Abstract
Sylviornis neocaledoniae (Galliformes, Sylviornithidae), a recently extinct bird of New-Caledonia (Galliformes, Sylviornithidae) is the largest galliform that ever lived and one of the most enigmatic birds in the world. Herein, for the first time, we analyze its neuroanatomy that sheds light on its lifestyle, its brain shape and patterns being correlated to neurological functions. Using morphometric methods, we quantified the endocranial morphology of S. neocaledoniae and compared it with extinct and extant birds in order to obtain ecological and behavioral information about fossil birds. Sylviornis neocaledoniae exhibited reduced optic lobes, a condition also observed in nocturnal taxa endemic to predator-depauperate islands, such as Elephant birds. Functional interpretations suggest that S. neocaledoniae possessed a well-developed somatosensorial system and a good sense of smell in addition to its specialized visual ability for low light conditions, presumably for locating its food. We interpret these results as evidence for a crepuscular lifestyle in S. neocaledoniae.
Similar content being viewed by others
Introduction
Avian evolution on islands has produced some remarkable species exhibiting unique characters and odd morphologies and behaviors, often associated with gigantism: this is particularly the case of the endemic flightless birds such as the iconic dodo (Raphus cucullatus) from Mauritius1, the impressive moa (Dinornithiformes) from New Zealand2 and the giant elephant birds (Aepyornithiformes) from Madagascar3. Sylviornis neocaledoniae Poplin, 1980, an enigmatic extinct bird from New Caledonia (France), is one such species: the only known representative of its genus, this giant flightless bird (1.3 m in height, 1.7 m in length and 30–35 kg in weight4,5) lived on La Grande Terre (the main island of the Archipelago) and L’Île des Pins (small southern island) until the late Holocene4,5. It went extinct shortly after the colonization of these islands by humans4, which dates to slightly more than 3000 BP6,7. Despite extensive osteological studies4,8 S. neocaledoniae remains enigmatic: vague information is available concerning its overall morphology and behavior has been transmitted thanks to the oral tradition of the autochthonous people4,9,10,11. According to the Kanak tradition, this giant and aggressive bird laid a single egg per year and appeared stealthily during sunset or sunrise11: this is the only and unverified “data” on its behavior available so far. The phylogenetic position of S. neocaledoniae is the subject of debate5,8. Initially thought to be a possible ratite12, S. neocaledoniae was later considered to be a megapode (Megapodiidae)4,12. Later, Mourer-Chauviré & Balouet8 erected the family Sylviornithidae for this unique species, based on multiple cranial characters interpreted as autapomorphies. Most analyses have suggested that it is a galliform5,8,12,13, possibly a stem galliform2, or Galloanserae5,14,15. In the latest phylogenetic analyses S. neocaledoniae and its sister species Megavitiornis altirostris were recovered sister to all galliforms16,17. However, its precise phylogenetic position remains to be determined. Moreover, no molecular analysis (e.g., palaeogenomics) has been carried out on sufficiently fresh material. Consequently, S. neocaledoniae remains anatomically, ecologically, ethologically and phylogenetically one of the most problematic extinct birds in the world. Here, for the first time we analyze its endocranial anatomy in order to reconstruct its anatomy and behavior. To do so, we compared S. neocaledoniae with galliforms and other extant and extinct large birds (Aepyornithiformes, Casuariiformes, Dinornithiformes, Rheiformes, Struthioniformes, Gastornithiformes, Sphenisciformes, Cariamiformes), so as to throw light on its paleoecology. We used non-destructive and non-invasive conventional X-ray computed microtomography (µCT). We targeted internal cranial structures because they are well known to yield precious information about behavior and phylogeny in both avian and non-avian theropods18,19. In the absence of the brain endocranial reconstructions are a good alternative to neuroanatomy because, in various extinct and extant birds, there exists a strong correlation and parallelism between the endocranial structures and the underlying brain structures (e.g.,20,21,22,23): birds generally have a high cranial cavity-brain correlation index (BEC index) resulting from their exceptionally thin meningeal tissues, meaning that inferences regarding sensory capacities can be made21,24,25,26. Moreover, S. neocaledoniae is larger than other galliforms. We also consider the size ranges observed in S. neocaledoniae because Sayol et al.27 showed that cognitive skills in birds are partly related to brain size, which is strongly correlated with body size. This observation stems from the now well-established principle of proper mass, which is used in the study of the brain25,28. The relative size of each brain region has also been calculated because it has been showed that it reflects the relative importance of the sensory modalities and specific cognitive processes of the regions of the brain concerned, that is its relative function 25,29,30,31,32. We are aware that these relative measurements could be biased by allometric factors related to the great size of the animal25: in order to minimize this bias, we also compare the endocranium of S. neocaledoniae with those of all other large birds (i.e., of body mass greater than 8.750 kg, for the statistical delineation) in addition to extant galliforms. All these comparisons are based on our own 3D-scans realized on various skulls of diverse species (see list in Supplementary Data) and on data extracted from the literature22,33,34,35,36.
The results of these comparisons are interpreted in terms of sensorial capacities and possible related behavior based on quantitative comparisons of relative cerebral areas.
Results
Endocast description and comparison
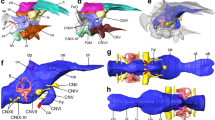
For ease of reference, the morphological terms specific to the brain used here are defined and described for the different regions of the endocast. The virtual endocasts of two specimens of S. neocaledoniae revealed no overall noticeable intraspecific variation (Figs. 1a,c–e) but a morphological similarity to the set of galliforms studied, as opposed to various non-galliform birds, starting from the phylogenetically closest neognaths (see “Materials and methods”) The endocasts of extant galliforms and S. neocaledoniae studied revealed interspecific and interfamilial morphological variation (Figs. 1, S3, S4, S5, S7).
