Abstract
Mango (Mangifera indica) is the second most internationally traded tropical fruit in the world. The fruit has high nutritional value. Its susceptibility to postharvest diseases and chill injuries increases its storage cost and put stress on exploring natural products that can increase its shelf-life. Our team has previously described Prosopis juliflora water-soluble leaf ethanolic (PJ-WS-LE) extract with fungicidal effectiveness against spoiling fungi. The present study explores P. juliflora genetic diversity in the state of Qatar and the antifungal effectiveness of the leaf extract of plants collected from different locations. The study also evaluates PJ-WS-LE extract efficacy against Alternaria. alternata and Colletotrichum gloeosporioides inoculated in mango samples and the power of the extract as coating material. P. juliflora samples collected from six different locations showed genetic and antimicrobial effectiveness similarities. They showed also similarity to the sequence representing P. juliflora 18S ribosomal RNA partial sequence, accession number JX139107.1 originated from India. PJ-WS-LE extract (8 mg/ml) has 80% efficacy in controlling A. alternata in mango and it lowers C. gloeosporioides disease severity by 53.4%. PJ-WS-LE extract (8 mg/ml) embedded in 1% chitosan maintained mango quality for 5 weeks. In vivo results of PJ-WS-LE extract highlights the potentials of the extract as chemical fungicides replacement.
Similar content being viewed by others
Introduction
Prosopis (mesquite; Fabaceae) is found in hot, dry and semi-arid areas around the world, either as native species or as introduced ones1. Prosopis is known as major invasive species in many countries2. Prosopis juliflora (Mimosaceae), the focus of this study, is a small tree, native to Mexico, South America and Caribbean, it is an increasingly spreading invasive species in Asia, Austria and in other places around the world including Qatar3. When considering the exploration of P. juliflora as source of biological controllers, it is important to learn first about its biodiversity. When introduced to the country, the tree may originate from a single source which implies homogeneity in active phytochemicals or it may arrive from different sources which makes it necessary to investigate the effectiveness of extracts of trees of various origins.
Globally, around 33% of the food produced is lost and not consumed. A high percentage of harvested fresh produce is wasted yearly by microbial spoilage4,5. Mango (Mangifera indica) is a tropical fruit rich with fibers, vitamin C, vitamin A, polyphenols, carotenoids, carbohydrates, and calcium. Various types of mangoes have wide ranges of nutritional facts6. India is the largest producer of mango in the world7. Mango availability in the market is affected by its susceptibility to postharvest diseases and to chill injuries. The short shelf life of mango increases its prices in markets that depend totally on imported fruit8. Mango is the second most important internationally traded tropical fruit straight after banana, which gives it a high economical value9. In 2020, imported mangoes costed Qatar 70.8MM QR making 8.12% of the total cost of imported fruits, this number highlights the significance of wastage of mango in storage for the country economy10.
During the storage time, the physiological changes that occurs on fruit make them more prone to the development of microorganisms11. Postharvest losses in mango can reach 50% in some developing countries such as Ethiopia9. The main fungal species that causes losses in mango are Alternaria. alternata and Colletotrichum gloeosporioides. Infection of mango by A. alternata can occur through the lenticels and cause rot mainly on the side of the fruit or through the ends of the stem to cause stems rot12. On the other hand, C. gloeosporioides is the causative agent of mango anthracnose, one of the most devastating and hard to control diseases in this fruit13.
A high percentage of the spoilage controllers currently used in the agricultural domain are either polluting or costly14. Chemical antifungals and antimicrobials used for spoilage control increase fruit and vegetables shelf-life, but they also leave their damaging fingerprints on the environment and pose, in many cases, potential risks on human health15. In addition, the controlled microorganisms are acquiring, with time, resistance to the commonly used antimicrobials, which is a problem that requires immediate attention16,17. This lead many scientists to explore plants and other sources of natural products to replace chemicals used in-fields and at post-harvest level18.
Within natural products, higher plants phytochemicals have valuable characteristics that help in curing several diseases19. Various researches have documented antifungal effectiveness of higher plants extracts at the level of spores germination and/or mycelium growth. Among the tested plants, Prosopis species in general and P. juliflora in particular have shown antioxidant, anti-inflammatory, antibacterial, antifungal, and anti-tumor activities20. The stability of active phytochemicals with time is one of the desired characteristics when exploring natural products21. P. juliflora leaf ethanolic extract has been proven by our team as an effective antimicrobial against a list of fungi and bacteria. The previous study showed also stability of the antimicrobial activity over time, for up to six months, which encouraged the team to explore the in vivo value of the extract22. This study aims to construct the phylogeny tree of P. juliflora in the municipality of Doha, Qatar, to evaluate genetic relatedness among different samples, variation of in vitro antifungal effectiveness among samples collected from different places was also evaluated. The study aims then to test P. juliflora water-soluble leaf ethanolic (PJ-WS-LE) extract curative and preventive effect against artificially inoculate A. alternata and C. gloeosporioides in mango. Moreover, It aims finally at evaluating the effect of the extract on its own and when embedded in chitosan, as a protective coating material for mango samples. Chitosan (poly β-(1–4)N-acetyl-d-glucosamine), used as stabilizer of our extract, is a non-toxic biodegradable polymer that has shown in previous studies an antifungal activity against many fungal types including C. gloeosporioides23. PJ-WS-LE extract is a solvent free solution that can be prepared using an economically feasible method, its antimicrobial activity results makes it a promising replacement of chemically synthesized fungicides. The extract has shown high efficacy in increasing strawberry and cucumber samples shelf-lives and in maintaining their quality storage parameters when used as coating material24,25.
