.org/10.1111/J.1600-0501.2011.02327.X (2012).
Sicilia, A. et al. Titanium allergy in dental implant patients: A clinical study on 1500 consecutive patients. Clin. Oral Implants Res. 19, 823–835. https://doi.org/10.1111/J.1600-0501.2008.01544.X (2008).
Guglielmotti, M. B., Olmedo, D. G. & Cabrini, R. L. Research on implants and osseointegration. Periodontology 79, 178–189. https://doi.org/10.1111/PRD.12254 (2019).
Mishra, S. K. & Chowdhary, R. Heat generated by dental implant drills during osteotomy-a review: Heat generated by dental implant drills. J. Indian Prosthodont. Soc. 14, 131–143. https://doi.org/10.1007/S13191-014-0350-6 (2014).
Hernández-Bule, M. L., Toledano-Macías, E., Naranjo, A., de Andrés-Zamora, M. & Úbeda, A. In vitro stimulation with radiofrequency currents promotes proliferation and migration in human keratinocytes and fibroblasts. Electromagn. Biol. Med. 40, 338–352. https://doi.org/10.1080/15368378.2021.1938113 (2021).
Costantino, C., Pogliacomi, F. & Vaienti, E. Cryoultrasound therapy and tendonitis in athletes: A comparative evaluation versus laser CO2 and t.e.ca.r. therapy. Acta Biomed. 76, 37–41 (2005).
Osti, R., Pari, C., Salvatori, G. & Massari, L. Tri-length laser therapy associated to tecar therapy in the treatment of low-back pain in adults: A preliminary report of a prospective case series. Lasers Med. Sci. 30, 407–412. https://doi.org/10.1007/s10103-014-1684-3 (2015).
Takahashi, K. et al. Clinical effects of capacitive electric transfer hyperthermia therapy for cervico-omo-brachial pain. J. Phys. Ther. Sci. 12, 43–48. https://doi.org/10.1589/jpts.12.43 (2004).
Takahashi, K. et al. Clinical effects of capacitive electric transfer hyperthermia therapy for lumbago. J. Phys. Ther. Sci. 11, 45–51. https://doi.org/10.1589/jpts.11.45 (2004).
López-De-Celis, C. et al. Thermal and non-thermal effects off capacitive-resistive electric transfer application on the achilles tendon and musculotendinous junction of the gastrocnemius muscle: A cadaveric study. BMC Musculoskelet. Disord. https://doi.org/10.1038/s41598-020-78612-8 (2020).
Rodríguez-Sanz, J. et al. Is tecar therapy effective on biceps femoris and quadriceps rehabilitation? A cadaveric study. J. Sport Rehabil. 1, 1–8. https://doi.org/10.1123/jsr.2021-0458 (2022).
Hernández-Bule, M. L., Trillo, M. Á. & Úbeda, A. Molecular mechanisms underlying antiproliferative and differentiating responses of hepatocarcinoma cells to subthermal electric stimulation. PLoS ONE. https://doi.org/10.1371/journal.pone.0084636 (2014).
Rodríguez-Sanz, J. et al. Thermal and non-thermal effects of capacitive–resistive electric transfer application on different structures of the knee: A cadaveric study. Sci. Rep. https://doi.org/10.1038/s41598-020-78612-8 (2020).
Hernández-Bule, M. L., Paíno, C. L., Trillo, M. Á. & Úbeda, A. Electric stimulation at 448 kHz promotes proliferation of human mesenchymal stem cells. Cell. Physiol. Biochem. 34, 1741–1755. https://doi.org/10.1159/000366375 (2014).
Rodríguez-Sanz, J. et al. Temperature and current flow effects of different electrode placement in shoulder capacitive-resistive electric transfer applications: A cadaveric study. BMC Musculoskelet. Disord. 22, 139. https://doi.org/10.1186/s12891-020-03918-7 (2021).
López-de-Celis, C. et al. Thermal and current flow effects of a capacitive–resistive electric transfer application protocol on chronic elbow tendinopathy a cadaveric study. Int. J. Environ. Res. Public Health 12, 1012. https://doi.org/10.3390/ijerph18031012 (2021).
Tashiro, Y. et al. Effect of capacitive and resistive electric transfer on haemoglobin saturation and tissue temperature. Int. J. Hyperthermia 33, 696–702. https://doi.org/10.1080/02656736.2017.1289252 (2017).
Buser, D., Sennerby, L. & De Bruyn, H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontology 73, 7–21. https://doi.org/10.1111/PRD.12185 (2017).
Stadelmann, W. K., Digenis, A. G. & Tobin, G. R. Physiology and healing dynamics of chronic cutaneous wounds. Am. J. Surg. 176, 26S-38S. https://doi.org/10.1016/S0002-9610(98)00183-4 (1998).
Gharaibeh, B. et al. Musculoskeletal tissue injury and repair: Role of stem cells, their differentiation, and paracrine effects. Muscle 2, 881–897. https://doi.org/10.1016/B978-0-12-381510-1.00062-4 (2012).
Wei, S. & Huard, J. Tissue therapy implications of regenerative medicine for skeletal muscle. Princ. Regener. Med. https://doi.org/10.1016/B978-012369410-2.50074-7 (2008).
Friedl, P. & Bröcker, E.-B. The biology of cell locomotion within three-dimensional extracellular matrix. Cell. Mol. Life Sci. 57, 41–64. https://doi.org/10.1007/s000180050498 (2000).
Acknowledgements
The authors express our sincere gratitude to the body donors; thanks to their generosity, science can advance.
Author information
Authors and Affiliations
Contributions
All authors have read and approved the manuscript. P.B.A. wrote the introduction and discussion sections, and he prepared the cadaver and performed superficial temperature measurements. C.S.J. Performed all dental procedures for the study and wrote introduction and discussion. J.R.S. wrote and designed the methods section. H.G.C. made the CRet interventions and assisted in the drafting of the article. G.R.V. worked on sample collection and review of article and critical review. G.V.S. worked on sample collection and review of article and critical review. Z.C.D. worked on sample collection and review of article. L.d.C.C. He performed the statistical analysis, the writing of results section and the calibration of the instruments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pérez-Bellmunt, A., Caballé-Serrano, J., Rodríguez-Sanz, J. et al. Comparison of resistive capacitive energy transfer therapy on cadaveric molars and incisors with and without implants. Sci Rep 12, 11845 (2022). https://doi.org/10.1038/s41598-022-16189-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-16189-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.