Abstract
The purpose of this study is to verify the clinical applicability by applying a mouthwash containing Sambucus williamsii var. coreana extract for preventing periodontal disease. A randomized, double-blind, placebo-controlled study was conducted on 64 patients, excluding those with insufficient data, who visited M dental clinic located in Busan, Korea. Thirty-two people were assigned respectively to the saline solution gargle group and the Sambucus williamsii var. coreana extract gargle group to conduct the O'Leary index, plaque index (PI), gingival index (GI), and subgingival plaques. For the homogeneity of the two groups, scaling was carried out one week before the experiment, and the participants were taught for oral care to conduct during the study period. SPSS 24.0 for Windows (IBM Corp., Armonk, NY, USA) was used to compare the saline solution gargle group and the Sambucus williamsii var. coreana extract gargle group as well as to analyze Baseline (before gargle application), Treatment (immediately after gargle application), and After 5 Days (5 days after gargle application). There was a significant difference in the O'Leary index, PI, GI and subgingival plaques after Treatment and After 5 days (p < 0.05). Also, the periodontal-related indexes improved as the application time increased in the Sambucus williamsii var. coreana extract gargle group. The antibacterial effect was also shown for gram-positive bacteria and gram-negative bacteria in subgingival plaques as the application time increased. The use of the mouthwash containing Sambucus williamsii var. coreana extract was found to be effective for oral periodontal-related indicators and bacteria causing periodontal disease. Therefore, using a mouthwash containing Sambucus williamsii var. coreana extract, a natural drug, will possibly maintain healthy periodontal health by inhibiting and preventing the progression of periodontal disease.
Similar content being viewed by others
Introduction
Periodontal disease is a non-communicable chronic disease that destroys soft and hard tissues due to local bacterial infection and imbalanced host immune responses1. As periodontal disease progresses, the collagen fibers of the periodontal ligament are destroyed to form a periodontal pocket, which creates a favorable ecological environment for anaerobic bacteria to thrive2. Most periodontal disease bacteria are anaerobic and sensitive to oxygen, so they stay in the subgingival biofilm, where oxygen supply is poor than in the supragingival biofilm3. The anaerobic bacteria associated with periodontal disease include Parvimonas micra (P. micra), Porphyromonas gingivalis (P. gingivalis), Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans), Treponema denticola (T. denticola), Prevotella nigrescens (P. nigrescens), Fusobacterium nucleatum (F. nucleatum)4.
The lipopolysaccharides (LPS) endotoxins or metabolites formed by these anaerobic bacteria increase the secretion of proinflammatory cytokines from tissues and immune cells5. In particular, Interleukin-1 (IL-1β) and Tumor necrosis factor-α (TNF-α) are representative cytokines involved in tissue destruction. IL-1β induces the followings: recruitment of inflammatory cells, priming degranulation of polymorphonuclear leukocytes, production of inflammatory mediators such as prostaglandin, production of matrix metalloproteinases (MMP), inhibiting collagen synthesis, activating T and B lymphocytes6. TNF-α increases cellular apoptosis, bone resorption, MMP secretion, intercellular adhesion molecule (ICAM) expression, and IL-6 production7. In addition, IL-6 is involved in the tissue destruction process by promoting osteoclast formation, bone resorption, and T lymphocyte differentiation8. Therefore, it is crucial to suppress anaerobic bacteria as soon as possible to prevent periodontal disease.
When inhibiting bacteria, mouthwash, one of the chemical plaque control practices, is becoming popular as it is easier to carry and use than toothbrushing, a mechanical and cumbersome way of controlling plaque9. Above all, the SARS-CoV-2 virus of COVID-19, a cause of the pandemic, was found in 91.7% of saliva samples of patients with COVID-1910. Accordingly, mouthwash is recommended as an effective measure to reduce the risk of viral infection, and people's attention to mouthwash increases11—however, most mouthwashes with intensive chemical agents remove resident bacteria, not only pathogens in the mouth. Therefore, natural extracts are recommended rather than synthetic chemical agents to suppress only pathogens while maintaining healthy oral bacteria12.
Nowadays, many studies on natural extracts have been actively ongoing; practical use of natural antibacterial action varies accordingly13,14,15,16. Studies on Glycyrrhiza uralensis extract17 and bamboo extract18 said that these are effective natural agents showing antibacterial effects related to periodontal disease. Onion extract also inhibits strains of periodontal pathogens P. gingivalis and Prevotella intermedia (P. intermedia) at a concentration of 40 μg/ml19. In addition, pulsatilla extract has an antibacterial effect in the layer separated by butyl alcohol20. Asafoetida mouthwash has been studied as an effective mouthwash to improve the gingival health index15, and saffron mouthwash has been reported to have an anti-inflammatory effect in the treatment of periodontitis patients and to improve the periodontal period16. Even though researches on the antibacterial effects of various natural medicines continue, clinical studies about natural medicines preventing periodontal disease are insufficient. Therefore, it is necessary to prove the clinical effects of natural medicines to develop them as mouthwashes.
