Abstract
Through long-term training, music experts acquire complex and specialized sensorimotor skills, which are paralleled by continuous neuro-anatomical and -functional adaptations. The underlying neuroplasticity mechanisms have been extensively explored in decades of research in music, cognitive, and translational neuroscience. However, the absence of a comprehensive review and quantitative meta-analysis prevents the plethora of variegated findings to ultimately converge into a unified picture of the neuroanatomy of musical expertise. Here, we performed a comprehensive neuroimaging meta-analysis of publications investigating neuro-anatomical and -functional differences between musicians (M) and non-musicians (NM). Eighty-four studies were included in the qualitative synthesis. From these, 58 publications were included in coordinate-based meta-analyses using the anatomic/activation likelihood estimation (ALE) method. This comprehensive approach delivers a coherent cortico-subcortical network encompassing sensorimotor and limbic regions bilaterally. Particularly, M exhibited higher volume/activity in auditory, sensorimotor, interoceptive, and limbic brain areas and lower volume/activity in parietal areas as opposed to NM. Notably, we reveal topographical (dis-)similarities between the identified functional and anatomical networks and characterize their link to various cognitive functions by means of meta-analytic connectivity modelling. Overall, we effectively synthesized decades of research in the field and provide a consistent and controversies-free picture of the neuroanatomy of musical expertise.
Similar content being viewed by others
Introduction
Decades of research in psychology, cognitive and translational neuroscience have attempted to deepen our understanding of the cognitive and neural processes which allow individuals to reach high levels of mastery within a given domain. For instance, expert musicians (M) seem to develop, through long-term training, complex and specialized auditory and sensorimotor skills, which enable them to achieve the highest levels of performance in playing a musical instrument (thus, the label of “virtuoso”)1,2,3,4. Notably, such debate extends to the association between the acquisition of such fascinating skills and the continuous neuro-anatomical and -functional changes in auditory, motor and higher-order cognitive control regions since childhood4,5,6,7,8. Furthermore, other non-music-specific cognitive functions seem to benefit from such trainings, as revealed by increased performance in working memory, intelligence, executive functions and inhibitory control tests6,9,10,11. These effects may represent, on the one hand, ‘neuroplastic’ adaptations to environmental demands and successful gene-environment interactions3,12. On the other hand, many background and environmental factors may influence observed neurocognitive changes, as we shall discuss later.
Neuroplasticity generally refers to the brain’s capacity to modulate its anatomical and functional features during maturation, learning, skill acquisition, environmental challenges, or pathology. Interestingly, these effects seem to be salient enough to be also observed macroscopically with magnetic resonance imaging (MRI). However, whole-brain analyses in humans often fail to convey a link between complex behaviour and neuroplastic mechanisms. Such difficulty might emerge due to methodological and sample differences. For instance, background variables such as genome, psychological (motivation, reward), and socio-economical characteristics are not always considered13,14 but may determine the predisposition to engage in specialized trainings (e.g., music or dance) and/or to possess fine-grained sensory processing and sensorimotor skills which are independent of, or precede the training. Consequently, the adoption of cross-sectional designs, as opposed to longitudinal designs, may lead to spurious observations of training-related neuroplasticity mechanisms as they may fail to isolate background and confounding factors from the ‘true’ training-related effects. In contrast, longitudinal studies allow to zoom into individual differences and to follow specific trajectories of neurocognitive development. However, longitudinal research is rather sparse4,7.
A common approach adopted to strengthen the link between observed neuro-anatomical and -functional changes and specialized trainings in cross-sectional designs is to quantify their correlation. Thus, the relation with the length of specialized trainings, is interpreted as evidence for a link between expertise and neuroplasticity (in various populations from athletes, chess-players, golfers15 and musicians6). These correlational approaches are usually further extended to investigate the link between specific trainings and higher-order cognitive functions6. Thus, improved intelligence, working memory and executive functions are commonly associated with duration and intensity of music training6,9,10,11. These studies should be interpreted with caution as they not necessarily allow to dissociate pre-existing and training-independent neurocognitive differences from real neuroplasticity effects. Additionally, musical training represents a stimulating experience which engages highly-specialized perceptual, motor, emotional and higher-order cognitive abilities16, ranging from multimodal (auditory, visual, motor) sensory perception, integration, predictions and fine movement control17,18. Furthermore, it stimulates mnemonic processes associated with the acquisition of long and complex bimanual finger sequences, as well as fine-grained auditory perception (absolute pitch)19. Thus, the daily and intensive training of such complex, varied and specialized sensory, sensorimotor and higher-order cognitive skills represents a very appealing scenario to investigate neuroplasticity mechanisms and to monitor the continuous underlying neuro-anatomical and -functional adaptations4,20.
Anatomical and functional resonance imaging studies (fMRI) have correlated audio-motor, parietal and occipital brain structures (the dorsal stream) to the ability to play an instrument via automatic and accurate associations between motor sequences and auditory events leading to multimodal predictions17, the simultaneous integration of multimodal auditory, visual and motor information, and fine-grained skills in auditory perception, kinaesthetic control, visual perception, and pattern recognition18. Also, dorsolateral prefrontal structures, basal ganglia and mesial temporal structures have further been related to musicians’ ability to memorize long and complex bimanual finger sequences and to translate musical symbols into motor sequences19. Importantly, however, music experts do not show an overall pattern of increased structure and/or task-based functional activity. While surely appealing, such assumption would narrow down the complexity of the rather heterogenous neurocognitive mechanisms of musical expertise21,22. Hence, some works reported co-occurrent patterns of increase and decrease of grey matter (GM) volumes when comparing musicians to non-musicians23,24, and other research highlighted negative correlations between GM volumes and musical expertise24,25. Similarly, investigations focusing on white matter (WM) reported increased fractional anisotropy (i.e., a WM micro-structural organisation measure informing about the diffusion within the predominant direction in a given pathway26) in expert musicians as compared to non-musicians in the corticospinal tract27,28, internal capsule bundles29 and corpus callosum30, while others found reduced fractional anisotropy and increased radial diffusivity31. fMRI studies are similarly characterized by variegated observations, with musicianship being exclusively associated with stronger activity in, e.g., either premotor cortices32, right auditory cortex33, or prefrontal cortex34, ultimately failing to converge into a common functional network for music expertise.
Despite a growing interest in the topic, we here highlight that there has never been a quantitative meta-analytic attempt to summarize existing findings and provide a unified picture of the neuroanatomy of musical expertise. To address this limitation in the field, we conducted a comprehensive coordinate-based meta-analysis (CBMA) of (f)MRI studies using the anatomic/activation likelihood estimation (ALE) method35, to investigate the neuro-anatomical and -functional signatures associated with musical training on healthy humans. Specifically, we first provide a detailed overview of the studies included and their methods, paradigms, sample details and backgrounds so to guide the reader into a critical consideration of the results. Then, we characterize the topographical (dis)similarities between the identified functional and anatomical networks and link them to various cognitive functions by means of meta-analytic connectivity modelling (MACM).
Results
A total of 1169 records was identified through database searching, and 679 records were initially screened by title and abstract after removing duplicates. Next, 145 articles were assessed for eligibility in the full-text screening stage. From these, 84 studies fulfilled criteria for eligibility and were thus included in the qualitative synthesis. Finally, from the 84 studies, only 58 reported results in stereotactic coordinates (foci), either Talairach or Montreal Neurological Institute (MNI) three-dimensional-coordinate system which were therefore included in the quantitative synthesis (ALE meta-analyses) (Supplementary Fig. 1). This study used the BrainMap platform36, a large-scale database storing results obtained from published brain activation (functional) and brain structure (voxel-based morphometry) studies. ALE meta-analyses were conducted using GingerALE37, a software that tests the convergence of coordinates across independently conducted MRI studies investigating the same construct. To complement our findings, meta-analytic connectivity modelling (MACM)38 was performed using Sleuth39, to explore the co-activation patterns of regions-of-interest (ROIs) resulted from the ALE meta-analysis, and to functionally segregate each region’s contribution to behavioural domains and paradigm classes according to the BrainMap platform40,41,42.