Digital endocranial reconstructions of Sylviornis neocaledoniae (a, b, MNHN NCP 241; c, d, MNHN unnumbered), Odontophorus guttatus (e, f), Mitu tuberosum (g, h), Gallus gallus (i, j) and Megapodius cumingii (k, l). In dorsal (a, c, e, g, i, k) and left lateral (b, d, f, h, j, l) views. Optic lobes are highlighted in purple to aid in comparison of relative size of this structure across taxa. Colors: blue, endocast; yellow, cranial nerves; red, vasculature. ca carotid artery canal, cer cerebellum, ff floccular fossa, if interhemispheric, mca medial carotid artery canal, ob olfactory bulb, ol optic lobe, pf pituitary fossa, rho rhombencephalon, tel telencephalon, vt vallecula telencephali, wslt wulst, I olfactory nerve canal, II optic nerve canal, III oculomotor nerve canal, V1 ophthalmic nerve canal, V2–3 maxillomandibular nerve canal, VI abducens nerve canal, VII–VIII facial and vestibulocochlear nerves canal, IX–XI glossopharyngeal nerve canal and accessory nerves, XII hypoglossal nerve canal. †Fossil taxon. Scale bars = 1 cm.
Olfactory bulb
The olfactory bulb, located at the anterior tip of the telencephalon, is composed of two olfactory lobes. Sylviornis neocaledoniae shows a slight rostral pinch/tightening of the telencephalon, which delimits the olfactory bulb (Figs. 1, S3–S5). This is also the case in extant galliforms although the shape of their olfactory bulbs varies; individually it is either of the one-lobe type (true fusion of the two bulbs into a single midline bulb) or of the biantennary type (the two bulbs are quite separate)37,38,39. Among S. neocaledoniae and studied extant galliforms, the two olfactory lobes are fused as a single lobe but only S. neocaledoniae shows an appreciable sulcus delimiting the two lobes, in dorsal view (Fig. S3). The olfactory bulb of S. neocaledoniae is narrow and becomes wider at the rostral extremity, from which the olfactory nerves (I) bifurcate. In lateral view, the orientation of the long axes of the olfactory bulbs is different from that of the cerebral hemispheres, as in some galliforms. Unlike Phasianidae and Odontophorus guttatus (Odontophoridae), S. neocaledoniae, Megapodiidae (Alectura lathami, Megapodius cumingii) and Cracidae (Mitu tuberosum, Penelope pileata) have an olfactory bulb located slightly rostroventrally and not in the sub-horizontal position in continuity with the telencephalon. In addition, the olfactory bulb in these groups, has a more ventral inclination (Figs. 1, S4, S5).
Telencephalon
In dorsal view, the telencephalon of S. neocaledoniae exhibits a morphology similar to that of many extant galliforms22,36,40: in dorsal and ventral view, the shape of the two cerebral hemispheres gives the rostral part of the endocast a pear-shaped or heart-shaped contour wider caudally. This morphology is much less marked than that of Acryllium vulturinum, Gallus gallus, O. guttatus, P. pileata and Guttera plumifera. In S. neocaledoniae, the mediolateral expansion of the telencephalon begins where the medial cerebral artery traverses the telencephalic hemisphere and in dorsal view, it occludes the rounded optic lobe (Figs. 1, S3). This limit is much less visible in extant galliforms. In dorsal view, this mediolateral expansion in S. neocaledoniae is relatively narrower and less caudally developed than that of extant galliforms. In lateral view, the general contour of the telencephalon is sub-rounded, as in G. gallus and Tetrao urogallus. In other galliforms, the mediolateral expansion of the telencephalon is slightly ventrally pointed, approximately at the rostrodorsal level of the optical lobe. Thus the general contour of the telencephalon is slightly sub-triangular ventrally. In S. neocaledoniae, M. tuberosum and T. urogallus, the mediolateral width of the telencephalon is more developed than the optic lobes. Whereas in other extant galliforms, the mediolateral width of the telencephalon is similar to that of the optic lobes.
Most modern birds have a wulst (sagittal eminence corresponding to the hyperpallium), but it differs in size, shape and position depending on the species23,41. The wulst is well defined and delimited dorsolaterally by the vallecula telencephali (Figs. 1, S3). The wulst of S. neocaledoniae is relatively developed and as in all galliforms, is in a rostral position (i.e., type A of Walsh and Milner41), thus it does not overlap the cerebellum (Figs. 1, S3). In all galliforms, the wulst is delimited from the telencephalon by a shallow groove, the vallecula telencephali. The wulst of S. neocaledoniae and that of M. tuberosum are more developed than those of other galliforms (Fig. 1). In dorsal view, the wulst of S. neocaledoniae is regularly oval in shape, as in A. vulturinum, A. lathami and M. cumingii, whereas in the other galliforms it is dorsolaterally expanded (Fig. S3).
Diencephalon
The external surface of the endocranial diencephalon is limited to the optic nerve (II) and the pituitary fossa. The optic nerve canal in S. neocaledoniae is well developed (Figs. S4, S5). In ventral view, the pituitary fossa is relatively wider in S. neocaledoniae and G. gallus, than in the other galliforms. The pituitary fossa of S. neocaledoniae widens rostrally and it is narrows posteriorly, like all the galliforms studied here, except for O. guttatus and P. pileata. These have a more rounded pituitary fossa, in lateral view. (Figs. 1, S4). Sylviornis neocaledoniae has a unique morphology of the carotid artery: it remains linked to a point well posterior of the pituitary before subdividing into two distinct branches (Fig. S4). This is not the case in extant galliforms, where the carotid artery is subdivided into two distinct branches at the exit of the pituitary fossa (G. plumifera, M. cumingii, G. gallus, T. urogallus) or slightly more posteriorly (A. lathami, M. tuberosum, A. vulturinum, P. pileata, O. guttatus) (Figs. 1, S4).