Materials and methods
Prosopis juliflora leaves collection and processing for Ribotyping
Prosopis juliflora species of the genus Prosopis, family of Fabaceae had its genetic variation in Doha evaluated. Seven samples of P. juliflora leaves were collected from six different locations in Doha, Qatar, during five field trips. Plant leaves were collected after proper permissions and all methods were carried out in accordance with relevant guidelines and regulations. Trees in all locations were naturally growing around urbanization areas in their normal arid habitat without artificial irrigation, samples were collected from fully mature trees. Table 1 shows the samples details. Figure 1 shows the location sites of where the samples were collected on the map of Qatar, Doha. Leaf samples were kept in sterile labeled bags until having reached the laboratory where few leaflets were washed with sterile distilled water and sterilized using 70% ethanol to be used for DNA extraction.
Ribotyping analysis
The leaflets of each sample were transferred into a sterile mortar previously cooled at -20 ˚C and used for DNA extraction following the kit manufacturer instructions (DNeasy Plant Mini Kit-QIAGEN-USA).
Extracted DNA of each sample were subject to PCR using ITS1 and ITS4 primers. PCR products obtained were purified using the Invitrogen Quick PCR Purification Kit (QIAGEN, Germany) as indicated by the manufacturer and sequenced using Sanger sequencer (3130/3130xl DNA Analyzers, Thermofisher Scientific, USA) as previously described22.
Sanger sequencer raw data was read using BioEdit software. Basic Local Alignment Search Tool (BLAST) network services of the National Centre for Biotechnology Information (NCBI) database were used to compare the obtained sequences to the existing sequences. Sequences were submitted to NCBI for accession numbers. The various P. juliflora ribosomal sequences obtained were also uploaded on MEGA-X software and the phylogeny tree was generated using the neighbor-joining algorithm26.
Minimum inhibitory concentrations of PJ-WS-LE extracts prepared using leaf samples collected from various locations against A. alternata and C. gloeosporioides
Preparation of PJ-WS-LE extract
Fresh, young full leaves of P. juliflora were collected from various locations as indicated in Fig. 1. Samples were washed, dried and ground into powder to be used in the preparation of PJ-WS-LE extract as previously described22. Briefly, every 20 g of the leaf powder were incubated in 200 mL of 70% ethanol for 48 h. The supernatant has its solvent evaporated, the extract was then re-dissolved in sterile distilled water. Only water-soluble phytochemicals were tested by centrifuging the final preparation tubes and excluding the pellet. Stock solution of 100 g L−1 was stored at 4 °C to be used for later experiments. PJ-WS-LE extract concentration used in treatments was 8 g L−1 which is double the highest minimum inhibitory concentration of the extract against spoiling microorganisms as previously determined22.
Determination of minimum inhibitory concentration
The MIC test was conducted in a sterile 96-well plate, with each well containing 100 μl of potato dextrose broth (PDB) (HIMEDIA-India). Every four wells made one replication, nine different concentrations of the crude extracts were tested (1:1 dilutions) ranging from 42 to 0.16 g L−1. Wells were then inoculated with one of the two tested microorganisms’ spore suspensions (A. alternata and C. gloeosporioides). The last three rows are control rows: no spores and no extract control wells, negative control with spores but no extract wells, and positive control with spores and 10 µl of the fungicidal Clatrimazole (1%) wells.
Fungal spore suspensions were adjusted to the range of 104 spores L−1 using a 10 day old fungal plate and sterile distilled water, the spore concentration was calculated using a heamatocytometer.
Fungal growth in each well was monitored using Resazurin (HIMEDIA-India) dye. Upon cells division, Resazurin changes its color from blue to pink and fluorescent27. Results were taken within 48 h of incubation at 25 °C. MIC was recorded as the last extract concentration that shows no change in the color of Resazurin within the incubation period.
Curative and preventive effects of PJ-WS-LE extract against A. alternata and C. gloeosporioides induced infection in mangoes
Pathogens
The two fungal strains used C. gloeosporioides and A. alternata were obtained from our laboratory collection, Department of Biological and Environmental Sciences, Qatar University, Qatar. Both fungal isolates were previously isolated from locally collected fruit samples. Isolates were molecularly identified by sequencing the Internal Transcribed Spacer (ITS) regions of fungal ribosomal DNA (rDNA) that was amplified by PCR. Identified fungal isolates were given the strains code of AaltQU17 for A. alternata and CgloQU17 for C. gloeosporioides22. Preserved cultures were sub-cultured on potato dextrose agar (PDA) plates and incubated at 25 °C for 10 days. Plates were then flooded with 10 mL of sterile distilled water each, to prepare the needed spores suspension solutions. The concentrations of spores suspensions were adjusted to 106 spores L−1 using a heamatocytometer18.
Fruit
The mango (Mangifera indica) type known as Neelam imported from India was used in the experiments. Fruit were bought from the whole sale market upon their arrival to the country. Only undamaged mature fruit were used in the experiment. Fruits chosen were ripen but not yet soft with firmness average of 20 ± 5.1 N, weight average of 177.61 ± 0.2 g and TSS average of 70 ± 5.3%. Fruit were first washed with sink water and sterilized twice with 70% ethanol to be then washed with sterile distilled water and left to air dry.