Characteristics of studies
Details of the studies included in our work are provided in Table 1. Eighty-four publications met inclusion criteria and were included in the qualitative synthesis which was comprised of 3005 participants, with 1581 musicians (M) and 1424 non-musicians (NM). Eighteen studies (21%) included amateur musicians, and only 7 studies (8.3%) reported absolute pitch possessors (n = 97). Musical instruments were reported in most of the studies (81%): piano or keyboard (62%), string instruments (41%), wind instruments (26%), percussion instruments (17%), voice (8%), and 19% studies failed to report musicians’ instrument. Years of education was described only in 8% of the included studies. Years of musical training was reported in 63% of the studies, with a mean of 15.6 ± 5.9 years. The age of onset of musical training was reported in 49% of the studies, with a mean of 7.4 ± 2.3 years old. Weekly hours of training were reported in 32% of the studies, with a mean of 16.7 ± 8.9 h per week.
MRI quality
MRI quality of the included studies in the meta-analysis was assessed following a set of guidelines for the standardized reporting of MRI studies43,44. All studies included in the qualitative synthesis (n = 84) reported their MRI design, software package and image acquisition, pre-processing, and analyses. Overall, all the studies followed good MRI practices. Neuroimaging data was acquired in either 1.5 T (39%), or 3 T (56%) scanners, while 5% of studies did not report the magnetic field strength. MRI scanners included Siemens (40%), General Electric (25%), Philips (21%), Bruker (5%), while 7% did not report it. Analysis methods included fMRI (52%), VBM (29%), DTI (18%), and CT (10%). Finally, 84% of studies included GM analyses, while 23% included WM analyses (Supplementary Tables 1, 2, and 3).
ALE meta-analysis
The quantitative synthesis of the primary outcome included 58 publications with 675 foci, 79 experiments and a total of 2780 participants. Separate ALE meta-analyses were conducted in GingerALE37 for structural and functional foci, focusing on the comparison between M and NM.
Structural studies
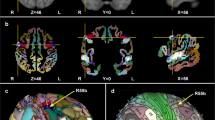
The structural ALE meta-analysis included 33 experiments and 1515 participants. The contrast M > NM in GM resulted in significant peak clusters in the bilateral superior temporal gyrus (primary auditory cortex), including the bilateral Heschl’s gyrus and planum temporale, and the postcentral gyrus (somatosensory cortex, SI), including area 4a of the primary motor cortex (M1 4a). Conversely, the comparison NM > M in GM resulted in a significant peak cluster located in the right precentral gyrus (primary motor cortex, M1). In WM, musicians showed larger tracts of the internal capsule bundle (extending to the thalamus) and corticospinal tract. No significant clusters were identified in the comparison NM > M for WM (Fig. 1, Table 2).
Anatomic likelihood estimation meta-analytic results for studies comparing brain structure and function between M and NM at cluster level inference p < 0.05 (FWE). The primary outcome included ALE meta-analysis of the contrast M vs NM for structural and functional modalities, independently. M > NM = higher volume/activity in musicians; NM > M = lower volume/activity in musicians; GM grey matter, WM white matter, L left, R right, A anterior, P posterior, Z peak Z-value, IC internal capsule, INS insula, IPL inferior parietal lobule, PostCG postcentral gyrus (primary somatosensory cortex, or S1), PreCG precentral gyrus (primary motor cortex, or M1), STG superior temporal gyrus (primary auditory cortex).
Functional studies
The functional ALE meta-analysis included 46 experiments and 1265 participants. The contrast M > NM resulted in large and significant peak clusters of the bilateral superior temporal gyrus (the bilateral primary auditory cortices) extending to the left insula, the left inferior frontal gyrus, and the left precentral gyrus (primary motor cortex, M1). The comparison NM > M resulted in smaller peak clusters of the left inferior parietal lobule and the left precentral gyrus (Fig. 1, Table 2).
Meta-analytic connectivity modelling (MACM)
MACM was performed to functionally segregate the behavioural contribution and the patterns of co-activation of each music-related region-of-interest (ROI) resulted from the structural (n = 5) and functional (n = 5) ALE meta-analyses. Five-millimetre ROIs were created using Mango41 and imported into the BrainMap42 database separately using Sleuth39. Foci from each identified study were extracted and a secondary GingerALE meta-analysis was performed aiming to identify the functional network of each ROI, namely its functional connectivity (FC)38 (Supplementary Table 4). Finally, the functional characterization of each ROI was described using Sleuth and focused on behavioural domains (e.g., action, perception, emotion, cognition and interoception) and paradigm classes (e.g., pitch discrimination, finger tapping, music comprehension, go/no-go) included in the BrainMap platform (Supplementary Table 5).
Structural ROIs
The right superior temporal gyrus ROI (Fig. 2a) showed co-activation with left superior temporal gyrus, right precentral gyrus, left medial frontal gyrus, left cerebellum, and left thalamus. Relevant behavioural domains within its boundaries include execution, speech, memory, music, emotion, reward, and auditory perception; and experimental paradigms including emotion induction, finger tapping, music comprehension and production, passive listening, reasoning/problem solving, and phonological, pitch, semantic, syntactic, and tone discrimination.
Meta-analytic connectivity modelling of regions-of-interest that resulted from the structural ALE meta-analysis, at cluster level inference p < 0.05 (FWE). ROIs music-related regions-of-interest, P p-value, Z peak z-value, R right, L left. ROIs: (a) STG-R right superior temporal gyrus; (b) STG-L left superior temporal gyrus; (c) PostCG-R right postcentral gyrus; (d) PreCG-R right precentral gyrus; (e) IC/THA-R internal capsule (including right thalamus). Co-activated areas: claustrum; CRBL cerebellum, IFG inferior frontal gyrus, IPL inferior parietal lobule, INS insula, MedFG medial frontal gyrus (pre-motor), MidFG middle frontal gyrus (pre-frontal), PostCG postcentral gyrus (primary somatosensory cortex or S1), PreCG precentral gyrus (primary motor cortex or M1), PUT putamen, STG superior temporal gyrus (primary auditory cortex), THA thalamus. To conduct MACM, music-related ROIs were created in Mango (http://rii.uthscsa.edu/mango//userguide.html) with a 5 mm-radius sphere. For visualization purposes, the music-related ROI radius was increased to 10 mm, while co-activated areas were created with a 5 mm-radius sphere. Last search in Sleuth, 10.10.2021 (http://www.brainmap.org/sleuth/).
The left superior temporal gyrus ROI (Fig. 2b) showed co-activation with right superior temporal gyrus, right insula, right inferior frontal gyrus, bilateral precentral gyrus, and left medial frontal gyrus. Relevant behavioural domains within its boundaries include execution, speech, motor learning, attention, language, speech, memory, music, emotion, and auditory perception; and experimental paradigms including emotion induction, finger tapping, music comprehension and production, passive listening, reasoning/problem solving, visuospatial attention, and oddball, orthographic, phonological, pitch, semantic, and tone discrimination.
The right postcentral gyrus ROI (Fig. 2c) showed co-activation with left medial frontal gyrus, left parietal lobule, right middle frontal gyrus, right thalamus, right superior temporal gyrus, and right cerebellum. Relevant behavioural domains within its boundaries include execution, motor learning, attention, respiration regulation, and auditory perception; and experimental paradigms including finger tapping, passive listening, visuospatial attention, and oddball, tactile, and tone discrimination.
The right precentral gyrus ROI (Fig. 2d) showed co-activation with claustrum and insula. Relevant behavioural domains within its boundaries include execution, attention, speech, temporal processing, and emotional processing; and experimental paradigms including finger tapping and visuospatial attention.
The right internal capsule ROI (Fig. 2e), including the right thalamus as the nearest grey matter, showed co-activation with left thalamus, right medial frontal gyrus, right insula, and left cerebellum. Relevant behavioural domains within its boundaries include execution, speech, attention, memory, reasoning, emotion, reward, and auditory perception; and experimental paradigms including emotion induction, finger tapping, passive listening, reward, and tone discrimination.
Functional ROIs
The left inferior frontal gyrus ROI (Fig. 3a) showed co-activation with right inferior frontal gyrus, left inferior and superior parietal lobules, left medial frontal gyrus, left fusiform gyrus, and right caudate. Relevant behavioural domains within its boundaries include execution, speech, attention, language, memory, music, reasoning, social cognition, emotion, and auditory perception; and experimental paradigms including encoding, finger tapping, music comprehension and production, passive listening, reasoning/problem solving, reward, and phonological, semantic, tactile, and tone discrimination.