Mesencephalon
The optic lobes (i.e., the visible parts of the mesencephalon), are located underneath and posterior to the telencephalon. In S. neocaledoniae they are somewhat visually inconspicuous structures and seem smaller in comparison to those in the extant galliforms (Fig. 1). They are characterized by a slight lateral expansion of the ventromedial endocast (Fig. S4). In lateral view, the optic lobe of S. neocaledoniae is ovoid, as in T. urogallus. The other galliforms have a more developed optic lobe rostroventrally (Fig. S4).
Metencephalon
The morphology of the cerebellum varies individually among the galliforms. The cerebellum of S. neocaledoniae is compressed mediolaterally, expanded rostrocaudally, like that of M. tuberosum and G. gallus (Fig. S3) and it narrows at its rostral and caudal extremities, like that of T. urogallus (Fig. S3). In lateral view, the cerebellum of S. neocaledoniae is less dorsally domed than that of the other galliforms (Fig. 1). In lateral view, the main axis of the brain of most bird species deviates from the main axis of the spinal cord, resulting a rostral flexion mainly located in the region of the mesencephalon41,42 (Fig. 1). Sylviornis neocaledoniae and T. urogallus are characterized by the absence of rostral flexion of the cerebellum, reflecting the dorsoventral compression and the elongated shape of their skull.
Among galliforms, the floccular fossa is highly variable in morphology and size. The floccular fossa of S. neocaledoniae is parallel to the axial plane and has a lateral projection oriented at 45° angle to the sagittal plane. Sylviornis neocaledoniae has a type 5 flocculus (the base is not twisted but is rostrocaudally compressed)43. A sheet of bone between the arterial loop and the surface of the fossa is visible: it causes a “fenestra” in the flocculus endocast (Fig. 1). Following Walsh et al.43, this structure, covered by an amount of vascular tissue, is characteristic of low manoeuvrability.
Rhombencephalon
The rhombencephalon forms the caudoventrolateral areas of the hindbrain and defines the structures of the medulla oblongata and pons. In S. neocaledoniae and T. urogallus, the rhombencephalon is relatively flat rostrocaudally and compressed mediolaterally, unlike those of the other galliforms (Figs. 1, S4). In S. neocaledoniae, the cerebellum and associated ventral rhombencephalon form a distinctive hindbrain, which overall morphology differs compared with that of the extant galliforms (Figs. 1, S4).
Myelencephalon
In S. neocaledoniae and all the extant galliforms, the trigeminal ganglion and the base of the trigeminal nerve are contiguous with the optic lobe. This represents the most common condition in extant birds44. The trigeminal nerve complex divides into three branches: the ophthalmic nerve (V1) and the maxillomandibular complex nerve (V2–3) comprising the maxillary nerve (V2) and the mandibular nerve (V3), and insert on the ventral surface of the optic lobe. The myelencephalon is distinctive in S. neocaledoniae, in that the maxillomandibular branch (V2–3) is more developed than that of the other galliforms and is practically perpendicular to the ophthalmic nerve (V1) (Figs. 1, S4).
Inner ear
S. neocaledoniae and the Cracidae (M. tuberosum and P. pileata) have more sinuous semi-circular canals than those of other galliforms (Fig. S7). In S. neocaledoniae, the semi-circular canals are slightly thicker than those of most galliforms (Fig. S7). Benson et al.45 have shown that large non-volant birds have shorter labyrinths. Sylviornis neocaledoniae supports this hypothesis in having labyrinths that are proportionately shorter than those of the other galliforms (Fig. S6). A detailed description is given in the electronic supplementary material and is illustrated in Supplementary Fig. S7.
Endocast orientation
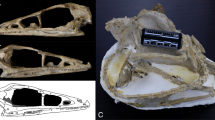
Like for the external skull described by Mourer-Chauviré and Balouet8 (and Fig. 2c), the endocast of S. neocaledoniae does not show a great dorsoventral expansion. When the skull is oriented in a normal position, that is following the horizontal of the lateral semi-circular canal, the reconstructions show that the flexion (the endocranial axis from the tip of the olfactory bulb to the foramen magnum through the midpoint of the isthmic constriction) of the endocranial cavity of the two S. neocaledoniae specimens was significantly lower (averaged 17.7°) than that of the other galliforms examined (averaged 30.9°) (Fig. S10). This low brain flexion of S. neocaledoniae is associated with a particular orientation of the skull (∽ 15°) relative to the horizontal (Fig. 2). These two observations are consistent with a horizontal posture/position of the upper neck as in the more recent skeletal reconstructions2. This horizontal posture and its effect on the orientation of the endocast and the relatively dorsal position of the foramen magnum are illustrated in Fig. 2. These observations support the hypothesis of a correlation between skull size, brain orientation, foramen magnum position and posture of the head in birds46,47. Most endocasts of adult neognath birds are steeply inclined upwards, ventrally flexed or narrower and more anteriorly inclined30 whereas in S. neocaledoniae it is narrower and weakly inclined downwards. Ashwell and Scofield48 showed that most moa also tend to have low values for endocast flexion, induced by horizontal deportment from their upper neck. The entire endocast has a relatively elongated general morphology like that of T. urogallus.