Williams Elder (Sambucus williamsii var. coreana) is a deciduous broad-leaved tree distributed in Korea, China, and Japan. This tree has been helpful as medicine for bones and musculoskeletal injuries in traditional oriental medicine21. There was a study of triterpenes, phenols, and lignans isolated from Sambucus williamsii var. coreana used in proliferation experiments on mouse osteocytes (UMR106) and alkaline phosphatase (ALP) activity experiments on UMR106 cells22. Based on the research results related to osteogenesis, Sambucus williamsii var. coreana possibly regenerates destroyed alveolar bones and periodontal tissues caused by periodontal disease. In terms of a study checking the relationship between Sambucus williamsii var. coreana and oral disease, there was a study showing the antibacterial effect of Sambucus williamsii var. coreana extract against oral pathogens23. However, no studies in the dental field verified the clinical usefulness of Sambucus williamsii var. coreana based on clinical effectiveness.
Therefore, we evaluated the clinical applicability of mouthwash using Sambucus williamsii var. coreana extract, a phytotherapeutic medication, to develop an effective medicine against periodontal-related clinical indicators and pathogens that cause periodontal disease.
Materials and methods
Ethical consideration
This study followed the International Council for Harmonization of Technical Requirements for Pharmaceuticals for human use (ICH) guideline. The Silla University Institutional Review Board (1041449-202008-HR-001, Busan, Korea) had approved the human study and WHO International Clinical Trial Registry Platform was registered by clinical trial registration (10/03/2022, registration number: KCT0007064; https://cris.nih.go.kr/cris/search/detailSearch.do/21436). All participants had noticed relevant information (purpose, procedures, and risks) of this study. Participants were free to withdraw from the study at any time. An informed consent form was provided to all participants prior to enrollment in the clinical trial and written informed consent was obtained.
Study participants
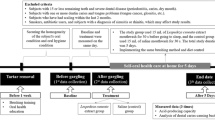
The G* Power 3.1 program calculated sample sizes. 68 participants were required for an independent t-test with significance level a = 0.05 two-tailed, power = 0.8, and effect size = 0.7. An initially planned sample size was 96 to consider a dropout rate of 40%, and actual participants were 98 in this study. The dropout rate was set high as the subjects were college students or working adults. 98 subjects were screened, but 74 participants were randomly assigned to the saline group or Sambucus williamsii var. coreana extract group after excluding 24 subjects who did not meet inclusion criteria or refused to participate during 5 days. 64 subjects were selected as final subjects, excluding 6 subjects who did not follow the guidelines for 5 days and 4 subjects who did not complete the final analysis (Fig. 1).
Study design and protocol
An experiment was randomized, double-blind, and placebo-controlled. In this study, a dental hygienist with more than ten years of experience directly stated the aim of the study to the patients who visited M dental clinic in Busan from October 2020 to June 2021. These patients agreed to participate in the study on December 15, 2020. Among the subjects, the following subjects who met the exclusion criteria were excluded from this study: a person with severe dental disease (e.g., periodontitis, dry mouth, dental caries), a person with a disease that may cause bad breath (e.g., Sjogren's syndrome, rheumatism, renal disease, and hepatic disease), a smoking person, a person diagnosed with sinusitis or rhinitis, a person taking antibiotics, a person with tongue problems (e.g., tongue cancer, glossitis), a person received scaling within 2 months. Among dental caries, patients with enamel caries were eligible to participate in the study, but patients with more than one dentin caries were excluded. Accordingly, the subjects of this study were those who agreed to complete the questionnaire, were not included in the exclusion criteria, and had 16 or more remaining teeth. 74 patients who satisfied the inclusion criteria were selected as the study subjects. Finally, the participants in this study were analyzed with a result of 64 subjects.
Clinical examination
Participants who agreed to this study visited the M dental clinic in Busan one week before the start and received an oral examination by a dentist. Two dental hygienists trained under the guidance of a dentist performed light scaling on the participants for the same oral environment and provided the same toothbrush and toothpaste to them. After a recovery period of 1 week after scaling, the study started. As representative teeth for an oral examination, maxillary right first molar (#16), maxillary left central incisor (#21), maxillary left first premolar (#24), mandibular left first molar (# 36), mandibular right central incisor (#41), and mandibular right first premolar (#44) were selected and measured.
After scaling, two dental hygienists trained under the guidance of a dentist obtained the following data: before the application of mouthwash as Baseline, immediately after the application of mouthwash as Treatment, and five days after the application of mouthwash as After 5 Days. Participants were provided with labeled items so that they could not tell whether they were in the experimental group or the control group. They were also educated on how to use mouthwash and how often and how to brush their teeth using the provided toothpaste and toothbrush. For five days, the experimental group held 15 ml of mouthwash containing Sambucus williamsii var. coreana extract for 30 s and then spat it out before going to bed after brushing, while the control group did the same with 15 ml of saline. After gargling, intake of food, including water, was prohibited. O'Leary index, plaque index (PI), gingival index (GI), and microbiological analysis were evaluated as indicators related to periodontal disease.