Preventive and curative effects of PJ-WS-LE extract
Wounded mango fruit were used during the experiment, the wounds were made through three needle pricks (2 mm deep) in three different places for each plant using a sterile syringe. A completely randomized design was used and each treatment was made of a triplicate of 10 fruit each. The experiment was repeated twice.
PJ-WS-LE extract of leaves collected from Qatar university field was first tested for its efficacy in preventing fungal contamination in wounded mango fruit (preventive effect). Therefore, the wounded zone of each fruit was sprayed with 8 g L−1 PJ-WS-LE extract and then left to air-dry. Once dry the fruit were sprayed again with the extract at the same concentration and left to dry. Control fruit were only treated with sterile distilled water without the plants extract. After two hours all wounds were inoculated with 20 μL of conidia aqueous solution (106 spores mL−1) of one of the tested fungi. The extract was then tested for its ability to cure fungal contamination in wounded fruit. Therefore, wounds were inoculated first with 20 μL of conidia aqueous solution (106 spores mL−1) and left to dry. Wounds were then sprayed twice with 8 g L−1 PJ-WS-LE extract.
All mangoes were stored in sterilized plastic trays inside an incubator at 25 °C and 75% humidity. Fruit were observed every 24 h for 5 days for C. gloeosporioides inoculated fruit and for 10 days for A. alternata inoculated fruit. Three parameters were recorded at the end of the experiment: disease incidence (DI), disease severity (DS), and percent plant extract efficacy (%EE). To calculate disease severity, the diameter of the infected area of each fruit was measured in two perpendicular directions and mean diameter mycelial growth was calculated28,29.
End of the trial samples firmness
At the end of the trial, remaining mango fruit were tested for their flesh quality using a penetrometer (Agriculture Solutions, USA) to test the flesh firmness. Fruit were peeled, then the stainless steel probe of the instrument was inserted in three different points towards the equator of the fruit. Firmness in Newton was recorded and compared with standard fruit firmness to judge fruit quality18.
Effectiveness of PJ-WS-LE extract as long-term coating material and the preservative value of its chitosan-embedded form
Coating solutions preparation
Chitosan solution of 1% concentration was prepared by stirring chitosan powder (CAS 9012-76-4, Himedia, India) in 1% glacial acetic acid (IsoLab, Germany) overnight. The final chitosan solution pH was adjusted to 5.6 using 0.1 M NAOH (Sigma-Aldrich, Germany). To prepare PJ-WS-LE extract chitosan-embedded coating material, filter-sterilized PJ-WS-LE extract stock solution was added to 1% chitosan to achieve a final concentration of 8 g L−130.
Samples preparation
Eighty-four mango samples chosen as described above, were divided into four groups of 18 samples each. Samples were divided into four treatment batches and treated as following:
-
Batch A: non-treated fruit.
-
Batch B: PJ-WS-LE extract at 8 g L–1 was used to spray the fruit.
-
Batch C: 1% chitosan was used to spray the fruit.
-
Batch D: 8 g L−1 PJ-WS-LE extract embedded in 1% chitosan was used to spray the fruit.
Every experimental replicate was made up of three mango samples that were stored together in one sterile bag at 4 °C. The number of replications per treatment was six. The experiment was repeated twice31.
Evaluation of sensory quality
A five-points scale was used for the evaluation of the sensory quality of the samples for overall quality, smell, and color change. The attributes were evaluated weekly using the fruit of one experimental replicate. Scores were given using the following scale: 5 points indicate “extremely liked”, 4 points indicate “liked”, 3 points indicate “acceptable” 2 points indicate “disliked” and 1 point indicates “extremely disliked”. The weekly average score per batch was also calculated32.
Estimation of weight loss
Upon treatment at day zero, all mango samples were weighed and their weights were recorded as initial weights. Weights of all remaining samples were measured at the end of every week. The variation between the start weight and weekly weights is calculated as weekly weight loss. The average percent of weekly weight loss of each batch was calculated32.
Determination of samples firmness
The samples of each experimental replicate evaluated on a weekly basis had their firmness measured as previously described. The weekly average samples firmness (N) of every treatment batch was also calculated33.
pH measurement
Mango fruit of each experimental replicate were blended weekly into juice, after filtration, a digital pH meter (Jenway, UK) was used to measure pH. The weekly average fruit pH of every treatment batch was also calculated. The pH meter was calibrated using a buffer solution of pH 734.
Total soluble solids (TSS) measurement
Total soluble solids of the prepared mango juice samples were measured in percent brix using a refractometer (ANTAHI, New Zealand). The weekly average fruit TSS (%) for each treatment batch was also calculated. The refractometers was calibrated using distilled water35.
DPPH radical scavenging assay
A 1/10 mango juice dilution was prepared using sterile distilled water. 100 μL of each dilution was mixed with 1 mL of 2,2-diphenyl-1-picrylhydrazyl (DPPH) (100 mg L−1) to be incubated in the dark at 37 °C for 45 min. After incubation, samples were centrifuged and the pellet was discarded. The intensity of the change in color of the supernatant was measured by spectrophotometry at 517 nm using methanol as a blank. 100 μL of methanol in 1 mL DPPH was used as the control for the experiment. Percent radical scavenging activity was calculated as per the below formula:
The weekly average % radical scavenging activity for each treatment batch was finally calculated31.