Carriage of the head and orientation of the endocranial axis in Sylviornis neocaledoniae (a) contrasted with that in Gallus gallus (b). The endocranial cavity and endocranial axis (orange dashed lines) are superimposed on lateral views of the head and neck in the two species. The attachment of the neck to the back of the skull in S. neocaledoniae is accompanied by a dorsally positioned foramen magnum (c) and particular orientation of the skull (∽ 15°) relative to the horizontal (orange line, d). Scale bars = 2 cm.
Size of endocast structures
Olfactory bulb size
The relative development of the olfactory bulb in S. neocaledoniae is the largest of all galliforms and positions itself close to O. guttatus and T. urogallus (Fig. 3, Tables S1, S3). When the ratio of olfactory bulb is compared to that of other large birds, it is positioned close to the median: S. neocaledoniae has an olfactory bulb almost as developed proportionately as in the giant moa (Pachyornis elephantopus) and more developed than that of the ostrich (Struthio camelus) (Tables S1, S3). In birds, the relative size of the olfactory bulb is correlated with olfactory capacity28,38,49. Sylviornis neocaledoniae therefore probably had strong olfactory abilities and the best sense of smell among galliforms measured here.
Violin plots showing the position of Sylviornis neocaledoniae (black star) relative to other Galliformes, with n = 11 (a, c) and large birds (body mass greater than 8.750 kg), with n = 9 for the ratio of wulst and n = 15 for the two other ratios (b), according to a series of ratios. Heavy black line indicates the median. Distribution of certain species: circle, Tetrao urogallus; square, Mitu tuberosum; pentagon, Odontophorus guttatus; rhombus, Meleagris gallopavo; triangle, Casuarius casuarius; hexagon, Llallawavis scagliai; ellipce, Pachyornis elephantopus. CHA cerebral hemisphere surface area, CHL cerebral hemisphere length, CrbA cerebellum surface area, OLA optic lobe surface area, OBL olfactory bulb length, nV2–3A maxillomandibular nerve canal cross-section area, TBA total brain surface area, WA wulst surface area.
Optic lobe size
In contrast, the optic lobes of S. neocaledoniae are relatively the smallest of all galliforms (Fig. 3a, Tables S1, S3). When the relative size of the optic lobe is compared with that of all birds or large birds33,34,50,51, it is positioned close to the medians and means in both cases (Fig. 3b, Tables S1, S3): S. neocaledoniae has an optic lobe as reduced as that of the cassowary (Casuarius casuarius, Casuariidae), an extinct penguin (Paraptenodytes antarcticus, Spheniscidae) and the magnificent bird of Scaglia (Llallawavis scagliai, Phorusrhacidae). In birds, the relative size of the optic lobe is correlated with optical capacity22,33,50,51. Sylviornis neocaledoniae therefore probably had a weak visual acuity, as is also suggested by its proportionately reduced optic nerve and its small orbits (see electronic supplementary material, Fig. S11).
Wulst size
Relative to endocast, the wulst of S. neocaledoniae is one of the largest among galliforms, with T. urogallus and Meleagris gallopavo (Fig. 3a, Tables S1, S3). However, when the relative size of this wulst is compared to that of large birds34,50,51, it is one of the smallest wulsts of large birds (Fig. 3b, Table S3).
Maxillomandibular nerve (V2–3) section
The relative size of the maxillomandibular nerve canal cross-section (V2-3), provides information about the development of the somatosensory system (sense of touch) and presumably mechanoreception. The comparison does not include large birds because of the lack of data. In galliforms, the maxillomandibular nerve is weakly developed in general (average ratio of: 0.0097 against 0.023 for S. neocaledoniae). Sylviornis neocaledoniae presents a much more developed nerve than that of extant galliforms (Fig. 3c, Tables S1, S3), except for that of Mitu tuberosum (Tables S1, S3). Apart from S. neocaledoniae another Galloanserae shows an exceptionally well developed maxillomandibular nerve: the extinct anseriform Talpanas lippa of the Hawaiian Islands (Mole-duck, Oxyurini)52,53 (Fig. S11). Sylviornis neocaledoniae shows a relatively smaller maxillomandibular nerve than T. lippa proportional to the size of the endocast, but slightly more developed compared to the surface of the foramen magnum (Fig. S11).
Discussion
The endocranium of S. neocaledoniae is similar to that of a typical galliform, but, like its general osteology, it also shows a mosaic of derived characters, galliform synapomorphies and probably some plesiomorphies. A precise phylogenetic analysis of S. neocaledoniae is outside the scope of this study, however, the cranial and endocranial anatomy of S. neocaledoniae reveals very interesting traits concerning its behaviour and lifestyle:
The shape and orientation of the endocast is caused in part by the foramen magnum being located caudally and facing caudally on the cranium, and it is not directed somewhat ventrally as in most birds. The fact that its cerebellum has no rostral flexion is linked to the general shape and orientation of the endocast, and the high dorso-caudal position of the foramen magnum, also observed exclusively in some moa48 and a giant terror bird54. Gussekloo and Cubo55 have shown that the inability to fly in modern birds affects the morphology of the skull. They suggest that this relationship is related to a reduced selective force for light skull in these birds, allowing the development of strong muscle insertions of a heavier skull and robust neck in walking birds that are unable to fly. These authors confirmed the relationship between flight incapacity and a peramorphic (i.e., overdeveloped) skull in relation to the standard ontogenetic trajectory of G. gallus, suggested by morphological studies56. We can assume that the cranial morphology of S. neocaledoniae follows this same trend (e.g. see the morphology of the palatine-pterygoid complex8), but a more detailed morphological and quantitative study is needed to test this hypothesis. Other parts of the skeleton such as the reduced keel and wings are typically interpretable as paedomorphic, and such a mosaic of different heterochronic conditions is observed in other flightless birds57.