O’Leary index
We conducted O'Leary, Drake, and Naylor’s dental plaque test (O'Leary index)24, where all teeth in the oral cavity were discolored with a dental surface discoloration agent and estimated the status of adherence (%) using the plaque control score (O'Leary index). Score 1 if the dental plaque adheres to the dental surfaces of 4 teeth (mesial, efferent, facies, and lingual), and score 0 if not.
PI
Using the PI technique of Loe and Silness25, dividing all teeth surface into two parts; the gingival margin and the tooth surface. Then, measuring plaque accumulation and its thickness with a red colorant. The evaluation criteria are as follows; 0 = no plaque; 1 = thin plaque attached to the gingival margin and visible when rubbing gently with a probe or applying a tooth colorant; 2 = moderate plaque visible along the gingival margin; 3 = thick plaque collection in gingival margin and tooth surface as well as in the gingival pockets. PI is the average quantity of plaque per measured tooth surface, and its score for each tooth was calculated using the average value.
GI
Gingival health status at the proximal, distal, buccal, and lingual regions of all teeth was evaluated using the GI technique26. Each section was assigned from score 0 to 3: 0 = normal gingiva; 1 = a slight color change and swelling of gingivitis showing no bleeding with a mild stimulus; 2 = red and swollen gingivitis showing bleeding with a mild stimulus; 3 = remarkably red and swollen gingivitis due to progressed inflammation and showing the possibility of ulceration and natural bleeding. The values for each tooth were added up to calculate the individual's total mean GI.
Microbiological analysis
To obtain subgingival microbiome samples from periodontal pockets, sterile #15 paper points were placed in the gingival sulcus at four sites: two maxillary teeth (#16 and #21) and two mandibular teeth (#36 and #41) with pocket depth (PD) less than 4 mm for 10 s. They were then placed in sterile 1.5 mL tubes and frozen at − 20 °C until right before an analysis. DNA was dragged from collected #15 paper points using the AccuPrep Universal RNA Extraction Kit (Bioneer, Daejeon, Korea) following the manufacturer's instructions. As shown in Supplementary Table 1, we used three oligonucleotides (forward primer, reverse primer, probe) and OligoMix (YD Global Life Science Co., Ltd., Seongnam, Korea) that react particularly to each bacterium27. Also, to prepare reaction samples of polymerase chain reaction (PCR), we combined the following: 9 µL of OligoMix, 10 µL of 2 × probe qPCR mix (Takara Bio Inc., Shiga, Japan), and 1 µL of template DNA. A 96-well plate with PCR reaction samples was placed in a CFX96 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, USA) for DNA amplification. The cycle requirements of PCR were as follows: PCR initial activation step at 95 °C for 30 s, denaturation at 95 °C for 10 s, annealing at 62 °C for 30 s with 40 repeated cycles. The Bio-Rad CFX Manager Software program was used to calculate the cycle threshold (Ct) parameter, and Ct values were plotted on a standard curve for each bacterium to figure out the number of copies.
Statistical analysis
An analysis of frequency was conducted on the demographic characteristics of the participants in this study. SPSS 24.0 for Windows (IBM Corp., Armonk, NY, USA) was used to verify the significance of the measured O'Leary index, PI, GI, and subgingival plaques between the saline group and the Sambucus williamsii var. coreana extract group performing independent t-test at level 5%. One-way ANOVA was conducted on ‘Baseline’, ‘Treatment’, and ‘After 5 Days’, which are changes according to the application time of gargling. Tukey's test was analyzed as a posteriori test.
Ethics approval and consent to participate
The study was approved by Institutional Review Board of Silla University (1041449-202008-HR-001, Busan, Korea). Informed consent was obtained from all individual participants included in the study. All methods have been carried out in accordance with the relevant guidelines and regulations.
Results
Population characteristics
Table 1 shows the general features of the study subjects. The saline group consisted of 19 females and 13 males, and the Sambucus williamsii var. coreana group consisted of 20 females and 12 males, so there was no significant difference in gender distribution (p > 0.05). The saline group was 36.13 ± 16.48 years old, while the Sambucus williamsii var. coreana group was 38.06 ± 17.68 years old, so there was no significant difference between the two groups concerning the average age of the subjects (p > 0.05)., There were 29 patients without systemic disease and 3 patients with systemic disease in both groups; two had hypertension and one had diabetes. In both groups, 17 were single, and 15 were married, and there was no significant difference between the two groups in systemic disease and marriage (p > 0.05).
Clinical indicators related to periodontal disease
Table 2 shows the measured results for the O’Leary index, PI, and GI of the saline gargle group and the Sambucus williamsii var. coreana extract gargle group. There was no difference concerning the ‘Baseline’ (p > 0.05), but there was a significant difference between the two groups concerning ‘Treatment’ and ‘After 5 Days’ (p < 0.05). When comparing the two groups concerning periodontal disease-related indexes, such as O'Leary index, PI, and GI, the application of mouthwash containing Sambucus williamsii var. coreana extract resulted in lower values, indicating that the clinical improvements in the oral environment. In the case of the saline gargle group, all indicators in ‘Baseline’, ‘Treatment’, and ‘After 5 days’ did not show a significant difference (p > 0.05). On the other hand, in the Sambucus williamsii var. coreana extract gargle group, all clinical indicators showed significantly lower values as the application time increased (p < 0.05).