The right superior temporal gyrus ROI (Fig. 3b) showed co-activation with bilateral inferior frontal gyrus, bilateral inferior parietal lobule, left medial frontal gyrus, left fusiform gyrus, and caudate. Relevant behavioural domains within its boundaries include execution, speech, attention, language, memory, music, reasoning, social cognition, emotions, and auditory perception; and experimental paradigms including finger tapping, music comprehension and production, passive listening, reasoning/problem solving, reward, theory of mind, and phonological, semantic, tactile, and tone discrimination.
The left superior temporal gyrus ROI (Fig. 3c) showed co-activation with right superior temporal gyrus, right middle temporal gyrus, right claustrum, left insula, and left medial frontal gyrus. Relevant behavioural domains within its boundaries include execution, speech, attention, language, memory, reasoning, social cognition, emotion, and auditory perception; and experimental paradigms including divided auditory attention, emotion induction, emotional body language perception, encoding, finger tapping, music comprehension and production, reasoning/problem solving, theory of mind, visuospatial attention, and oddball, phonological, pitch, semantic, and tone discrimination.
The left inferior parietal lobule ROI (Fig. 3d) showed co-activation with left medial frontal gyrus, right inferior frontal gyrus, and left precentral gyrus. Relevant behavioural domains within its boundaries include execution, speech, motor learning, attention, memory, music, reasoning, social cognition, emotion, and auditory perception; and experimental paradigms including emotion induction, finger tapping, motor learning, reasoning/problem solving, reward, visuospatial attention, and phonological, semantic, tactile and tone discrimination.
The left precentral gyrus ROI (Fig. 3e) showed co-activation with left precuneus, left superior frontal gyrus, right inferior frontal gyrus, right inferior parietal lobule, right claustrum, left fusiform gyrus, left thalamus, and right middle frontal gyrus. Relevant behavioural domains within its boundaries include execution, speech, motor learning, attention, language, memory, music, reasoning, social cognition, temporal processing, emotion, sleep, and auditory perception; and experimental paradigms including divided auditory attention, emotion induction, encoding, finger tapping, music comprehension, reasoning/problem solving, reward, theory of mind, visuospatial attention, and oddball, phonological, pitch, semantic, tactile, and tone discrimination.
Meta-analytic connectivity modelling of regions-of-interest that resulted from the functional ALE meta-analysis, at cluster level inference p < 0.05 (FWE). ROIs music-related regions-of-interest, P p-value, Z peak z-value, R right, L left. ROIs: (a) IFG-L left inferior frontal gyrus; (b) STG-R right superior temporal gyrus; (c) STG-L left superior temporal gyrus; (d) IPL-L left inferior parietal lobule; (e) PreCG-R right precentral gyrus. Co-activated areas: CAU caudate; claustrum, CRBL cerebellum, FusG fusiform gyrus, IFG inferior frontal gyrus, IPL inferior parietal lobule, INS insula, MedFG medial frontal gyrus (pre-motor), MidFG middle frontal gyrus (pre-frontal), PostCG postcentral gyrus (primary somatosensory cortex or S1), PreCG precentral gyrus (primary motor cortex or M1), PCN precuneus, PUT putamen, SFG superior frontal gyrus, STG superior temporal gyrus (primary auditory cortex), THA thalamus. To conduct MACM, music-related ROIs were created in Mango (http://rii.uthscsa.edu/mango//userguide.html) with a 5 mm-radius sphere. For visualization purposes, the music-related ROI radius was increased to 10 mm, while co-activated areas were created with a 5 mm-radius sphere. Last search in Sleuth, 10.10.2021 (http://www.brainmap.org/sleuth/).
Discussion
The link between musical expertise and humans’ cognitive functions has been explored with great interest since the times of Pythagoras. Recent years reveal a renewed and more than vivid attention to the topic, as reflected in the rising number of empirical research in the past half-century1,2. Decades of investigations in psychology, cognitive and translational neuroscience have attempted to foster our understanding of the neurocognitive processes underlying musical expertise. Thus, long-term musical training has been associated with neuro-anatomical and -functional specializations in brain regions engaged in multimodal (audio-visual) sensory and sensorimotor perception, integration and predictions as well as fine movement control17,18. Furthermore, the duration and intensity of training has been associated with improvements in general cognition, ranging from working memory, intelligence, executive functions and inhibitory control6,9,10,11. However, as mentioned before, this rapidly growing field of research is also characterized by some methodological inconsistencies (e.g., sample differences and neglected background variables), and sometimes shows discrepant results and controversial interpretations of the findings. Such limitations, alongside with the absence of a meta-analysis, has prevented the plethora of variegated findings to ultimately converge into a unified picture of the neuroanatomy of musical expertise.
To address this lack in the literature, we performed a comprehensive and quantitative meta-analysis of neuro-anatomical and -functional studies investigating brain changes associated with long-term musical training. Our coordinate-based anatomic/activation likelihood estimation (ALE) meta-analysis effectively summarizes decades of research in the field and finally provides a consistent and controversies-free picture of the core brain regions engaged in and influenced by long-term music processing and production. To better characterize the emergent neural network of musical expertise, we performed meta-analytic connectivity modelling analyses (MACM) and functionally linked each node of the music network to specific cognitive functions. By discussing the main results of the meta-analysis alongside with the observations derived from MACM, we ultimately provide a comprehensive view of the anatomical, functional, and cognitive substrates of musical expertise. This discussion is organized in three main paragraphs: ‘the ear’, ‘the body’ and ‘the heart’, elaborating on the emergent fronto-temporal, sensorimotor and interoceptive networks respectively. Notably, MACM further allows us to strengthen the notion that musical training represents a stimulating multisensory experience engaging not only sensory and motor functions strictly related to acoustic and motor processes, but a wide variety of high-order cognitive functions from working memory, attention, executive functions and emotional regulation11,16,17,18,45. To conclude, we argue that the observed music-related neuroanatomical and -functional changes represent an interface between nature and nurture effects. Namely, gene-environment interactions and other background variables likely interacted with brain maturation processes ultimately influencing the neuroplasticity mechanisms responsible for the observed training-specific neuroanatomical and -functional changes.
Characteristics of the included studies
The publications included in this systematic review and meta-analysis reported a clear research question, inclusion and exclusion criteria for participants, description of methods and explicit results. Most of the studies used state-of-the-art techniques and computational MRI-tools, important for the support of standardization and reproducibility of neuroimaging studies. However, some of the studies lacked important demographic data such as the years of education, age of musical training onset, and current time of musical practice, which may influence behavioural tasks and neuroimaging data. Thus, our research encourages to adopt in future studies standardized tools specifically designed and validated for assessing musical expertise46.
Structural and functional neuroplasticity in musical expertise
Our results highlight that expert musicians exhibited higher GM volume in the bilateral superior temporal gyri and right postcentral gyrus and greater WM volume in the right internal capsule bundle and corticospinal tract, as compared to non-musicians. Additionally, musicians exhibited higher activity of the bilateral superior temporal gyri, left inferior frontal gyrus, left precentral gyrus, and left insula. On the other hand, musicians had lower GM volume in areas of the sensorimotor cortex and no WM structure was found to have larger volume in non-musicians as compared to musicians. Finally, musicians exhibited lower neurofunctional activation of the inferior parietal lobule and motor cortex during a variety of cognitive tasks.
The ear: enhanced frontotemporal auditory network in musicians
One of our main findings shows enlargement of GM volume in musicians located in medial and posterior superior temporal regions, with clusters extending into primary and secondary auditory cortices. These regions include neuronal assemblies dedicated to encoding of spectro-temporal features of sounds relevant to music47, such as the discrete pitches forming the Western chromatic scale and fine changes in pitch intervals48. More specifically, it seems that the posterior supratemporal regions are more involved in encoding the height of pitch, whereas the anterior regions are representing the chroma, that is the pitch category irrespectively of the octave49. Moreover, these areas participate in auditory imagery of melodies50 and in the processing of the contour and Gestalt patterns of melodies, allowing for recognition and discrimination of mistakes51.
Beyond music-related functions, functional characterization analyses of our ROIs (Supplementary Table 5) show that superior temporal regions are usually recruited for phonological processing and multimodal integration of sensory information. Accumulating evidence has shown that the superior temporal sulcus and posterior superior temporal gyrus, together with early auditory regions (HG), are involved for the processing of speech sounds, abstract representation of speech sounds, as well as more general language, phonology and sematic processing and audio-visual integration. Therefore, temporal regions seem to represent fundamental structures for both language and music processing52. MACM further revealed that auditory cortices tend to co-activate with insula, (pre)motor regions, inferior and medial frontal gyri, thalamus and cerebellum, confirming the relevance of extended cortico-subcortical audio-motor coupling for rhythm processing in language and music53,54,55,56. Further supporting this view, MACM showed that the inferior frontal gyrus co-activates with motor areas in the cortex and the basal ganglia (see next paragraph, ‘The body’), and with parietal areas related to the dorsal auditory pathway.