The relative importance of sensory modalities and specific cognitive processes is reflected in the relative sizes of brain regions25,29,30,31. Our reconstructions of the S. neocaledoniae endocasts, compared with both extant galliforms and extant and extinct “large” birds (i.e., above 8.570 kg), show that this bird had relatively small optic lobes associated with small orbits and optic nerves (Figs. 3, S11). The visual information is processed along three different pathways in the avian brain: accessory optic, thalamofugal, and tectofugal pathways of which the last two are most important and different. The optic lobe is the equivalent of the visible part of the optic tectum and optic tract and is the relay point for sensory information to the anterior brain22,58. The optic lobe comprises most of retinal afferents, is related to retinal summation and hence acuity and corresponds to the main visual pathway in birds59,60,61. Early et al.22 showed that the relative size of the optic lobes is representative of the size of the underlying brain structure. Thus we supposed that behavioral inferences can be made from brain-structure size. Among extant birds, relatively small optic lobes are observed only in crepuscular or nocturnal birds such as the kiwi62, the nocturnal parrot Pezoporus occidentalis63, and the cassowary33. Optic lobe reductions in the elephant birds and the Mole duck Talpanas lippa, have been interpreted as revealing a nocturnal lifestyle pattern for these extinct birds33,52,64. This correlation is consistent with the proper mass principle25, which predicts that the relative development of a brain structure is proportional to the complexity of the associated behaviours, which is confirmed by studies on extant species22,33,38,41,62,64. Therefore, as evidenced by the small optic nerves and lobes associated with the small orbits, the visual system of S. neocaledoniae implies reduced visual performance. Compared to the extant galliforms, S. neocaledoniae did not have a good ability to discern information brought to the brain by sight and thus poor vision. Biometrically, S. neocaledoniae exhibits endocranial ratios similar to those of the cassowary33 and, among the galliforms studied, it has the smallest relative surface of the optic lobes (Fig. 3, Tables S1, S3). Additionally, S. neocaledoniae also has an optical lobe ratio (to the endocast area) similar to that of the diurnal emu (Dromaius novaehollandiae), as given in the Torres & Clarke33 study, but is lower than that given for the emu in Corfield et al.51 (Table S3). This assumes some relatively large intra-species variation in emu for ratios of optic lobe and the other different brain structures (Table S3), or more likely differences between how different workers measured these data. The cassowary has a greater activity at the beginning and the end of the day, which corresponds to a crepuscular lifestyle. Assuming that the correlation between this structural reduction and this lifestyle is valid for S. neocaledoniae, then it is inferred that it had reduced visual acuity and probably a crepuscular lifestyle.
To respond to the transition from a mainly diurnal towards a crepuscular or nocturnal lifestyle, the bird’s visual system can either increase its visual sensitivity or reduce it in favour of other senses62. Increased sensitivity characterizes flying nocturnal birds (e.g., owls, oil birds, nightjars), which specialize in manoeuvrability and feeding in low light conditions, often characterized by large eyes and hyperdeveloped wulsts65. Only nocturnal flightless insular birds are known to have reduced the visual system in favour of other senses, including the Malagasy elephant birds, New Zealand moa, and the kakapo33,62,66,67. However, the last species appears to have atypical eye anatomy for diurnal or nocturnal activity64. The relatively small size of the eyes and optical lobe in the Moa, have been recently interpreted in terms of a nocturnal ecology for these giant flightless birds from New Zealand33. However, the study of Megalapteryx didinus (Dinornithiformes); the only species for which sclerotic rings are known64, indicated cathemeral (even diurnal/nocturnal) activity.
The adaptation of S. neocaledoniae to a crepuscular activity corroborates the hypothesis of Torres and Clarke33, in which reduced vision in birds (that are nocturnal or crepuscular and which use their other senses) is an adaptation that is probably an option only for flightless species (or really advanced only in those species). This hypothesis is based on the fact that despite the observation that a visual capacity that is adapted to low light conditions exists in various clades across many terrestrial non-avian vertebrates, only birds exhibit a correlation with changes in locomotion strategy, that is, loss of flight. But this might be easily explained by the correlation between loss of flight, living in predator-free or predator-scarce, insular environments, and viability of reduced vision. Indeed, high-performance vision is rendered less indispensable as soon as there are no more flight requirements (manoeuvrability etc.) nor any (or reduced) need to escape predators.
It can be hypothesized that vision capacities have decreased in favour of the use of other senses, such as smell, touch or hearing62,68,69. These non-visual senses may also have been used by S. neocaledoniae in adapting to a crepuscular lifestyle. The relatively large size of the olfactory bulb of S. neocaledoniae seems to attest to a well-developed sense of smell. Zelenitsky et al.28 showed that the olfactory bulb ratio and body mass are uncorrelated in birds. However, they observed that in large species of Neornithes (more than 4.85 kg, in Zelenitsky et al.28), the olfactory ratios tend to be lower than those predicted by the regression. Sylviornis neocaledoniae, being a large bird, has a smaller olfactory bulb ratio than predicted by this regression and so confirms this tendency. However, it has proportionately the largest olfactory bulb of all galliforms and its ratio is larger than average, among large birds. Therefore, we consider that S. neocaledoniae had a relatively well-developed sense of smell. This well-developed sense of olfaction was perhaps crucial while foraging or was correlated with a particular, closed, forested habitat in which vision (and even hearing) were hampered (as Torres and Clarke33, have hypothesized with elephant birds).