Statistical analysis
The experimental design used was Completely Randomized Design (CRD). One-way ANOVA followed by Tukey Post-Hoc test was used to evaluate the significance of the weekly percent change in weight among treatment batches at P ≤ 0.05. The significances of pH and TSS variation within different treatment batches were evaluated using One-way ANOVA test at P ≤ 0.05. Data was presented as average ± standard error of the Means (SEM). SPSS (Ver. 27, SPSS Inc. Chicago, USA) was used to perform the statistical analysis tests.
Results
DNA fingerprinting results
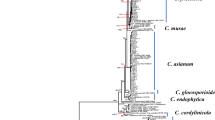
Upon submission to NCBI, the seven P. juliflora rDNA sequences were given accession numbers (Table 2). The blasting of the sequences on NCBI, in order to compare them with the database, showed percent similarities between 96 and 99% to the sequence representing P. juliflora 18S ribosomal RNA gene, partial sequence; internal transcribed spacer 1, 5.8S ribosomal RNA gene, and internal transcribed spacer 2, complete sequence; and 28S ribosomal RNA gene, partial sequence with accession number JX139107.1 (originated from India). Neighbor joining phylogeny tree of the sequences shows very low variation among the P. juliflora isolates collected from different places in Doha (Fig. 2).
MICs evaluation of crude extract prepared using P. juliflora leaves samples from various locations against A. alternata and C. gloeosporioides
The MICs of the extracts prepared using leaves of trees from different locations showed homogeneity in their results. The slight one raw variation shown in the case A. alternata is due to the difficulty in preparing extracts of exactly the same concentration at every run, which in turn is due to the difficulty in controlling how much of the extract is water-soluble. The MICs of the extract against A. alternata are within the range obtained previously in our laboratory, which is 1 g L−1 (Fig. 3). As previously described, PJ-WS-LE extract did not show a total growth inhibition of C. gloeosporioides. However, the change in color in the 96-well plate experiment shows a dose-dependent effectiveness in lowering fungal growth (Fig. 3).
MICs of PJ-WS-LE extracts prepared using P. juliflora leaves collected from different locations in Qatar against A. alternata (a) and C. gloeosporioides (b). S0: old extract from the laboratory. S1 to S7: extract prepared using leaves of sample S1 to S7. Rows 1 to 9 contains the following concentrations of PJ-WS-LE extract in g L−1: 42, 21, 10.5, 5.2, 2.6, 1.3, 0.6, 0.3 and 0.16. Row 10 contains no spores, row 11 contains no extract and row 12 contains 1% Clatrimazole.
Curative and preventive effects of PJ-WS-LE extract against A. alternata and C. gloeosporioides induced infection in mangoes
In the case of A. alternata artificially inoculated mango fruit, PJ-WS-LE extract showed high efficacy in reducing infection. The incidence rate went down from 51.7% in control samples to 10.3% in treated samples with preventive treatment and to 25% in treated samples with curative treatment (Fig. 4). PJ-WS-LE extract at 8 g L−1 had an efficacy of 80% against A. alternata infection by the end of the 10 d storage period. Disease severity was also reduced in treated samples. Samples with preventive treatment (average lesion diameter = 3.7 mm) that showed infection demonstrated a disease severity of 16% when compared to untreated control samples (average lesion diameter = 11.5 mm) (Fig. 5). The effect of PJ-WS-LE extract on mango firmness was evaluated at day 10. ANOVA test showed a significant average firmness conservation (P = 0.00 ≤ 0.01) between plants exposed to the extract (curative and preventive treatment) (14.93 N) and the untreated plants (7.4 N).
The efficacy of PJ-WS-LE extract in reducing C. gloeosporioides incidence on artificially inoculated fruit was low, preventive treatment showed a disease incidence rate reduction of 25% (extract efficacy). Although extract efficacy in controlling mango anthracnose does not account for total eradication, PJ-WS-LE extract exhibited significant reduction in disease severity, which was reduced by more than 50%, from a control average lesion diameter of 14.7 mm to a preventive treatment average lesion diameter of 7.0 mm by the end of the 5 d storage period (Fig. 5). The effect of PJ-WS-LE extract on mango firmness was also evaluated at day 5. One-way ANOVA test showed a significant average firmness conservation (P = 0.00 ≤ 0.01) in samples exposed to the extract (curative and preventive treatments) (26.74 N) compared to the control samples firmness average (12.62 N).
PJ-WS-LE extract long-term efficacy as coating material and the preservative value of its chitosan-embedded form
Sensory evaluation
The weekly changes of mangoes sensory characteristics stored at 4 °C were evaluated. Figure 6 shows the sensory scores changes of the fruit of each of the treatment batches.
Weekly sensory evaluation of mango fruit exposed to four different treatments (A negative control non-treated fruit; B PJ-WS-LE extract at 8 g L−1 was used to treat the fruit; C 1% chitosan was used to treat the fruit; D 8 g L−1 PJ-WS-LE extract embedded in 1% chitosan was used to treat the fruit) stored at 4 °C for 5 weeks.
The overall mangoes’ sensory scores declined with storage time. Nevertheless, samples of treatment batches B and D coated with 8 g L−1 of PJ-WS-LE extract individually and embedded in 1% chitosan conserved better sensory scores during the five week storage time.