Distribution of periodontal disease-causing bacteria in the study subjects
Table 3 shows the results of confirming the distribution of bacteria in the oral cavity of the study subjects. Among Gram-positive bacteria, those with P. micra had the highest proportion in the oral, and the average of bacteria was also high. Among Gram-negative bacteria, those with F. nocleatum had the highest proportion in the oral, and the average of bacteria was also high.
Gram-positive bacteria in subgingival plaques
Gram-positive bacterial data in subgingival plaques was detected as three types of bacteria: P. micra, Staphylococcus aureus (S. aureus), and Eubacterium nodatum (E. nodatum). In addition, the effect difference between the two groups was verified according to the maxillary and mandible (Table 4). When comparing the saline gargle group and the Sambucus williamsii var. coreana extract gargle group against P. micra, there was a significant difference only in the mandible at the time of ‘Treatment’ and ‘After 5 days’ (p < 0.05). There was a significant difference in ‘After 5 days’ in the maxilla (p < 0.05) regarding S. aureus; it was not detected in both groups in the mandible. There was no significant difference between the two groups for E. nodatum in the oral cavity (p > 0.05). Also, based on the ‘Baseline’, there was a significant difference from ‘Treatment’ to ‘After 5 days’ in the mandible of the Sambucus williamsii coreana var. extract gargle group in the case of P. micra (p < 0.05). As for E. nodatum, there was a significant difference in both groups in the maxilla (p < 0.05).
Gram-negative bacteria in subgingival plaques
The following seven types of bacteria were detected as gram-negative bacteria in the oral cavity: P. gingivalis, T. denticola, F. nocleatum, P. intermedia, P. nigrescens, Eikenella corrodens (E. corrodens), and Campylobacter rectus (C. rectus). In addition, the effect difference between the two groups was verified according to the maxillary and mandible (Table 5). When comparing the saline gargle group and the Sambucus williamsii var. coreana extract gargle group, P. gingivalis showed a significant difference at ‘Treatment’ and ‘After 5 days’ in maxilla and mandible (p < 0.05). T. denticola was detected only in the mandible, showing a significant difference between the two groups at ‘Treatment’ and ‘After 5 days’ (p < 0.05). F. nocleatum differed between groups only in the maxilla ‘After 5 days’ (p < 0.05). As for P. intermedia showed a significant difference between the groups at ‘Treatment’ and ‘After 5 days’ only in the mandible (p < 0.05). In the case of P. nigrescens, there was a significant difference between the two groups ‘After 5 days’ in both maxilla and mandible (p < 0.05). E. corrodens was not significantly different between the two groups in the maxilla and mandible. C. rectus showed a difference between the two groups at ‘Treatment’ and ‘After 5 days’ in the case of maxilla but showed a significant difference only at ‘Treatment’ in the mandible (p < 0.05). According to the application time, the changes were not significantly different in the saline gargle group from ‘Baseline’ to ‘After 5 days’ except for F. nocleatum in the maxilla and E. corrodens in the mandible, among the seven types of gram-negative bacteria (p > 0.05). On the other hand, in the Sambucus williamsii var. coreana extract gargle group, all bacteria in the maxilla and mandible were significantly reduced according to the application time, except for F. nocleatum in the mandible; P. intermedia and P. nigrescens of the maxilla. In particular, based on ‘Baseline’, P. gingivalis and C. rectus showed a marked differences from ‘Treatment’ to ‘After 5 days’ in the maxilla and mandible, verifying that gram-negative bacteria decreased clearly. From ‘Treatment’ to ‘After 5 days’, T. denticola, P. intermedia, and E. corrodens were reduced in the mandible only, and T. denticola was not even detected at ‘Treatment’ and ‘After 5 days’.
Discussion
About 700 types of microorganisms live in the oral cavity; because the nutrients necessary for the growth of microorganisms are continuous, the bacteria proliferate to form dental plaques that cause oral bacterial diseases28. Therefore, the best way to prevent this is to remove the dental plaque, representatively using Tetracyclines, Metrodinidazole, Penicillin, Clindamycin, and Ciprofloxacin out of the various types of antibacterial agents29. These chemical antibacterial agents inhibit bone loss and are effective for periodontitis30, but their use is limited due to side effects such as the generation of resistant bacteria and mycosis31.
Therefore, this study verified the O’Leary index, PI, and GI as periodontal-related indicators; and antibacterial effects on pathogens causing periodontal diseases to develop a natural mouthwash using Sambucus williamsii var. coreana extract, which has a natural antibiotic effect.
The high baseline values of the PI, GI, and bacteria seem to come out due to the lack of oral care before applying mouthwash. After measuring the baselines, two examiners (dental hygienists) also educated the subjects about how to use mouthwash and how often and how to brush teeth using the provided toothpaste and toothbrush. As a result of this study, O'Leary index, PI, and GI all showed marked decreases from ‘Treatment’ to ‘After 5 days’ compared to the saline gargle group when applying mouthwash containing Sambucus williamsii var. coreana extract. Also, as the application time of the mouthwash containing Sambucus williamsii var. coreana extract increased, the values of periodontal-related indicators were effectively lowered, verifying that there was an effect of improving the oral environment.