For some species, the adoption of a crepuscular or nocturnal lifestyle may lead to a development of the second visual pathway; the thalamofugal pathway, which projects from the retina to the dorsal thalamus and onto the wulst70, resulting in a relatively enlarged wulst. This peculiarity is present and well known in Strigiformes especially, unlike kiwi (Apterygiformes) that lack a developed wulst. However, some diurnal species also possess a relatively enlarged wulst, e.g., emus, and therefore probably had a developed thalamofugal visual pathway. The thalamofugal pathway is devoted to integrating visual information71, for example, associated with stereopsis65, spatial orientation72, pattern discrimination at a distance73. Nocturnal or crepuscular taxa therefore require an increased sensitivity of the visual system, especially in low light environments65,70. Sylviornis neocaledoniae seems to have a relatively well-developed wulst (among all birds, it is the best-developed within galliforms). Therefore, its stereopsis was apparently developed. Early et al.34 speculated that the position of the wulst is correlated with the use of the tactile sense when feeding. Birds with a wulst in the caudal position (type B sensu Walsh & Milner41), use the tactile sense while foraging, unlike birds with a wulst of type A (in rostral position). We suppose that the latter have a developed sense of touch that is not used for foraging but could help to understand and perceive their environment, thus compensating for the possible reduction of other senses. As T. lippa among anseriforms, S. neocaledoniae also has a maxillomandibular nerve that is proportionately wider than in the extant galliforms examined. However, it should be noted that the enlargement in T. lippa was extraordinary, and enlargement is not so great in S. neocaledoniae. This nerve is directly related to the somatosensory system (the perception of touch, temperature, body position (proprioception), and pain) of the beak. Sylviornis neocaledoniae would therefore have had a well-developed somatosensory system of the beak to compensate for its reduced visual acuity. This developed sense agrees with the large size of its beak, its vascularization and the presence of numerous foramens at the rostral extremity of the beak8. This sense may have served it to investigate its environment, using its beak as a tester. It is difficult to be more precise, but if there were Herbst receptors associated to the numerous foramens at the rostral extremity of the beak, such a tactile sense could correspond to foraging in the soil, the humus of the forested habitats, or under leaves. Enhanced beak somatosensory capacities could also relate to enhanced mechanoreception, possibly in relation to special use of the beak that might have exerted particular mechanical constraints. The maxillomandibular nerve could relate to the presence of pressure receptors for skillful manipulation of food. The mechanoreceptors are just one of the many potential things that this nerve transmits, and it can be bound to taste receptors. Indeed, the gustative information from the tongue is conveyed, within the lingual branch of the maxillomandibular (V2-3) ramus, by the facial (VII) nerve to the trigeminal principal sensory nucleus, which also receives input from glossopharyngeal (IX) and hypoglossal (XII) nerves36. But we consider it to be quite possible that the beak, with its peculiar morphological characteristics8 of a greatly enlarged bony casque dorsally, was used for other and still unknown purpose(s) (mate selection for example?). Further analyses will be carried out to try to answer these questions.
The ecology of S. neocaledoniae seems to have been highly peculiar, and more complex than thought earlier. The adoption of a crepuscular lifestyle by S. neocaledoniae seems to be correlated to a reduction in visual acuity and specialised visual ability for low light conditions, associated to the development of smell and somatosensory system of the beak, linked to the loss of flight permitted by the absence of predators in an oceanic insular environment. Moreover, S. neocaledoniae seems to have substantially developed its somatosensory system, the only other such example known being the extinct, highly peculiar Hawaiian Mole duck T. lippa52. Sylviornis neocaledoniae stands out as one of the most eccentric recently extinct bird species known, highlighting the fact that the most divergent species, morphologically, ecologically and behaviourally speaking, went preferentially extinct as a result of the arrival of humans on the islands where it lived, and that such a threat is still extremely great for the surviving insular endemics all over the world74.
Materials and methods
Endocast reconstruction
We reconstructed two digital endocasts of S. neocaledoniae with high-resolution X-ray computed microtomographic methodology, based on two well-preserved cranial specimens from the collections of the Muséum National d’Histoire Naturelle (Paris, France); MNHN-NCP 241 and an MNHN-NCP unnumbered). For our comparative analysis, we also reconstructed the endocrania of at least one representative of all extant galliform families, one extant species of anseriform (the extant sister group of galliforms75,76) and representatives of other orders: Phoenicopteriformes, Podicipediformes, Gruiformes, Charadriiformes and Opisthocomiformes. Virtual endocasts were reconstructed using Avizo 9 lite (FEI Visualization Sciences Group, Berlin, Germany), following the reconstruction suggestions of Balanoff et al.21. Only practices to smooth and connect the separate portions of the endocasts were used. Scanning parameters and details of methods are provided in the electronic supplementary material (see Table S2). For all specimens, skull, endocast, base of the major nerves and labyrinths (anterior (ASC), lateral (LSC) and posterior (PSC) semi-circular canals, vestibule and cochlear canal) were segmented. Data for other species are derived from other studies22,33,36,50,51,77 and their data and endocast reconstructions contributed to this study (see Tables S1, S3).
Measurements of the olfactory bulb, optic lobe, and semicircular canals
The olfactory bulb of extant species has been difficult to reconstruct, because its surrounding is not completely ossified. In some species, the olfactory nerves could not be reconstructed and the lower limit of the olfactory bulb is not always well defined. We were therefore unable to carry out surface measurements for the olfactory bulb. The measurements of the olfactory bulb were therefore carried out according to the method described of Bang and Cobb38: the ratio of length of the olfactory bulb over the length of the corresponding cerebral hemisphere, and in the axis of the maximum length for each measure. To investigate olfactory bulb relative size across galliforms and other birds, this ratio was then compared with the species sampled by Bang and Cobb38, Torres and Clarke33 and Zelenitsky et al.28.