The inferior frontal gyrus has been described as an important hub of both the dorsal and ventral auditory streams. The dorsal auditory stream connects the auditory cortex with the parietal lobe, which projects in turn to the inferior frontal gyrus pars opercularis (Brodmann area 44). The inferior frontal gyrus has been related to the articulatory network, dedicated to specific functions of speech comprehension and production, and highly connected to premotor and insular cortices57. The ventral auditory stream connects the auditory cortex with the middle temporal gyrus and temporal pole, which in turn connects to the inferior frontal gyrus pars triangularis (Brodmann area 45). This area has been associated with semantic processing58. These two regions within the inferior frontal gyrus constitute Broca’s area. The supramarginal gyrus is also a relay of the dorsal auditory stream involved in processing of complex sounds, including language and music59. As such, it is considered an integration hub of somatosensory input60.
The parietal lobe has been also described as an integration area of sensory inputs. The superior parietal lobule includes Brodmann areas 5 and 7, which are involved in somatosensory processing and visuomotor coordination, respectively. The inferior parietal lobule includes Brodmann areas 39 and 40, the angular gyrus and supramarginal gyrus, respectively. The angular gyrus has been related to projection of visual information to Wernicke’s area, memory retrieval and theory of mind61. MACM revealed that the parietal lobe co-activates with sensorimotor cortices and the inferior frontal gyrus.
The body: enhanced sensorimotor functions in musicians
The precentral and postcentral gyri represent the primary motor and somatosensory cortex, respectively. These two areas are divided by the central sulcus, whose extension represent the sensation and motion of segregated body parts. Our findings show both convergent and divergent effect of musical training in these areas, suggesting a more complex picture than previously thought. For example, neuroadaptations in the sensorimotor system may vary depending on the musical instrument of use62. MACM revealed that the primary motor cortex co-activates with an extensive network that includes the frontal pole, limbic areas such as the anterior cingulate cortex and insula, and parietal areas such as the precuneus. It also revealed that the primary somatosensory cortex co-activates with motor and pre-motor areas, basal ganglia, thalamus, and the cerebellum. A dedicated temporal processing network has been described by Kotz and Schwartze54 including such areas, which are important for implementing sequential actions, as well as to form predictions about the timing of external events. Healthy motor performance relies on a functional loop established by the basal ganglia and supplementary motor area that maintains adequate preparation for sequential movements. The supplementary motor area prepares for predictable forthcoming movements, keeping the system “ready”. Once the movement starts, the supplementary motor area’s readiness activity stops. This cycle engages with BG discharges after each sub-movement within an automatized sequence63. The loop requires an internal cue to coordinate the cycle.
The basal ganglia are nuclei of neurons important for the initiation and suppression of movements. In the motor loop of the basal ganglia (BG), inputs from motor cortices project to the dorsal striatum, composed by the putamen and caudate. In the presence of adequate dopaminergic signalling, the ‘direct pathway’ (cortex—striatum—internal pallidum—thalamus—cortex) works to facilitate movement, while the ‘indirect pathway’ suppresses it (cortex—striatum—external pallidum—subthalamic nucleus—thalamus—cortex). Zooming into inhibitory processes, the striatum transiently inhibits the pallidum, and in turn, the motor area of the thalamus is disinhibited and is free to project back to the motor cortex, initiating a motor program that flows down the corticospinal tract. Similarly, the subthalamic nucleus in the indirect pathway is transiently inhibited when suppressing movement, increasing the inhibition of the pallidum over the thalamus, therefore blocking the motor cortex activity64.
Our findings show neuroadaptive processes in the putamen and caudate of musicians (striatum), presumably reflecting effective inhibitory mechanisms as seen by fine movement control. Furthermore, our findings strengthen the notion that basal ganglia circuits are involved in motor sequence learning, and in particular in the learning and control of fine-movement sequences acquired through music practice65,66.
The cerebellum has been shown to play a crucial role in multiple cognitive processes such as sensory discrimination, rhythmic perception and production, working memory, language, and cognition67. Previous fMRI studies in humans suggest that the cerebellum shows segregated activations for motor and cognitive tasks. Motor tasks seem to activate lobules IV-VI in the superior parts of the anterior cerebellum. In contrast, attentional or working memory tasks activate posterior cerebellar hemispheres, namely lobule VIIA, which is divided to crus I and crus II, as well as lobule VIIB68. Musicians and non-musicians show GM volume differences in the cerebellum, specifically in area Crus I. In our study, this area did not survive correction for multiple comparisons, however MACM revealed that the cerebellum is functionally connected to auditory cortices, somatosensory cortices, and the thalamus. It has been demonstrated that the activity in crus I/II has a specific relationship with cognitive performance and is linked with lateral prefrontal areas activated by cognitive load increase69. In other words, the crus I/II seems to optimize the response time when the cognitive load increases. Additionally, it has been suggested that crus I/II is associated with beat discrimination thresholds. Thus, there is a positive correlation between GM volume in crus I and beat discrimination performance, evidenced by enhanced ability in musicians70.
The heart: enhanced interoceptive areas in musicians
Among the other results, our meta-analysis reported higher functional activation of left insula in musicians as compared to non-musicians. MACM analyses reported the left insula in a functional network that connects inferior frontal gyrus with precentral gyrus, middle frontal gyrus and parietal lobule bilaterally (Supplementary Table 4).
It has been proposed that the insula and the anterior cingulate cortex (ACC) are part of the salience network, and coordinate interactions between the default-mode network and the central executive network71. The ACC has been related to cognitive and emotional processing. The cognitive component projects to prefrontal, motor, and parietal areas to process top-down and bottom-up stimuli. The emotional component features connections from the insula to amygdala, nucleus accumbens, hypothalamus and hippocampus, with the scope to assess the salience of emotional and motivational information72. Moreover, the insula integrates information from the internal physiological state, and projects to the ACC, ventral striatum and prefrontal cortex to initiate adaptive responses73. Thus, enhanced function of these areas after musical training may be associated with a more efficient coordination between interoceptive, emotional, salience and central executive networks.
White matter
M exhibited larger clusters of WM as compared to NM in the internal capsule and cortico-spinal tract. While previously thought to be rather passive tissues, WM tracts are now consistently associated with an active modulatory role in information flow between brain regions64. Indeed, myelin regulates the speed of action potential transfer within and between GM structures and further provide metabolic support to local neural cells. WM changes are commonly observed during learning and associated with fast, accurate and coordinated motor sequences27.
The internal capsule is a WM structure which connects basal ganglia regions and carries information from and to surrounding cerebral cortex. Connecting fibres in basal ganglia might be thickened by musical expertise because of their involvement in motor control, rhythmic processing, sequence learning, reinforcement learning and memory processes65. In general, basal ganglia structures are recruited during working memory processing for musical motifs74 and the most ventral regions are a core structure of the reward circuit. Interestingly, they are found to be more active in musicians as compared to non-musicians while listening to expressive music75.
The corticospinal tract allows the motor plans originated in the cortex to be transferred to motor nuclei in the spinal cord and to finally regulate the activity of muscle effectors. The myelination and integrity of the corticospinal tract has been observed to be increased in expert musicians27,28, and is further influenced by the time of onset of musical practice31 with early onset musician showing the greatest axial diffusivity.
Structural connectivity analyses comparing M vs NM are scarce in the literature with high variability of designs, methods, and results76. However, it is suggested that neuroadaptive effects of musical expertise relies on effective structural and functional communication between cortical and subcortical sensorimotor areas through thalamic radiations and the internal capsule. Moreover, differences in the corpus callosum connecting both hemispheres have been reported in studies using DTI77,78, which may reflect the bimanual coordination and related inter-hemispheric connections required for playing most musical instruments. Notably, such differences appear to be more salient in musicians that started musical training before the age of seven27,79,80. Taken together, our results and previous results suggest that the acquisition of musical skills will develop structural and functional connections between auditory, sensorimotor, timing, and reward areas of the brain reflecting the network-like nature of the human brain.