The immediate improvement in GI in this study is shown as a statistical result by a small number of subjects with a large number of subjects with healthy gingiva and a large change in GI improvement.
According to Cai et al.’s study32, herb mouthwash has mentioned potential benefits in plaque and inflammation control as a supplement to routine oral hygiene in patients with gingivitis, and in particular, Balappanavar et al.’s study33 and Meena Priya et al.’s study34 showed a greater reduction in gingivitis after intervention.
According to a previous study, Sambucus williamsii var. coreana extract had antioxidant and anti-inflammatory effects and had no cytotoxicity35. Therefore, applying it in the oral cavity as a mouthwash is considered appropriate to suppress and prevent periodontal disease.
P. micra, which is known as a bacterium associated with periodontal disease, is a gram-positive, non-motile, anaerobic coccus36 that is more common in patients with severe or moderate periodontitis than those with gingivitis and mild periodontitis37. In this study, a mouthwash containing Sambucus williamsii var. coreana extract was effective in the mandible; the antibacterial effect appeared immediately after application and continued until ‘After 5 days’. P. micra is known to act as a core pathogen in the development of the periodontal disease38; signaling molecules derived from P. micra stimulate the expression of the P. gingivalis proteolytic gingipain enzyme thirteen times more to enhance the growth and toxicity of Porphyromonas gingivalis, a major periodontal pathogen39. P. gingivalis, a gram-negative anaerobic bacterium, is associated with periodontal destruction and increasing risk of disease recurrence40; it is the foremost periopathogen for periodontal disease progression41. After applying the mouthwash containing Sambucus williamsii var. coreana extract to P. gingivalis, it decreased sharply in the maxilla and the mandible compared to the saline gargle group. The mouthwash containing Sambucus williamsii var. coreana extract was verified to be very effective against P. gingivalis as it decreased continuously right after applying the mouthwash. Therefore, a mouthwash using Sambucus williamsii var. coreana extract can inhibit and block the growth of core pathogens of periodontal disease. P. gingivalis is also considered the major pathogen because of its ability to change the normal microflora into a dysbiotic community42. Among dysbiotic species, the increase in P. gingivalis, a major periodontal disease-related pathogen, is accompanied by various bacteria, including P. intermedia and F. nucleatum43. Since the antibacterial effect was shown by a marked reduction of P. gingivalis in this study, mouthwash containing Sambucus williamsii var. coreana extract will play a huge role in reducing various anaerobic bacteria. P. intermedia, the anaerobic black bacteria, is one of the powerful causative agents of adult-type periodontitis and various types of periodontal disease44. In addition, since the expression frequency of P. nigrenscens was reported to be more than double that of P. intermedia in the inflammatory site of patients with periodontal disease45, dealing with P. nigrenscens is also necessary. Compared with the saline gargle group, P. intermedia effectively decreased in the mandible immediately after applying a mouthwash containing Sambucus williamsii var. coreana extract. There was no effect on P. nigrenscens immediately after using a mouthwash containing Sambucus williamsii var. coreana extract, but it became effective in both the maxilla and mandible after using the mouthwash for five days. Both P. intermedia and P. nigrenscens more effectively decreased over time in the mandible as applying a mouthwash containing Sambucus williamsii var. coreana extract. As a result, a mouthwash containing Sambucus williamsii var. coreana extract reduced P. gingivalis immediately after applying a mouthwash. P. nigrenscens and P. intermedia also decreased as the application time increased. F. nocleatum, one of the oral microflora and a causative agent of periodontitis, is also related to preterm birth, colorectal cancer, inflammatory bowel disease, respiratory tract infections, cardiovascular disease, rheumatoid arthritis, and Alzheimer’s disease46. T. denticola, a bacterium that causes progressive periodontitis, is associated with losing attached gingiva and bleeding when measuring the periodontal pocket and its depth3. In the results of this study, F. nocleatum was reduced compared to the saline gargle group at ‘After 5 days’ in the maxilla upon using a mouthwash containing Sambucus williamsii var. coreana extract. On the other hand, in the case of T. denticola, the mouthwash containing Sambucus williamsii var. coreana extract was effective until ‘After 5 days’ as the bacteria completely disappeared from ‘Treatment’ in the mandible. In addition, E. corrodens is a bacterium that causes acute periodontitis and promotes complex infection. C. rectus is a bacterium involved in root canal infection and periodontal disease47. Both bacteria appear in patients with severe periodontal disease. When comparing the saline gargle group and the Sambucus williamsii var. coreana extract gargle group, there was no significant difference between the two groups regarding E. corrodens. In the case of the saline gargle group, there was a significant difference in the mandible at 'After 5 days,' the increased application time; if the application time increases, the reduction of acute periodontitis-causing bacteria can occur even with a saline. The number of C. rectus decreased in Sambucus williamsii var. coreana extract gargle group showing the antibacterial effect. From immediate applying a mouthwash containing Sambucus williamsii var. coreana extract, bacteria showed differences continuously in both the maxilla and the mandible. According to these oral clinical results, we verified Sambucus williamsii var. coreana extract as an excellent natural substance with antibacterial effects for improving periodontal disease.