Score from 1 to 5 are: 5 points indicate “extremely liked”, 4 points indicate “liked”, 3 points indicate “acceptable” 2 points indicate “disliked” and 1 point indicates “extremely disliked”.
Weight loss
The weekly percent change in weight of every treatment batch was calculated (Fig. 7). Weight loss increased with time, nevertheless, PJ-WS-LE extract embedded in chitosan showed efficacy in reducing weight decline in the fruits.
Weekly average percentage change in weight of the overall mangoes exposed to various treatments ± SE (A negative control non-treated fruit; B PJ-WS-LE extract at 8 g L−1 was used to treat the fruit; C 1% chitosan was used to treat the fruit; D 8 g L−1 PJ-WS-LE extract embedded in 1% chitosan was used to treat the fruit). abTreatment columns marked with different letters have their percent change in weight values significantly different as per the results of the one-way ANOVA Post-Hoc Tukey test for weekly data.
A significant increase of percent change of weight of mango samples was observed in all treatment categories with the passage of storage time. Mangoes coated with 8 g L−1 of PJ-WS-LE extract in 1% chitosan had the lowest percent change in weight with an observable weekly increase. One-way ANOVA test showed a significant effect of treatment type on percent weight change in the overall data of all weeks, post-Hoc Tukey test shows that treatment D (8 g L−1 PJ-WS-LE extract embedded in 1% chitosan) lead significantly to lower percent weight loss compared to the control fruit (batch A) in the overall data (p = 0.012 ≤ 0.05).
Weekly changes of physical and chemical properties (firmness, pH, TTS, antioxidant activity, and ascorbic acid level)
Mango samples treated differently had their weekly physical parameters monitored. Table 3 shows the weekly results of averages firmness, pH and total soluble solids.
Mango samples of batches A, B and C showed a decrease in their firmness during the storage time although samples of batch D showed a kind of firmness stability. The results of the mango juice pH of all treatment batches increased weekly, except for batch D which samples showed pH levels decrease. When all of the weeks’ pH results for each batch were combined and tested using one-way ANOVA followed by Tukey test, the overall results showed significant differences in pH levels of mango juices of different batches (P = 0.00 ≤ 0.01). Tukey test shows that batch D samples had significantly lower pH than the other samples (average pH 4.51).
Total soluble solids results showed fluctuation in TSS levels throughout the five weeks of storage. One-way ANOVA test, followed by Tukey test done on the overall results show a significant difference in the average TSS levels (P = 0.009 ≤ 0.01). Tukey test results show that samples of batch D had significantly higher TSS levels when compared to others with an average TSS level of 81.7% compared to an average TSS level of 69.4% in the case of the control samples (batch A).
DPPH radical scavenging activity of mango samples of different treatment batches was measured for four weeks. The antioxidant activity increased with time in all mango samples. The highest increase was observed in mango samples coated with chitosan and PJ-WS-LE extract (batch D) followed by samples coated with chitosan alone (batch C).
Discussion
Sequence number JX139107.1 has originated from India. Knowing that P. juliflora is not a native species of Qatar, the similarity between the indicated sequence and our sequences could mean that workers of Indian origin may have brought the first seeds of the plant to the country, hence it is resistant to similar climatic conditions and due to its pharmaceutical importance among Indian society20. The low variation in climate among the different places in Qatar does not allow for genetic variation among the different isolates. Considering the possibility that all the trees in the country could originate from the same country (India) explains also the similarity among the sequences. A previous study on the diversity within and among the populations of P. cineraria and P. juliflora collected from different locations in Qatar was conducted on 12 samples, six of each species. Genetic variation was explored using Inter Simple Sequence Repeat (ISSR) and Random Amplified Polymorphic DNA (RAPD) markers. The analysis divided the twelve genotypes into two distinct clusters by species and showed genetic variations among samples of P. juliflora, however, the variation was inconsistent between the two methods used36. Collections of more samples and the usage of other genetic markers are needed to confirm genetic identity of P. juliflora in Qatar.
The results of MICs are consistent with the low genetic variation shown in the phylogeny analysis of P. juliflora in Qatar. The homogeneity in climate and in soil type in the collection areas, as well as the age of the tree that is relatively new to the country, support the obtained results and imply that the types of active phytochemicals are similar in all trees. Therefore, if to be utilized, all P. juliflora trees in Qatar would have the same antimicrobial effectiveness. The lack of variation in the in vitro results has led to the usage of PJ-WS-LE extract of a single source in the in vivo assays.
The MIC of PJ-WS-LE extract against A. alternata was shown to be around 1 g L−1 (1001 ppm) which is high compared to the concentration of chemical fungicides used in-field to control mango anthracnose. Systemic fungicides are usually used at concentration between 25 and 50 ppm, examples include Carbendazim, Propiconazole and others. Non-systemic fungicide are usually used at higher concentrations that might reach 1000 ppm, which is very close to our PJ-WS-LE extract MIC, examples include Mancozeb, Copper oxychloride and others37. Nevertheless, PJ-WS-LE crude extract MIC cannot be compared to the concentrations of chemical fungicides as the extract is a mixture of active and non-active phytochemicals. In addition, PJ-WS-LE extract might need to be used at higher concentration, yet it has the advantage of being new, which means that spoiling fungi haven not yet developed resistant to it.