(Dis)similarities between anatomical and functional studies
The meta-analyses on neuroanatomical and -functional changes coherently show greater GM volumes and increased functional engagement of superior temporal gyrus bilaterally, together with pre- and post-central gyri in expert musicians as compared to laypersons. Functional studies further agree on the pivotal involvement of left inferior frontal gyrus (BA9, BA44) along with superior temporal gyrus bilaterally in musicians. However, dissimilarities emerge when looking at pre- and post-central regions: right precentral gyrus (right primary motor cortex (M1)) is reduced in musicians, while the right postcentral gyrus [right primary somatosensory cortex (S1)] is increased. Functional studies show, instead, that there is increased activity in left precentral gyrus in the inferior frontal gyrus—superior temporal gyrus—insula network, and reduced activity of the left precentral gyrus in a cluster which extends into the left parietal lobule.
While results pertaining to the frontotemporal auditory network and the sensorimotor network have been discussed in ‘The ear’ and ‘The body’ paragraphs above, we here speculate that the enlargement of S1 in musicians is associated with a more sophisticated representation of the sensorimotor periphery19 and that the increased left inferior frontal gyrus—precentral gyrus—superior temporal gyrus—insula activation, at the expense of the M1-parietal lobule network may be related to the acquisition of accurate and automatized motor programs in musicians4. This view is further corroborated by the connectivity observed between primary somatosensory cortex, motor and pre-motor areas, basal ganglia, thalamus, and the cerebellum in the MACM analyses. In agreement with early studies, we lastly argue that the hemispheric asymmetry may be related to the music instrument played and the dominant hand of the musicians81, but interhemispheric transfer effects are possible with motor sequence learning82. However, longitudinal studies should further elucidate on the heterogeneity of structural and functional adaptations associated with intensive and long-lasting motor training.
Limitations and future perspectives
This comprehensive review and meta-analysis had the scope to summarize decades of research investigating neuro-anatomical and -functional changes associated with musical expertise. Our qualitative review highlights that previous studies in this field are characterized by heterogeneity of methods, paradigms, and sample backgrounds, as well as relevant missing information. While arguing that the field will benefit from more clarity (e.g., thorough description of methods) and consistency, we also delineate limitations for our meta-analysis. For example, we set a contrast based on the comparison M vs NM with the aim to narrow down the heterogeneity of the sample and methods in use. However, by doing so we relied on two assumptions: (1) the data we pool is based on best research practices; (2) the validity of the GingerALE method. Indeed, to conduct the ALE meta-analysis, we pooled peak coordinates derived from the included studies, rather than using original raw structural MRI images. Thus, the accuracy of our findings relies on the result of a statistical estimation of coordinate-based anatomic foci (input), treated as spatial probability distributions centred at the given coordinates. The heterogeneity of the methods in use in previous studies (ranging from pre-processing software, smoothing, statistical thresholds and participants’ characteristics) are not under our control and represent potential confounders for the results. Perhaps a regression-based assessment of the influence of those heterogenous factors on the findings would sharpen the results. However, meta-regression analysis is not compatible with GingerALE. When assessing publication bias using the Fail Safe-N analysis, we found adequate robustness of our results, with only 2 ROIs showing an FSN below of the minimum imposed in each of the ALE within contrasts (BA2, BA4 in the structural ALE and BA22, BA6 in the functional ALE), thus, indicating an overall robust convergence of foci our study (further information is reported in Supplementary Table 6).
Lastly, on a more theoretical perspective, our results contribute but do not solve the long-standing “nature vs nurture” debate. Indeed, based on evidence that musical training stimulates higher-cognitive functions, auditory-motor integration, attention, memory and engages reward networks, some have suggested that it may be particularly effective in driving neuroplastic mechanisms78. However, we are indeed blind to whether the highlighted differences emerging when comparing M vs NM are training-dependent or due to innate predispositions. Altogether, the most reasonable conclusion is that the observed neuro-anatomical and -functional changes may be attributed to the interaction between brain maturation processes and gene-environment interactions13,50,85. Notably, multiple studies demonstrated a strong correlational link between the length of musical training and neuroanatomical and -functional changes83,84. For instance, the study conducted by Gaser and Schlaug85 reported that amateur musicians showed an intermediate increase in gray matter volume when compared to NM and M, supporting the idea of use-dependent structural changes. The same pattern was found when comparing cognitive abilities, with amateurs showing higher cognitive abilities than NM, but lower than M11. To be noted, however, this research field suffers of the paucity of longitudinal (f)MRI studies conducted with children, which thus far amount only to seven4,5,6,7,8,86,87, next to one 15-week long study in adults88. Longitudinal studies are the only ones promising to better elucidate on the causal link between musical training and neural adaptations. Our work, on the other hand, pools a large quantity of anatomical and functional MRI studies conducted over > 20 years of world-wide research. By doing so, it bears the potential to achieve an unprecedented signal-to-noise ratio, so to filter out the mediating influence of background, psychological and other environmental factors, and to effectively isolate music-related neuroplastic changes. Thus, we here provide, within the delineated limits, a consistent view of the neuroanatomy of neural expertise. Furthermore, we explore the connections and functions of the brain areas that appear to be key in the acquisition of musical skills. Such regions include auditory, limbic, and sensorimotor regions that reflect the network-like nature of the human brain. We hope our work would better inform future basic and comparative research in the field of auditory and cognitive neuroscience and that we encouraged translational approaches bridging to the clinical field89,90.
Conclusions
The neuroanatomical and functional changes observed in the musician’s brain have been repeatedly regarded as the ideal scenario to investigate neuroplastic mechanisms. Yet, decades of research in cognitive neuroscience have provided a scattered and partially controversial series of findings. The present coordinate-based meta-analysis represents a comprehensive and quantitative attempt to summarize existing literature and provide a unified picture of the neuroanatomy of musical expertise. We show that music experts exhibit bilateral cortico-subcortical neuroanatomical and -functional differences as compared to laypersons. This systematic review and meta-analysis strengthens the view that musical training represents a beneficial and stimulating multisensory experience which engages a wide variety of neurocognitive functions.
Methods
Literature search, screening, and extraction
This systematic review and meta-analysis followed procedures from the Cochrane Handbook for Systematic Reviews91 and from the Centre for Reviews and Dissemination (Centre for Reviews and Dissemination, 2014). The review protocol was registered with PROSPERO No. [CRD42017060365]. This review was carried in accordance with the PRISMA statement92.
Systematic search was performed using PubMed, PsycInfo and Scopus, of publications that reported brain structural or functional differences between M and NM. The search (March 2021) included MeSH terms (“music”, “education”, “brain”, “motor skills”, “magnetic resonance imaging”) and key words (“musical training”, “musician”). No years or places of publication were imposed.
For qualitative synthesis, studies were included if they met the following criteria: (1) studies comparing brain structure and function between musicians and non-musicians, (2) in adult population, (3) by means of magnetic resonance imaging, in either structural modality (e.g., voxel-based morphometry [VBM]) or functional modality (e.g., functional magnetic resonance imaging [fMRI]). For the final quantitative synthesis (meta-analysis), studies were included only if the results were reported in stereotactic coordinates either Talairach or Montreal Neurological Institute (MNI) three-dimensional-coordinate system.
Studies were excluded using the following criteria: (1) review articles with no original experimental data, (2) neuroimaging data from non-MRI studies (e.g., PET), (3) pathological population, (4) longitudinal designs, (5) functional connectivity analyses, and (6) analyses based on region-of-interest (ROI) rather than whole-brain (only quantitative synthesis).
Two reviewers (AC and VP) independently screened by title and abstract and selected articles for full-text review and performed full-text reviews. Screening and data extraction were performed using the Covidence tool93. Any disagreements that arose between the reviewers were resolved through discussion or by a third and/or fourth reviewer (LB, EB).
From each study, the following variables were extracted: first author, year of publication, population of interest, number of participants, age, sex, absolute pitch, musical feature, years of education, years of musical training, age of musical training onset, weekly training, musical instrument, MRI-system, MRI-model, head-coil, image acquisition parameters of T1, T2* and DWI sequences, repetition time (TR), echo time (TE), voxel size, analysis method and software. The main outcome to extract was any difference in structure or function, in stereotactic coordinates, comparing a musician group and a non-musician group. If any of these points were not reported in the original article, authors were contacted to retrieve this information. Six authors were contacted, with 2 positive answers.
Quality assessment of MRI studies
Criteria for MRI quality reporting was selected from a set of guidelines for the standardized reporting of MRI studies43,44. Such guidelines dictate a more consistent and coherent policy for the reporting of MRI methods to ensure that methods can be understood and replicated.