The measurement of the optic lobe follows Torres and Clark33 but we used the ratio of the surface of the two optic lobes (and not a single optic lobe) on the total surface of the endocast. The measurement was carried out with the MeshLab software version 2020.06. Detailed methods of acquiring surface measurements are described in the electronic supplementary material and illustrated in Supplementary Fig. S1.
We measured the relative positions of the semi-circular canals (i.e., the angles between the canals) and their length using Avizo 9 lite (FEI Visualization Sciences Group, Berlin, Germany). The measurements follow Benson et al.45. All measurement data are provided in the electronic supplementary material (Table S1). Detailed methods are described in the electronic supplementary material and illustrated in Supplementary Fig. S2.
Nomenclature
We follow the anatomical nomenclature in Baumel and Witmer78, for osteology, external brain surface structure and innervation. Disparate terminologies have been used for the description of the surface morphology of the avian cranium and brain, in some instances with no consensus for any particular term (e.g.,78,79). The endocasts have been described in accordance with the terminology of Witmer et al.18, Early et al.23 and Handley and Worthy36, and are illustrated in the Figs. 1, S1, S3, S4 and S5.
Data availability
The original X-ray microtomography files for the specimens in this study are available on request and will be uploaded to MorphoSource.
References
Claessens, L. P., Meijer, H. J. & Hume, J. P. The morphology of the Thirioux dodos. J. Vertebr. Paleontol. 35, 29–187 (2015).
Worthy, T. H. & Holdaway, R. N. The Lost World of the Moa: Prehistoric Life of New Zealand (Indiana University Press, 2002).
Hansford, J. P. & Turvey, S. T. Unexpected diversity within the extinct elephant birds (Aves: Aepyornithidae) and a new identity for the world’s largest bird. R. Soc. Open Sci. 5(9), 181295 (2018).
Poplin, F. & Mourer-Chauviré, C. Sylviornis neocaledoniae (Aves, Galliformes, Megapodiidae), oiseau géant éteint de l’Ile des Pins (Nouvelle-Calédonie). Geobios 18(1), 73–105 (1985).
Worthy, T. H. et al. Osteology supports a stem-galliform affinity for the giant extinct flightless bird Sylviornis neocaledoniae (Sylviornithidae, Galloanseres). PLoS ONE 11(3), e0150841 (2016).
Anderson, A., Sand, C., Petchey, F. & Worthy, T. H. Faunal extinction and human habitation in New Caledonia: Initial results and implications of new research at the Pindai Caves. J. Pac. Archaeol. 1(1), 89–109 (2010).
Maurizot, P. & Campbell, H. J. Palaeobiogeography of New Caledonia. Geol. Soc. 51(1), 189–213 (2020).
Mourer-Chauviré, C., & Balouet, J. Description of the skull of the genus Sylviornis Poplin, 1980 (Aves, Galliformes, Sylviornithidae new family), a giant extinct bird from the Holocene of New Caledonia. In Proceedings of the International Symposium" Insular Vertebrate Evolution: the Palaeontological Approach": September, 16–19 Mallorca, 205–218. Societat d'Història Natural de les Balears (2005).
Dubois, M. J. Trouvailles à l’île des Pins, Nouvelle-Calédonie. J. Soc. Océan. 32(51), 233–239 (1976).
Dubois, M. J. Espèce disparue et tradition mélanésienne. Bull. Soc. Ét. Hist. 29, 7–8 (1976).
Griscelli, P. Deux oiseaux fossiles de Nouvelle-Calédonie. Bull. Soc. Ét. Hist. 29, 3–6 (1976).
Poplin, F. Sylviornis neocaledoniae n.g, n. sp. (Aves) Ratite éteint de la Nouvelle-Calédonie. C. R. Acad. Sci. 290, 691–694 (1980).
Poplin, F., Mourer-Chauviré, C. & Evin, J. Position systématique et datation de Sylviornis neocaledoniae, mégapode géant (Aves, Galliformes, Megapodiidae) éteint de la Nouvelle-Calédonie. C. R. Acad. Sci. 297(3), 301–304 (1983).
Mayr, G. Cenozoic mystery birds: On the phylogenetic affinities of bony-toothed birds (Pelagornithidae). Zool. Scr. 40(5), 448–467 (2011).
Elzanowski, A. & Boles, W. E. Australia’s oldest Anseriform fossil: A quadrate from the Early Eocene Tingamarra Fauna. Palaeontology 55(4), 903–911 (2012).
Worthy, T. H., Degrange, F. J., Handley, W. D. & Lee, M. S. The evolution of giant flightless birds and novel phylogenetic relationships for extinct fowl (Aves, Galloanseres). R. Soc. Open Sci. 4(10), 170975 (2017).
Tambussi, C. P., Degrange, F. J., De Mendoza, R. S., Sferco, E. & Santillana, S. A stem anseriform from the early Palaeocene of Antarctica provides new key evidence in the early evolution of waterfowl. Zool. J. Linn. Soc. 186(3), 673–700 (2019).
Witmer, L. M. & Ridgely, R. C. The paranasal air sinuses of predatory and armored dinosaurs (Archosauria: Theropoda and Ankylosauria) and their contribution to cephalic structure. Anat. Rec. 291(11), 1362–1388 (2008).