The protective effect of PJ-WS-LE extract against anthracnose caused by C. gloeosporioides in artificially inoculated mango samples was 25%. Similar anthracnose incidence inhibition rate was seen in inoculated avocadoes samples treated with Aloe vera extract. However, when Aloe vera extract was mixed with 1% chitosan, the disease incidence rate went down from 90% in the control samples to 20% in treated samples, similarly to our results, preventive treatment was more effective when it comes to C. gloeosporioides. Based on the results of the long term experiment involving chitosan, it is likely to have a higher extract efficacy against C. gloeosporioides when mixing PJ-WS-LE extract to chitosan18. As for the disease severity, PJ-WS-LE extract demonstrated a high success rate in lowering anthracnose lesion diameter by more than half with average lesion diameter of 7 mm in the preventive treatment samples. The result is somehow comparable to the final lesion diameter of treated avocado samples (diameter = 8.94 mm) as shown by Bill et al. Another study conducted on mangoes showed significant efficacy of P. juliflora leaf extracts in lowering anthracnose severity in inoculated samples. A comparison between the two extraction methods should be considered to improve our extract efficacy, however, it is vital to first examine the kind of solvent used by Deressa and Jalata, since it may have toxic antifungal activity all on its own9. A study conducted on Papaya samples, showed the effectiveness of different plant extracts, collected from Ambo and Haramaya, Ethiopia, in lowering anthracnose severity without totally inhibiting the fungal growth on plants, which indicates the difficulty in controlling mango anthracnose to a 100% level. In vivo experiments on mangoes showed much higher efficacy of PJ-WS-LE extract in protecting artificially inoculated samples from A. alternata (80%). Those results are in harmony with previous in vitro results showing total inhibition of A. alternata by PJ-WS-LE extract, while C. gloeosporioides showed only a decrease in mycelium growth38. One of the most common fungi causing mango postharvest diseases in the world is A. alternata, it can cause rot on the sides and stem ends, resulting in significant postharvest losses39. Controlling A. alternata using a naturally produced extract would add a valuable, safe and effective product to the agricultural world.
The experiment of long-term fruit preservation was conducted on mango samples stored at 4 °C and divided into four treatment batches. Samples coated with 8 g L−1 of PJ-WS-LE extract embedded in 1% chitosan (batch D) conserved sensory scores between acceptable and liked quality during the five week storage period, which highlighted the coating efficacy.
During the postharvest storage period, water evaporation leads to fruit shriveling and weight loss40. Mangoes coated with 1% chitosan and 8 g L−1 of the PJ-WS-LE extract showed the lowest percent change in weight with an observable weekly increase. At week 2, samples belonging to batch D showed significantly lower weight loss than samples of the control batch A. PJ-WS-LE extract increases water holding capacity when mixed with the edible coating of 1% chitosan. A recent study conducted on mangoes evaluated the effect of Aloe Vera gel embedded in 1% chitosan (CTS + AVG). Coated samples showed, similarly to our results, lower weight loss with time8.
Loss of firmness of fruit occurs during ripening by the activation of polygalacturonases (PG) and pectin methylesterase (PME) enzymes, which leads to the degradation of the middle lamella between parenchyma cells, loss of cellular turgidity, and cell wall disruption41. Samples firmness was evaluated with time, and all batches samples showed decrease in firmness with time except for the samples of batch D, which maintained their hardness during the five weeks of the experiment, thus giving a value to the coating material. Similarly, mango samples coated with (CTS + AVG) were significantly firmer than control samples8. The pH results of mango juices of different batches showed an increase in numbers throughout the storage weeks, except of batch D samples which had significantly lower pH values, thus indicating that different ripening chemical reactions are occurring inside the coated samples. Coating material also led to higher soluble sugar levels in mango juice samples, which might have played a role in quality maintenance. Tukey test results showed that samples of batch D have significantly higher TSS levels when compared to others. Finally, mango coating increased the DPPH radical scavenging activity. The highest increase was seen with mango samples of the coated batch D followed by samples of batch C. Similarly, (CTS + AVG) coated mango fruit showed higher antioxidant activity when compared to control samples8.
Conclusion
PJ-WS-LE extract with its novel extraction and preparation method has been described in earlier studies of the team. The crude extract has shown a strong antimicrobial activity, stability with time, fungicidal activity against A. alternata and no phytotoxicity when applied on fruit. All these previous results opened doors to discover further PJ-WS-LE extract in vivo applications. The efficacy of the extract against A. alternata in mangoes and the significant decrease of mango diseases severity, add a strong value to PJ-WS-LE extract as a future replacement of chemical fungicides both for in-field and for postharvest applications. PJ-WS-LE extract embedded in edible coating has also been demonstrated as an effective coating material that can maintain mangoes quality at low temperature for up to five week storage. An investigation on the genetic variation of P. juliflora in Qatar, shows similar in vitro effectiveness of leaves collected from various P. juliflora trees in the country and proved too little or no genetic variation among samples, in addition to a common origin. Future studies will involve more fruit and vegetable, and more pathogenic spoiling agents to get to determine all the possible roles of this natural biological controller in agriculture.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Shackleton, R. T., Le Maitre, D. C., Pasiecznik, N. M. & Richardson, D. M. Prosopis: A global assessment of the biogeography, benefits, impacts and management of one of the world’s worst woody invasive plant taxa. AoB Plants https://doi.org/10.1093/aobpla/plu027 (2014).