Activation likelihood estimation (ALE)
To test the convergence of findings from the neuroimaging studies, we used the anatomic/activation likelihood estimation (ALE) method implemented in the GingerALE software v3.0.235, a widely used technique for coordinate-based meta-analysis of neuroimaging data. Statistically significant foci from between-group contrasts were extracted and recorded for each study. If necessary, coordinates were converted from Talairach coordinates to MNI space using the Lancaster transform (icbm2tal) incorporated in GingerALE37,94. The ALE method uses activation foci (input) not as single points, but as spatial probability distributions centred at the given coordinates. Therefore, the algorithm tests to what extent the spatial locations of the foci correlate across independently conducted MRI studies investigating the same construct and assesses them against a null distribution of random spatial association between experiments46. Statistical significance of the ALE scores was determined by a permutation test using cluster-level inference at p < 0.05 (FWE), with a cluster-forming threshold set at p < 0.001. First, we used the ALE meta-analytic technique to identify brain differences measured by MRI between musicians (M) and non-musicians (NM) with the aim of comprehensively examine the neural signatures of musical expertise. Two independent ALE meta-analyses were conducted for structural studies and functional studies. To test the directionality of the M vs NM contrast, foci were pooled reporting higher volume/activity in musicians (M > NM) and lower volume/activity in musicians (NM > M) for both structural and functional studies.
Meta-analytic connectivity modelling (MACM)
Meta-analytic connectivity modelling (MACM) was performed to analyse co-activation patterns of music-related regions-of-interest (ROI) resulted from the structural (n = 5) and functional (n = 5) ALE meta-analyses, independently, and to functionally segregate each region’s putative contribution to behavioural domains and paradigm classes according to the BrainMap platform40,41,42.
Large-scale databases such as BrainMap store results obtained from published brain activation (functional) and brain structure (voxel-based morphometry) studies36,95. Such databases can be taken into advantage with a meta-analytic approach focusing on the co-activation of brain regions with a specific ROI across all kinds of different mental processes, rather than to a specific mental process. Thus, MACM identifies the functional network of the ROI, namely, its functional connectivity (FC). Traditionally, in fMRI studies, two brain regions are functionally connected when there is a statistical relationship between the measures of neuronal activity, by means of the blood-oxygen-level-dependent signal (BOLD), both during resting-state (task-free FC) or performing a specific task (task-dependent FC). In contrast, MACM relies on patterns of co-activation across many different tasks and allows to examine task-based FC in a general manner40,96,97. Thus, MACM provides a data-driven and unbiased approach to determine the connectivity “signature” of a given ROI.
Co-activation analyses were performed using Sleuth42 and GingerALE35 from the BrainMap platform. To identify regions of significant convergence, an ALE meta-analysis was performed over all foci retrieved after searching Sleuth by each music-related ROI independently and included the experiment level search criteria of “context: normal mapping” and “activations: activation only”. Music-related ROIs were created in Mango98 with a 5 mm-radius sphere. The results of each ROI search were exported to GingerALE, and a permutation test was conducted using cluster-level inference at p < 0.05 (FWE), with a cluster-forming threshold set at p < 0.001.
Finally, MACM allows to conduct functional profiling of ROIs to study brain-behaviour relationships at a meta-analytic level. In other words, through the BrainMap platform, it is possible to objectively characterize a given ROI in terms of its cognitive/behavioural function which are based on the meta-data that is stored in the database38. Thus, tasks in the database are coded in a way that is possible to conduct a behavioural profile of ROIs that resulted from an ALE meta-analysis. The tasks are coded in two dimensions: behavioural domains (BD) and paradigm classes (PC). As the present study has two independent meta-analyses, one for structural studies and one for functional studies, MACM was divided into ROIs that resulted from the structural ALE meta-analysis and ROIs that resulted from the functional ALE meta-analysis. The functional characterization of music-related ROIs was based on the BD meta-data categories available for each neuroimaging study in the database which include action, perception, emotion, cognition and interoception. PC refer to paradigms that have been used repeatedly by different researchers with only minor changes. Such paradigms have become widely known and accepted by the neuroimaging field (e.g., pitch discrimination, finger tapping, music comprehension, go/no-go). A BD refers to the categories and sub-categories of mental operations likely to be isolated by the experimental contrast; a PC is the experimental task isolated by the contrast of interest. Notably, multiple BDs and PCs may apply for a given experiment99.
All meta-analytic results (ALE maps) were visualized using Mango41 on the MNI152 1 mm standard brain, and resulting coordinates were cross-referenced to the Harvard–Oxford Cortical and Subcortical Atlas and the Juelich Histological Atlas via NeuroVault100 and FSLeyes101, respectively.
Fail-Safe N analysis (FSN)
As all meta-analyses, coordinate-based meta-analyses such as ALE can be subject to different forms of publication bias which may impact results and invalidate findings (e.g., the “file drawer problem”). Thus, the Fail-Safe N analysis (FSN)102 was performed as a measure of robustness against potential publication bias. It refers to the amount of contra-evidence that can be added to a meta-analysis before the results change and can be obtained for each cluster that survives thresholding in an ALE meta-analysis. For normal human brain mapping, it is estimated that a 95% confidence interval for the number of studies that report no local maxima varies from 5 to 30 per 100 published studies. Therefore, the minimum FSN was defined as 30% of total studies for each CBMA. A higher FSN indicates more stable results and hence a higher robustness.
Data availability
The data supporting the findings of this study is freely available at the Open Science Framework (OSF) website https://osf.io/5ekqr/?view_only=4416037a1b164e6287d95e7f24dd0a0a.
References
Vuust, P., Heggli, O. A., Friston, K. J. & Kringelbach, M. L. Music in the brain. Nat. Rev. Neurosci. https://doi.org/10.1038/s41583-022-00578-5 (2022).
Pando-Naude, V., Patyczek, A., Bonetti, L. & Vuust, P. An ALE meta-analytic review of top-down and bottom-up processing of music in the brain. Sci. Rep. 123, 20813 (2021).
Herholz, S. C. & Zatorre, R. J. Musical training as a framework for brain plasticity: Behavior, function, and structure. Neuron 76, 486–502 (2012).
Hyde, K. L. et al. The effects of musical training on structural brain development: A longitudinal study. Ann. N. Y. Acad. Sci. 1169, 182–186 (2009).
Wehrum, S. et al. Can you hear a difference? Neuronal correlates of melodic deviance processing in children. Brain Res. 1402, 80–92 (2011).
Sachs, M., Kaplan, J., Sarkissian, A. D. & Habibi, A. Increased engagement of the cognitive control network associated with music training in children during an fMRI Stroop task. PLoS One 12, e0187254 (2017).
Hennessy, S. L., Sachs, M. E., Ilari, B. & Habibi, A. Effects of music training on inhibitory control and associated neural networks in school-aged children: A longitudinal study. Front. Neurosci. 13, 1080 (2019).
Kausel, L. et al. Neural dynamics of improved bimodal attention and working memory in musically trained children. Front. Neurosci. 14, 1023 (2020).
Schellenberg, E. G. Music lessons enhance IQ. Psychol. Sci. 15, 511–514 (2004).
Swaminathan, S., Schellenberg, E. G. & Venkatesan, K. Explaining the association between music training and reading in adults. J. Exp. Psychol. Learn. Mem. Cogn. https://doi.org/10.1037/xlm0000493 (2018).
Criscuolo, A., Bonetti, L., Särkämö, T., Kliuchko, M. & Brattico, E. On the association between musical training, intelligence and executive functions in adulthood. Front. Psychol. 10, 1704 (2019).
Reybrouck, M. & Brattico, E. Neuroplasticity beyond sounds: Neural adaptations following long-term musical aesthetic experiences. Brain Sci. 5, 69–91 (2015).
Miendlarzewska, E. A. et al. How musical training affects cognitive development: Rhythm, reward and other modulating variables. Front. Neurosci. https://doi.org/10.3389/fnins.2013.00279 (2014).
Swaminathan, S., Schellenberg, E. G. & Khalil, S. Revisiting the association between music lessons and intelligence: Training effects or music aptitude?. Intelligence https://doi.org/10.1016/j.intell.2017.03.005 (2017).
Draganski, B. et al. Changes in grey, matter induced by training. Nature 427, 311–312 (2004).