As periodontal disease progresses, bacterial species diversify48, so continuous management of anaerobic bacteria related to periodontal disease is required. Based on the study results, a mouthwash containing Sambucus williamsii var. coreana extract reduced bacteria in the oral cavity such as P. gingivalis, C. rectus, T. denticola, P. intermedia, and E. corrodens immediately after application. As the application time of a mouthwash containing Sambucus williamsii var. coreana extract extended, a more marked antibacterial effect appeared, which means it is excellent in improving periodontal disease. According to the results of this study, mouthwash was more effective in the mandible; it seems that the teeth and periodontal tissues of the mandible were in contact with and stored more in the mouthwash when keeping the mouthwash in the mouth. Therefore, to maximize the effect of mouthwash, it will be more effective to gargle mouthwash enough to cover the entire oral cavity rather than simply holding it.
The limitation of this study is that a mouthwash containing Sambucus willamsii var. coreana extractwas applied only for five days to verify its effects. For this reason, it can be considered that the use of mouthwash for a short period of time alone has the effect of improving gingivitis. However, further research is needed through clinical efficacy verification to confirm the improvement of periodontitis by expanding the subjects to periodontal disease patients and lengthening the period of mouthwash use. In addition, more systemic disease variables will be corrected in order to secure the homogeneity of study subjects for future research, and the reliability of the results of clinical trials will be secured through 1:1 propensity score matching.
Based on this study verifying the practicality and development of oral health care products using Sambucus williamsii var. coreana extract, a mouthwash containing Sambucus williamsii var. coreana extract can inhibit and prevent periodontal disease. As a natural ingredient with sufficiently excellent effects, Sambucus williamsii var. coreana extract can be used for oral health by improving periodontal disease.
Conclusion
A mouthwash containing Sambucus williamsii var. coreana extract affects oral hygiene by reducing the O’Leary index, PI, and GI. It also has a potential advantage in inflammation control by reducing periodontal disease-causing bacteria, so alternative oral care products as the adjuvant treatment of periodontal disease can be possible. In addition, medicine using Sambucus williamsii var. coreana extract rather than chemicals can be a promising field for treating gingivitis.
Data availability
The data sets generated and/or analyzed during the current study are not publicly available for reasons of personal and organizational integrity but are available from the corresponding author on reasonable request.
Abbreviations
- PI:
-
Plaque index
- GI:
-
Gingival index
- P. micra :
-
Parvimonas micra
- S. aureus :
-
Staphylococcus aureus
- E. nodatum :
-
Eubacterium nodatum
- P. gingivalis :
-
Parvimonas micra
- T. denticola :
-
Treponema denticola
- F. nucleatum :
-
Fusobacterium nucleatum
- P. intermedia :
-
Prevotella intermedia
- P. nigrescens :
-
Prevotella nigrescens
- E. corrodens :
-
Eikenella corrodens
- C. rectus :
-
Campylobacter rectus
References
Kato, A., Imai, K., Ochiai, K. & Ogata, Y. Higher prevalence of Epstein-Barr virus DNA in deeper periodontal pockets of chronic periodontitis in Japanese patients. PLoS ONE 8, e71990. https://doi.org/10.1371/journal.pone.0071990.t002 (2013).
Könönen, E., Gursoy, M. & Gursoy, U. K. Periodontitis: A multifaceted disease of tooth-supporting tissues. J. Clin. Med. 8, 1135. https://doi.org/10.3390/jcm8081135 (2019).
Boutaga, K., van Winkelhoff, A. J., Vandenbroucke-Grauls, C. M. & Savelkoul, P. H. Comparison of real-time PCR and culture for detection of Porphyromonas gingivalis in subgingival plaque samples. J. Clin. Microbiol. 41, 4950–4954. https://doi.org/10.1128/JCM.41.114950-4954 (2013).
Borrell, L. N. & Papapanou, P. N. Analytical epidemiology of periodontitis. J. Clin. Periodontol. 32, 132–158. https://doi.org/10.1111/j.1600-051X.2005.00799.x (2005).
Garlet, G. P. Destructive and protective roles of cytokines in periodontitis: A re-appraisal from host defense and tissue destruction viewpoints. J. Dent. Res. 89, 1349–1363. https://doi.org/10.1177/0022034510376402 (2010).
Butler, L. D. et al. Interleukin 1-induced pathophysiology: Induction of cytokines, development of histopathologic changes, and immunopharmacologic intervention. Clin. Immunol. Immunopathol. 53, 400–421. https://doi.org/10.1016/0090-1229(89)90003-2 (1989).
Birkedal-Hansen, H. Role of cytokines and inflammatory mediators in tissue destruction. J. Periodont. Res. 28, 500–510. https://doi.org/10.1111/j.1600-0765.1993.tb02113.x (1993).