Low, T. Denial about dangerous aid. Biol. Invas. https://doi.org/10.1007/s10530-012-0264-3 (2012).
de Brito Damasceno, G. A. et al. Co-Evolution of Secondary Metabolites (eds. Merillon, J.-M., & Ramawat, K.G.). 1–21. (Springer, 2018).
Leyva Salas, M. et al. Antifungal microbial agents for food biopreservation-A review. Microorganisms. https://doi.org/10.3390/microorganisms5030037 (2017).
Mailafia, S., Okoh, G. R., Olabode, H. O. K. & Osanupin, R. Isolation and identification of fungi associated with spoilt fruits vended in Gwagwalada market, Abuja, Nigeria. Vet. World 10, 393–397. https://doi.org/10.14202/vetworld.2017.393-397 (2017).
Maldonado-Celis, M. E. et al. Chemical composition of mango (Mangifera indica L.) fruit: Nutritional and phytochemical compounds. Front. Plant Sci. 10, 1073–1073. https://doi.org/10.3389/fpls.2019.01073 (2019).
Jha, S. et al. Firmness characteristics of mango hybrids under ambient storage. J. Food Eng. 97, 208–212. https://doi.org/10.1016/j.jfoodeng.2009.10.011 (2010).
Shah, S. & Hashmi, M. S. Chitosan–aloe vera gel coating delays postharvest decay of mango fruit. Hortic. Environ. Biotechnol. 61, 279–289. https://doi.org/10.1007/s13580-019-00224-7 (2020).
Deressa, T. & Jalata, M. Antifungal activity of some invasive alien plant leaf extracts against mango (Mangifera indica) anthracnose caused by Colletotrichum gloeosporioides. Int. J. Pest Manag. https://doi.org/10.1080/09670874.2015.1016135 (2015).
Planning and Statistics Authority Qatar, P. Q. PSA Qata. https://www.mdps.gov.qa/.
Wenneker, M. & Thomma, B. P. H. J. Latent postharvest pathogens of pome fruit and their management: From single measures to a systems intervention approach. Eur. J. Plant Pathol. 156, 663–681. https://doi.org/10.1007/s10658-020-01935-9 (2020).
Konsue, W., Dethoup, T. & Limtong, S. Biological control of fruit rot and anthracnose of postharvest mango by antagonistic yeasts from economic crops leaves. Microorganisms 8, 317. https://doi.org/10.3390/microorganisms8030317 (2020).
N'Guettia, M., Kouassi, N., Diallo, H. & Kouakou, R. Evaluation of Anthracnose Disease of Mango (Mangifera indica L.) Fruits and Characterization of Causal Agent in Côte d'Ivoire. 2319–1473. (2014).
Garnier, L., Valence, F. & Mounier, J. Diversity and control of spoilage fungi in dairy products: An update. Microorganisms 5, 42. https://doi.org/10.3390/microorganisms5030042 (2017).
Mostafidi, M., Sanjabi, M. R., Shirkhan, F. & Zahedi, M. T. A review of recent trends in the development of the microbial safety of fruits and vegetables. Trends Food Sci. Technol. 103, 321–332. https://doi.org/10.1016/j.tifs.2020.07.009 (2020).
Basavegowda, N., Patra, J. K. & Baek, K.-H. Essential oils and mono/bi/tri-metallic nanocomposites as alternative sources of antimicrobial agents to combat multidrug-resistant pathogenic microorganisms: An overview. Molecules https://doi.org/10.3390/molecules25051058 (2020).
Saleh, Z. N. & Al-Ghouti, M. A. Removal of pesticides from water and wastewater: Chemical, physical and biological treatment approaches. Environ. Technol. Innov. 19, 101026. https://doi.org/10.1016/j.eti.2020.101026 (2020).
Bill, M., Sivakumar, D., Korsten, L. & Thompson, A. K. The efficacy of combined application of edible coatings and thyme oil in inducing resistance components in avocado (Persea americana Mill.) against anthracnose during post-harvest storage. Crop Protect. 64, 159–167. https://doi.org/10.1016/j.cropro.2014.06.015 (2014).
Halonen, N. et al. Bio-based smart materials for food packaging and sensors—A review. Front. Mater. https://doi.org/10.3389/fmats.2020.00082 (2020).
Henciya, et al. Biopharmaceutical potentials of Prosopis spp. (Mimosaceae, Leguminosa). J. Food Drug Anal. 25, 187–196. https://doi.org/10.1016/j.jfda.2016.11.001 (2017).
Raveau, R., Fontaine, J. & Lounès-Hadj Sahraoui, A. Essential oils as potential alternative biocontrol products against plant pathogens and weeds: A review. Foods (Basel, Switzerland) 9, 365. https://doi.org/10.3390/foods9030365 (2020).
Saleh & Abu-Dieyeh. Novel Prosopis juliflora leaf ethanolic extract as natural antimicrobial agent against food spoiling microorganisms. Sci. Rep. 11, 7871. https://doi.org/10.1038/s41598-021-86509-3 (2021).
Sharif, R. et al. The multifunctional role of chitosan in horticultural crops; A review. Molecules 23, 872. https://doi.org/10.3390/molecules23040872 (2018).
Saleh, I. & Abu-Dieyeh, M. Novel Prosopis juliflora leaf ethanolic extract coating for extending postharvest shelf-life of strawberries. Food Control 133, 108641. https://doi.org/10.1016/j.foodcont.2021.108641 (2022).