Reybrouck, M., Vuust, P. & Brattico, E. Music and brain plasticity: How sounds trigger neurogenerative adaptations. INTECH https://doi.org/10.5772/intechopen.74318 (2018).
Pantev, C. & Herholz, S. C. Plasticity of the human auditory cortex related to musical training. Neurosci. Biobehav. Rev. https://doi.org/10.1016/j.neubiorev.2011.06.010 (2011).
Barrett, K. C., Ashley, R., Strait, D. L. & Kraus, N. Art and science: How musical training shapes the brain. Front. Psychol. https://doi.org/10.3389/fpsyg.2013.00713 (2013).
Schlaug, G. The brain of musicians. A model for functional and structural adaptation. Ann. N. Y. Acad. Sci. 930, 281–299 (2001).
Schneider, P. et al. Morphology of Heschl’s gyrus reflects enhanced activation in the auditory cortex of musicians. Nat. Neurosci. 5, 688–694 (2002).
Kliuchko, M. et al. Fractionating auditory priors: A neural dissociation between active and passive experience of musical sounds. PLoS One 14, e0216499 (2019).
Müllensiefen, D., Gingras, B., Musil, J. & Stewart, L. The musicality of non-musicians: An index for assessing musical sophistication in the general population. PLoS One 9, e89642 (2014).
Vaquero, L. et al. Structural neuroplasticity in expert pianists depends on the age of musical training onset. Neuroimage 126, 106–119 (2016).
James, C. E. et al. Musical training intensity yields opposite effects on grey matter density in cognitive versus sensorimotor networks. Brain Struct. Funct. 219, 353–366 (2014).
Baer, L. H. et al. Regional cerebellar volumes are related to early musical training and finger tapping performance. Neuroimage 109, 130–139 (2015).
Figley, C. R. et al. Potential pitfalls of using fractional anisotropy, axial diffusivity, and radial diffusivity as biomarkers of cerebral white matter microstructure. Front. Neurosci. 15, 1855 (2022).
Bengtsson, S. L. et al. Extensive piano practicing has regionally specific effects on white matter development. Nat. Neurosci. 8, 1148–1150 (2005).
Rüber, T., Lindenberg, R. & Schlaug, G. Differential adaptation of descending motor tracts in musicians. Cereb. Cortex 25, 1490–1498 (2015).
Han, Y. et al. Gray matter density and white matter integrity in pianists’ brain: A combined structural and diffusion tensor MRI study. Neurosci. Lett. 459, 3–6 (2009).
Elmer, S., Hänggi, J. & Jäncke, L. Interhemispheric transcallosal connectivity between the left and right planum temporale predicts musicianship, performance in temporal speech processing, and functional specialization. Brain Struct. Funct. 221, 331–344 (2016).
Imfeld, A., Oechslin, M. S., Meyer, M., Loenneker, T. & Jancke, L. White matter plasticity in the corticospinal tract of musicians: A diffusion tensor imaging study. Neuroimage 46, 600–607 (2009).
Baumann, S. et al. A network for audio-motor coordination in skilled pianists and non-musicians. Brain Res. 1161, 65–78 (2007).
Bianchi, F. et al. Subcortical and cortical correlates of pitch discrimination: Evidence for two levels of neuroplasticity in musicians. Neuroimage 163, 398–412 (2017).
Chen, J. L., Penhune, V. B. & Zatorre, R. J. Moving on time: Brain network for auditory-motor synchronization is modulated by rhythm complexity and musical training. J. Cogn. Neurosci. 20, 226–239 (2008).
Eickhoff, S. B. et al. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. https://doi.org/10.1002/hbm.20718 (2009).
Fox, P. T. & Lancaster, J. L. Mapping context and content: The BrainMap model. Nat. Rev. Neurosci. 3, 319–321 (2002).
Eickhoff, S. B., Bzdok, D., Laird, A. R., Kurth, F. & Fox, P. T. Activation likelihood estimation meta-analysis revisited. Neuroimage 59, 2349–2361 (2012).
Langner, R. & Camilleri, J. A. Meta-analytic connectivity modelling (MACM): A tool for assessing region-specific functional connectivity patterns in task-constrained states. Brain Netw. Dysfunct. Neuropsychiatr. Illn. https://doi.org/10.1007/978-3-030-59797-9_5 (2021).
Laird, A. R. et al. User Manual for Sleuth 2.0.
Laird, A. R. et al. Networks of task co-activations. Neuroimage 80, 505–514 (2013).
Robinson, J. L., Laird, A. R., Glahn, D. C., Lovallo, W. R. & Fox, P. T. Metaanalytic connectivity modeling: Delineating the functional connectivity of the human amygdala. Hum. Brain Mapp. 31, 173–184 (2010).
Laird, A. R., Lancaster, J. L. & Fox, P. T. BrainMap: The social evolution of a human brain mapping database. Neuroinformatics 3, 065–078 (2005).
Poldrack, R. A. et al. Guidelines for reporting an fMRI study. Neuroimage 40, 409–414 (2008).
Nichols, T. E. et al. Best practices in data analysis and sharing in neuroimaging using MRI. Nat. Neurosci. 20, 299–303 (2017).
Schellenberg, E. G. Examining the association between music lessons and intelligence. Br. J. Psychol. 102, 283–302 (2011).
Müllensiefen, D., Gingras, B., Musil, J. & Stewart, L. Measuring the facets of musicality: The Goldsmiths Musical Sophistication Index (Gold-MSI). Pers. Individ. Diff. 60, S35 (2014).
Schneider, P. et al. Structural and functional asymmetry of lateral Heschl’s gyrus reflects pitch perception preference. Nat. Neurosci. 8, 1241–1247 (2005).
Brattico, E., Tervaniemi, M., Näätänen, R. & Peretz, I. Musical scale properties are automatically processed in the human auditory cortex. Brain Res. 1117, 162–174 (2006).
Warren, J. D., Uppenkamp, S., Patterson, R. D. & Griffiths, T. D. Analyzing pitch chroma and pitch height in the human brain. Ann. N. Y. Acad. Sci. 999, 212–214 (2003).
Zatorre, R. J. There’s more to auditory cortex than meets the ear. Hear. Res. 229, 24–30 (2007).
Hyde, K. L., Peretz, I. & Zatorre, R. J. Evidence for the role of the right auditory cortex in fine pitch resolution. Neuropsychologia 46, 632–639 (2008).
Peretz, I., Vuvan, D., Lagrois, M. -É. & Armony, J. L. Neural overlap in processing music and speech. Philos. Trans. R. Soc. B 370, 20140090 (2015).
Kotz, S. A. & Schwartze, M. Cortical speech processing unplugged: A timely subcortico-cortical framework. Trends Cogn. Sci. 14, 392–399 (2010).
Schwartze, M. & Kotz, S. A. A dual-pathway neural architecture for specific temporal prediction. Neurosci. Biobehav. Rev. 37, 2587–2596 (2013).
Kotz, S. A., Ravignani, A. & Fitch, W. T. The evolution of rhythm processing. Trends Cogn. Sci. 22, 896–910 (2018).
Kandylaki, K. D. & Criscuolo, A. Neural tracking of speech: Top-down and bottom-up influences in the musician’s brain. J. Neurosci. 41, 6579–6581 (2021).
Brown, S., Martinez, M. J. & Parsons, L. M. Music and language side by side in the brain: A PET study of the generation of melodies and sentences. Eur. J. Neurosci. 23, 2791–2803 (2006).
DeWitt, I. & Rauschecker, J. P. Phoneme and word recognition in the auditory ventral stream. Proc. Natl. Acad. Sci. U. S. A. 109, 2709 (2012).
Rauschecker, J. P. & Tian, B. Mechanisms and streams for processing of ‘“what”’ and ‘ “where”’ in auditory cortex. PNAS 97, 11800–11806 (2000).
Hartwigsen, G. et al. Phonological decisions require both the left and right supramarginal gyri. Proc. Natl. Acad. Sci. U. S. A. 107, 16494–16499 (2010).
Seghier, M. L. The angular gyrus: Multiple functions and multiple subdivisions. Neuroscientist 19, 43–61 (2013).
Schneider, P., Sluming, V., Roberts, N., Bleeck, S. & Rupp, A. Structural, functional, and perceptual differences in Heschl’s gyrus and musical instrument preference. Ann. N. Y. Acad. Sci. 1060, 387–394 (2005).
Mushiake, H., Inase, M. & Tanji, J. Experimental Brain Research Selective coding of motor sequence in the supplementary motor area of the monkey cerebral cortex. Exp. Brain Res. 82, 208–210 (1990).