Yamamoto, M. et al. Molecular and cellular mechanisms for periodontal diseases: Role of Th1 and Th2 type cytokines in induction of mucosal inflammation. J. Periodont. Res. 32, 115–119. https://doi.org/10.1111/j.1600-0765.1997.tb01391.x (1997).
Ciancio, S. G. Agents for the management of plaque and gingivitis. J. Am. Coll. Dent. 56, 14–20 (1989).
To, K. K. et al. Consistent detection of 2019 novel coronavirus in saliva. Clin. Infect. Dis. 71, 841–843. https://doi.org/10.1093/cid/ciaa149 (2020).
In the era of COVID-19, wise oral care. Ministry of Health and Welfare. (2021).
Lis-Balchin, M. Essential oils and aromatherapy: Their modern role in healing. J. R. Soc. Health. 117, 324–329. https://doi.org/10.1177/146642409711700511 (1977).
An, J. Y., Lee, S. S. & Kang, H. Y. Biological activities of essential oil from chamaecyparis obtusa. J. Soc. Cosmet. Scientists Korea 30, 503–507 (2004).
Palombo, E. A. Traditional medicinal plant extracts and natural products with activity against oral bacteria: Potential application in the prevention and treatment of oral diseases. Evid. Based Complement. Alternat. Med. 2011, 1–15. https://doi.org/10.1093/ecam/nep067 (2011).
Seyed Hashemi, M. et al. The efficacy of asafoetida (Ferula assa-foetida oleo-gum resin) versus chlorhexidine gluconate mouthwash on dental plaque and gingivitis: A randomized double-blind controlled trial. Eur. J. Integr. Med. 29, 100929–100933 (2019).
Rashidi Maybodi, F., Vaziri, F., Ghanbarnezhad, S. & Herandi, V. The effect of aqueous extract of Crocus sativus L. (Saffron) on periodontal indices of patients with generalized periodontitis. Tradit. integr. Med. 7, 13–19 (2022).
Villinski, J. R. et al. Pyrano-isoflavans from Glycyrrhiza uralensis with antibacterial activity against streptococcusmutans and porphyromonasgingivalis. J. Nat. Prod. 77, 521–526. https://doi.org/10.1021/np400788r (2014).
Jung, G. O., Seo, S. Y. & Yoon, S. U. Anti-microbial activity of bamboo extract against oral microbes. J. Koon. 20, 454–459. https://doi.org/10.5392/JKCA.2020.20.12.454 (2020).
Loe, H. Does chlorhexidine have a place in the prophylaxis of dental disease?. J. Periodontal Res. Suppl. 12, 93–99 (1973).
Haffajee, A. D. & Socransky, S. S. Microbial etiological agents of destructive periodontal diseases. Periodontol. 5, 78–111. https://doi.org/10.1111/j.1600-0757.1994.tb00020.x (2000).
Lee, S. C., Kang, H. M., Yu, S. B. & Choi, S. H. Vegetation characteristics and changes of evergreen broad-leaved forest in the cheomchalsan(Mt.) at Jindo(Island). Korean J. Ecol. 34, 235–248. https://doi.org/10.13047/KJEE.2020.34.3.235 (2020).
Yang, X. J., Wong, M. S., Wang, N. L., Chan, S. C. & Yao, X. S. Lignans from the stems of Sambucus williamsii and their effects on osteoblastic UMR106 cells. J. Asian Nat. Prod. Res. 9, 583–591. https://doi.org/10.1080/10286020500530433 (2007).
Nam, S. H. Antibacterial effect of Sambucus Williamsii Var. Coreana NAKAI (S. Williamsii) against Enterococcus faecalis (E. faecalis) for the Traditional Treatment of Oral Diseases. Indian J. Public Health Res. 10, 1098–1104 (2019).
Lang, N. P. & Tonetti, M. S. Periodontal risk assessment (PRA) for patients in supportive periodontal therapy (SPT). Oral Health Prev. Dent. 1, 7–16 (2003).
Loe, H. & Silness, J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol. Scand. 21, 533–551. https://doi.org/10.3109/00016356309011240 (1963).
Silness, J. & Loe, H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol. Scand. 22, 121–135. https://doi.org/10.3109/00016356408993968 (1964).
Lee, J.W., Kim, M.B. & inventors YD Global Life Science Company patentee. Composition and detection method for simultaneous detection of multiple oral disease-causing bacteria using multiplex real-time PCR. KR Patent 10–1706070.
Mashimo, P. A., Yamamoto, Y., Slots, J., Evans, R. T. & Genco, R. J. In vitro evaluation of antibiotics in the treatment of periodontal dissease. Pharmacol. Ther. Dent. 6, 45–56 (1981).
Slots, J. & Rams, T. E. Antibiotics and periodontal therapy: Advantages and disadvanyages. J. Clin. Periodontol. 17, 479–493. https://doi.org/10.1111/j.1365-2710.1992.tb01220.x (1990).
Botelho, M. A. et al. Antimicrobial activity of the essential oil from Lippia sidoides, carvacrol and thymol against oral pathogens. Braz. J. Med. Biol. Res. 40, 349–356. https://doi.org/10.1590/s0100-879x2007000300010 (2007).