Saleh, I. & Abu-Dieyeh, M. Evaluation of novel Prosopis juliflora water soluble leaf ethanolic extract as preservation coating material of cucumber. J. Food Process. Preserv. https://doi.org/10.1111/jfpp.16352 (2022).
Xu, Y. et al. Ribotypes of Polymyxa graminis in wheat samples infected with soilborne wheat viruses in China. Plant Dis. 102, 948–954. https://doi.org/10.1094/PDIS-09-17-1394-RE (2017).
Stefanovic, O. D., Tesic, J. D. & Comic, L. R. Melilotus albus and Dorycnium herbaceum extracts as source of phenolic compounds and their antimicrobial, antibiofilm, and antioxidant potentials. J. Food Drug Anal. 23, 417–424. https://doi.org/10.1016/j.jfda.2015.01.003 (2015).
Sayago, J. E., Ordoñez, R. M., Kovacevich, L. N., Torres, S. & Isla, M. I. Antifungal activity of extracts of extremophile plants from the Argentine Puna to control citrus postharvest pathogens and green mold. Postharvest Biol. Technol. 67, 19–24. https://doi.org/10.1016/j.postharvbio.2011.12.011 (2012).
Xiao, L., Zhou, Y. M., Zhang, X. F. & Du, F. Y. Notopterygium incisum extract and associated secondary metabolites inhibit apple fruit fungal pathogens. Pestic. Biochem. Physiol. 150, 59–65. https://doi.org/10.1016/j.pestbp.2018.07.001 (2018).
Chien, P.-J., Sheu, F. & Lin, H.-R. Coating citrus (Murcott tangor) fruit with low molecular weight chitosan increases postharvest quality and shelf life. Food Chem. 100, 1160–1164. https://doi.org/10.1016/j.foodchem.2005.10.068 (2007).
Mohammadi, A., Hashemi, M. & Hosseini, S. M. Postharvest treatment of nanochitosan-based coating loaded with Zataria multiflora essential oil improves antioxidant activity and extends shelf-life of cucumber. Innov. Food Sci. Emerg. Technol. 33, 580–588. https://doi.org/10.1016/j.ifset.2015.10.015 (2016).
Patel & Panigrahi. Starch glucose coating-induced postharvest shelf-life extension of cucumber. Food Chem. 288, 208–214. https://doi.org/10.1016/j.foodchem.2019.02.123 (2019).
Emamifar, A., Ghaderi, Z. & Ghaderi, N. Effect of salep-based edible coating enriched with grape seed extract on postharvest shelf life of fresh strawberries. J. Food Saf. 39, e12710. https://doi.org/10.1111/jfs.12710 (2019).
Naeem, A., Abbas, T. & Hasnain, A. Application of guar gum-based edible coatings supplemented with spice extracts to extend post-harvest shelf life of lemon (Citrus limon). Qual. Assur. Saf. Crops Foods https://doi.org/10.3920/QAS2018.1310 (2019).
Moradi, F., Emamifar, A. & Ghaderi, N. Effect of basil seed gum based edible coating enriched with echinacea extract on the postharvest shelf life of fresh strawberries. J. Food Meas. Character. 13, 1852–1863. https://doi.org/10.1007/s11694-019-00104-9 (2019).
Elmeer, K. & Almalki, A. DNA finger printing of Prosopis cineraria and Prosopis juliflora using ISSR and RAPD techniques. Am. J. Plant Sci. 2, 527–534. https://doi.org/10.4236/ajps.2011.24062 (2011).
Arunachalam, S. K. et al. Evaluation of fungicidal resistance among Colletotrichum gloeosporioides isolates causing mango anthracnose in agri export zone of Andhra Pradesh, India. Plant Pathol. Bull. 16, 469 (2007).
Saleh, I. & Abu-Dieyeh, M. H. Novel Prosopis juliflora leaf ethanolic extract as natural antimicrobial agent against food spoiling microorganisms. Sci. Rep. 11, 7871. https://doi.org/10.1038/s41598-021-86509-3 (2021).
Li, M. L., Zhang, Y., Zhang, L. & Jiang, H. Phytochemical changes in Mango fruit in response to Alternaria alternate infection. Czech J. Food Sci. https://doi.org/10.17221/328/2017-CJFS (2018).
Sogvar, O. B., Saba, M. K. & Emamifar, A. Aloe vera and ascorbic acid coatings maintain postharvest quality and reduce microbial load of strawberry fruit. Postharvest Biol. Technol. 114, 29–35. https://doi.org/10.1016/j.postharvbio.2015.11.019 (2016).
Harker, F., Redgwell, R., Hallett, I., Murray, S. & Carter, G. Hortic. Rev. 20, 121–224 (2010).
Acknowledgements
We would like to acknowledge Dr. Perumal Balakrishnan for his support in generating the samples collection locations map. Open Access funding provided by the Qatar National Library.
Author information
Authors and Affiliations
Contributions
I.S. and M.H.A. designed the study, I.S. and R.H. performed the experiments, I.S., T.A. and M.H.A. analyzed the results, I.S. wrote the manuscript. M.H.A. edited and reviewed the manuscript. M.H.A. and T.A. supervised the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saleh, I., Ahmed, T., Halboosi, R. et al. Genetic diversity of Prosopis juliflora in the state of Qatar and its valuable use against postharvest pathogen of mango fruits. Sci Rep 12, 11012 (2022). https://doi.org/10.1038/s41598-022-14871-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-14871-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.