Purves, D. et al. Neuroscience 5th edn. (Sinauer Associates Inc, 2012).
Lehericy, S. et al. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc. Natl. Acad. Sci. 102, 12566–12571 (2005).
Dayan, E. & Cohen, L. G. Neuroplasticity subserving motor skill learning. Neuron 72, 443–454 (2011).
Kim, S., Ugurbil, K. & Strick, P. Activation of a cerebellar output nucleus during cognitive processing. Science (80-). 265, 949–951 (1994).
Desmond, J. E., Chen, S. H. A. & Shieh, P. B. Cerebellar transcranial magnetic stimulation impairs verbal working memory. Ann. Neurol. https://doi.org/10.1002/ana.20604 (2005).
Salmi, J. et al. Cognitive and motor loops of the human cerebro-cerebellar system. J. Cogn. Neurosci. 22, 2663–2676 (2010).
Grahn, J. A. & Brett, M. Rhythm and beat perception in motor areas of the brain. J. Cogn. Neurosci. 19, 893–906 (2007).
Menon, V. & Uddin, L. Q. Saliency, switching, attention and control: A network model of insula function. Brain Struct. Funct. 214, 655–667 (2010).
Bush, G., Luu, P. & Posner, M. I. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 4, 215–222 (2000).
Brown, S., Martinez, M. J. & Parsons, L. M. Passive music listening spontaneously engages limbic and paralimbic systems. NeuroReport 15, 2033–2037 (2004).
Koelsch, S. et al. Functional architecture of verbal and tonal working memory: An fMRI study. Hum. Brain Mapp. 30, 859–873 (2009).
Brattico, E. et al. It’s sad but I like it: The neural dissociation between musical emotions and liking in experts and laypersons. Front. Hum. Neurosci. 9, 21 (2016).
Moore, E., Schaefer, R. S., Bastin, M. E., Roberts, N. & Overy, K. Can musical training influence brain connectivity? Evidence from diffusion tensor MRI. Brain Sci. 4, 405 (2014).
Schmithorst, V. J. & Wilke, M. Differences in white matter architecture between musicians and non-musicians: A diffusion tensor imaging study. Neurosci. Lett. 321, 57–60 (2002).
Steele, C. J., Bailey, J. A., Zatorre, R. J. & Penhune, V. B. Early musical training and white-matter plasticity in the corpus callosum: evidence for a sensitive period. J. Neurosci. 33, 1282–1290 (2013).
Schlaug, G., Jäncke, L., Huang, Y., Staiger, J. F. & Steinmetz, H. Increased corpus callosum size in musicians. Neuropsychologia 33, 1047–1055 (1995).
Imfeld, A., Oechslin, M. S., Meyer, M., Loenneker, T. & Jancke, L. White matter plasticity in the corticospinal tract of musicians: A diffusion tensor imaging study. Neuroimage https://doi.org/10.1016/j.neuroimage.2009.02.025 (2009).
Amunts, K. et al. Motor cortex and hand motor skills: Structural compliance in the human brain. Hum. Brain Mapp. 5, 206–215 (1997).
Hund-Georgiadis, M. & Yves Von Cramon, D. Motor-learning-related changes in piano players and non-musicians revealed by functional magnetic-resonance signals. Exp. Brain Res. 125, 417–425 (1999).
Hudziak, J. J. et al. Cortical thickness maturation and duration of music training: Health-promoting activities shape brain development. J. Am. Acad. Child Adolesc. Psychiatry 53, 1153-1161.e2 (2014).
Bailey, J. A., Zatorre, R. J. & Penhune, V. B. Early musical training is linked to gray matter structure in the ventral premotor cortex and auditory-motor rhythm Sybcrhonization performance. J. Neurosci. 26, 755–767 (2014).
Gaser, C. & Schlaug, G. Brain structures differ between musicians and non-musicians. J. Neurosci. 23(27), 9240–9245 (2003).
Habibi, A., Damasio, A., Ilari, B., Elliott Sachs, M. & Damasio, H. Music training and child development: A review of recent findings from a longitudinal study. Ann. N. Y. Acad. Sci. https://doi.org/10.1111/nyas.13606 (2018).
Habibi, A. et al. Childhood music training induces change in micro and macroscopic brain structure: Results from a longitudinal study. Cereb. Cortex 28, 4336–4347 (2018).
Stewart, L. et al. Brain changes after learning to read and play music. Neuroimage 20, 71–83 (2003).
Särkämö, T. et al. Music listening enhances cognitive recovery and mood after middle cerebral artery stroke. Brain https://doi.org/10.1093/brain/awn013 (2008).
Särkämö, T. et al. Structural changes induced by daily music listening in the recovering brain after middle cerebral artery stroke: A voxel-based morphometry study. Front. Hum. Neurosci. 8, 1–16 (2014).
Higgins, J. P. & Green, S. Cochrane Handbook for Systematic Reviews of Interventions. (2011).
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G. & Group, T. P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 6, 1–5 (2009).
Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. www.covidence.org. https://support.covidence.org/help/how-can-i-cite-covidence. (last access date: 03.03.2022)
Laird, A. R. et al. Comparison of the disparity between Talairach and MNI coordinates in functional neuroimaging data: Validation of the Lancaster transform. Neuroimage https://doi.org/10.1016/j.neuroimage.2010.02.048 (2010).
Yarkoni, T., Poldrack, R. A., Nichols, T. E., Van Essen, D. C. & Wager, T. D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods https://doi.org/10.1038/nMeth.1635 (2011).
Laird, A. R. et al. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J. Neurosci. https://doi.org/10.1523/JNEUROSCI.4004-09.2009 (2009).
Eickhoff, S. B. & Grefkes, C. Approaches for the integrated analysis of structure, function and connectivity of the human brain. Clin. EEG Neurosci. (2011).
Lancaster, J. L. et al. Automated regional behavioral analysis for human brain images. Front. Neuroinform. 6, 1–12 (2012).
Fox, P. T. et al. BrainMap taxonomy of experimental design: Description and evaluation. Hum. Brain Mapp. 25, 185–198 (2005).
Gorgolewski, K. J. et al. NeuroVault.org: A web-based repository for collecting and sharing unthresholded statistical maps of the human brain. Front. Neuroinform. 9, 1–9 (2015).
Jenkinson, M., Beckmann, C. F., Behrens, T. E. J., Woolrich, M. W. & Smith, S. M. FSL. Neuroimage 62, 782–790 (2012).
Acar, F., Seurinck, R., Eickhoff, S. B. & Moerkerke, B. Assessing robustness against potential publication bias in Activation Likelihood Estimation (ALE) meta-analyses for fMRI. PLoS One 13, e0208177 (2018).
Acknowledgements
The Center for Music in the Brain (MIB) is supported by the Danish National Research Foundation (Grant number DNRF 117). The authors wish to thank Hella Kastbjerg for assistance with language check and proof reading. Leonardo Bonetti is supported by the Carlsberg Foundation (CF20-0239) and Linacre College, University of Oxford.
Author information
Authors and Affiliations
Contributions
A.C., V.P.N. and E.B. designed the meta-analysis. V.P.N. guided A.C. in the initial steps of the meta-analysis after which A.C. and V.P.N. conducted the screening of the studies, and E.B. and L.B. controlled the final selection. A.C. performed the initial analyses, wrote the first draft of the manuscript, and prepared the initial versions of the tables and figures. V.P.N. performed analyses, prepared the figures and tables and edited paragraphs in the Methods, Results and Discussion sections. All authors contributed to the final version of the manuscript. PV contributed to financially support the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Criscuolo, A., Pando-Naude, V., Bonetti, L. et al. An ALE meta-analytic review of musical expertise. Sci Rep 12, 11726 (2022). https://doi.org/10.1038/s41598-022-14959-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-14959-4
This article is cited by
-
The role of auditory source and action representations in segmenting experience into events
Nature Reviews Psychology (2024)
-
Spatiotemporal brain hierarchies of auditory memory recognition and predictive coding
Nature Communications (2024)
-
Audiovisual integration in the McGurk effect is impervious to music training
Scientific Reports (2024)
-
Music reward sensitivity is associated with greater information transfer capacity within dorsal and motor white matter networks in musicians
Brain Structure and Function (2024)
-
Magnetoencephalography recordings reveal the spatiotemporal dynamics of recognition memory for complex versus simple auditory sequences
Communications Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.