Jin, H. J. et al. Relationship between periodontal status and chronic obstructive pulmonary disease. J. Korean Acad. Oral. 37, 147–153 (2013).
Cai, H., Chen, J., Perera, N. K. & Liang, X. Effects of herbal mouthwashes on plaque and inflammation control for patients with gingivitis: a systematic review and meta-analysis of randomised controlled trials. Evid. Based Complement Alternat. Med. https://doi.org/10.1155/2020/2829854 (2020).
Balappanavar, A. Y., Sardana, V. & Singh, M. Comparison of the effectiveness of 0.5% tea, 2% neem, and 0.2% chlorhexidine mouthwashes on oral health: a randomized control trial. Indian J. Dent. Res. 24, 26–34 (2013).
Meena Priya, B. et al. Efficacy of chlorhexidine and green tea mouthwashes in the management of dental plaque-induced gingivitis: A comparative clinical study. Contemp. Clin. Dent. 6, 505–509. https://doi.org/10.4103/0976-237X.169845 (2015).
Kim, K. T. Antioxidant activity and protective effects of extracts from Sambucus williamsii var. coreana on t-BHP induced oxidative stress in chang cells. J. Soc. Korean Med. Diagn. 17, 275–286 (2013).
Rams, T. E. & van Winkelhoff, A. J. Introduction to clinical microbiology for the general dentist. Dent. Clin. 61, 179–197. https://doi.org/10.1016/j.cden.2016.11.001 (2017).
Curtis, M. A., Diaz, P. I. & Van Dyke, T. E. The role of the microbiota in periodontal disease. Periodontol. 83, 14–25. https://doi.org/10.1111/prd.12296 (2020).
Al-Hebshi, N. N., Shuga-Aldin, H. M., Al-Sharabi, A. K. & Ghandour, I. Subgingival periodontal pathogens associated with chronic periodontitis in Yemenis. BMC Oral Health 18, 13. https://doi.org/10.1186/1472-6831-14-13 (2014).
Neilands, J., Davies, J. R., Bikker, F. J. & Svensäter, G. Parvimonas micra stimulates expression of gingipains from Porphyromonas gingivalis in multi-species communities. Anaerobe 55, 54–60. https://doi.org/10.1016/j.anaerobe.2018.10.007 (2019).
Socransky, S. S., Smith, C. & Haffajee, A. D. Subgingival microbial profiles in refractory periodontal disease. J. Clin. Periodontol. 29, 260–268. https://doi.org/10.1034/j.1600-051x.2002.290313.x (2002).
Socransky, S. S. & Haffajee, A. D. Periodontal microbial ecology. Periodontol 2000(38), 135–187. https://doi.org/10.1111/j.1600-0757.2005.00107.x (2005).
Romacho, T. et al. Visfatin as a novel mediator released by inflamed human endothelial cells. PLoS ONE 8, e78283. https://doi.org/10.1371/journal.pone.0078283 (2013).
Schwenzer, A. et al. Association of distinct fine specificities of anti-citrullinated peptide antibodies with elevated immune responses to Prevotella intermedia in a subgroup of patients with rheumatoid arthritis and periodontitis. Arthritis Rheumatol. 69, 2303–2313. https://doi.org/10.1002/art.40227 (2017).
Wennstrom, J. L., Dahlen, G., Svensson, J. & Nyman, S. Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Bacteroides intermedius: Predictors of attachment loss?. Oral Microbiol. Immunol. 2, 158–163. https://doi.org/10.1111/j.1399-302x.1987.tb00300.x (1987).
Teanpaisan, R., Douglas, C. W. I. & Walsh, T. F. Characterization of black-pigmented anaerobes isolated from diseased and healthy periodontal sites. J. Periodont. Res. 30, 245–251. https://doi.org/10.1111/j.1600-0765.1995.tb02129.x (1995).
Han, Y. W. Fusobacterium nucleatum: A commensal-turned pathogen. Curr. Opin. Microbiol. 23, 141–147. https://doi.org/10.1016/j.mib.2014.11.013 (2015).
Socransky, S., Haffajee, A., Cugini, M., Smith, C. & Kent, R. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 25, 134–144. https://doi.org/10.1111/j.1600-051x.1998.tb02419.x (1998).
Simon, D. T. & Rudney, J. D. Improved multiplex PCR using conserved and species-specific 16S rRNA gene primers for simultaneous detection of actinobacillus actinomycetemcomitans, Bacteroides forsythus, and Porphyromonas gingivalis. J. Clin. Microbiol. 37, 3504–3508. https://doi.org/10.1128/JCM.37.11.3504-3508.1999 (1999).
Funding
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea government (MSIT) (2020R1C1C1005306).
Author information
Authors and Affiliations
Contributions
Y.R.K. and S.H.N. participated in experiments, data analysis, and interpretation of the results. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, YR., Nam, SH. A randomized, placebo-controlled clinical trial evaluating of a mouthwash containing Sambucus williamsii var. coreana extract for prevention of gingivitits. Sci Rep 12, 11250 (2022). https://doi.org/10.1038/s41598-022-15445-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-15445-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.