Witmer, L. M., Ridgely, R. C., Dufeau, D. L. & Semones, M. C. Using CT to peer into the past: 3D visualization of the brain and ear regions of birds, crocodiles, and nonavian dinosaurs. In Anatomical Imaging 67–87 (Springer, 2008).
Balanoff, A. & Bever, G. The role of endocasts in the study of brain evolution. In Evolution of Nervous Systems 2nd edn 223–241 (Elsevier Incorporation, 2016).
Balanoff, A. M. et al. Best practices for digitally constructing endocranial casts: Examples from birds and their dinosaurian relatives. J. Anat. 229(2), 173–190 (2016).
Early, C. M., Ridgely, R. C. & Witmer, L. M. Beyond endocasts: Using predicted brain-structure volumes of extinct birds to assess neuroanatomical and behavioral inferences. Diversity 12(1), 34 (2020).
Early, C. M., Iwaniuk, A. N., Ridgely, R. C. & Witmer, L. M. Endocast structures are reliable proxies for the sizes of corresponding regions of the brain in extant birds. J. Anat. 237(6), 1162–1176 (2020).
Jerison, H. J. Brain evolution and dinosaur brains. Am. Nat. 103(934), 575–588 (1969).
Jerison, H. J. Evolution of the Brain and Intelligence (Academic Press, 1973).
Iwaniuk, A. N. & Nelson, J. E. Can endocranial volume be used as an estimate of brain size in birds?. Can. J. Zool. 80(1), 16–23 (2002).
Sayol, F., Lefebvre, L. & Sol, D. Relative brain size and its relation with the associative pallium in birds. Brain Behav. Evol. 87(2), 69–77 (2016).
Zelenitsky, D. K., Therrien, F., Ridgely, R. C., McGee, A. R. & Witmer, L. M. Evolution of olfaction in non-avian theropod dinosaurs and birds. Proc. R. Soc. B 278(1725), 3625–3634 (2011).
Dubbeldam, J. L. Birds In The Central Nervous System of Vertebrates 1525–1636 (Springer, 1998).
Butler, A. B. & Hodos, W. Comparative Vertebrate Neuroanatomy: Evolution and Adaptation (Wiley, 2005).
Striedter, G. F. Principles of Brain Evolution (Sinauer Associates, 2005).
Kawabe, S., Shimokawa, T., Miki, H., Matsuda, S. & Endo, H. Variation in avian brain shape: Relationship with size and orbital shape. J. Anat. 223(5), 495–508 (2013).
Torres, C. R. & Clarke, J. A. Nocturnal giants: Evolution of the sensory ecology in elephant birds and other palaeognaths inferred from digital brain reconstructions. Proc. R. Soc. B 285(1890), 20181540 (2018).
Early, C. M. Quantitative Assessments of Avian Endocasts as Tools for Inferring Neuroanatomical Traits and Potential Functional Capabilities (Doctoral dissertation, Ohio University) (2019).
Ksepka, D. T. et al. Tempo and pattern of avian brain size evolution. Curr. Biol. 30(11), 2026–2036 (2020).
Handley, W. D. & Worthy, T. H. Endocranial anatomy of the giant extinct Australian mihirung birds (Aves, Dromornithidae). Diversity 13(3), 124 (2021).
Cobb, S. A note on the size of the avian olfactory bulb. Epilepsia 1(1–5), 394–402 (1959).
Bang, B. G. & Cobb, S. The size of the olfactory bulb in 108 species of birds. Auk 85(1), 55–61 (1968).
Kawabe, S., Ando, T. & Endo, H. Enigmatic affinity in the brain morphology between plotopterids and penguins, with a comprehensive comparison among water birds. Zool. J. Linn. Soc. 170(3), 467–493 (2014).
Mayr, G., Goedert, J. L. & Rabenstein, R. Cranium of an Eocene/oligocene pheasant-sized galliform bird from western North America, with the description of a vascular autapomorphy of the Galliformes. J. Ornithol. 163, 1–12 (2022).
Walsh, S. & Milner, A. Evolution of the avian brain and senses. Living Dinosaurs: the Evolutionary History of Modern Birds, 282–305 (2011).
Walsh, S. A. & Knoll, F. The Evolution of Avian Intelligence and Sensory Capabilities: The Fossil Evidence. In Digital Endocasts 59–69 (Springer, 2018).
Walsh, S. A. et al. Avian cerebellar floccular fossa size is not a proxy for flying ability in birds. PLoS ONE 8(6), e67176 (2013).
Elzanowski, A. & Galton, P. M. Braincase of Enaliornis, an early cretaceous bird from England. J. Vertebr. Paleontol. 11(1), 90–107 (1991).
Benson, R. B., Starmer-Jones, E., Close, R. A. & Walsh, S. A. Comparative analysis of vestibular ecomorphology in birds. J. Anat. 231(6), 990–1018 (2017).
Duijm, M. On the head posture of some birds and its relation to some anatomical features. Proc. Koninkl. Nederl. Akad. Wetensch. 54, 260–271 (1951).
Kulemeyer, C., Asbahr, K., Gunz, P., Frahnert, S. & Bairlein, F. Functional morphology and integration of corvid skulls: A 3D geometric morphometric approach. Front. Zool. 6(1), 1–14 (2009).
Ashwell, K. W. S. & Scofield, R. P. Big birds and their brains: Paleoneurology of the New Zealand moa. Brain Behav. Evol. 71(2), 151–166 (2008).
Clark, L., Avilova, K. V. & Bean, N. J. Odor thresholds in passerines. Comp. Biochem. Physiol. A Physiol. 104(2), 305–312 (1993).
Iwaniuk, A. N., Heesy, C. P., Hall, M. I. & Wylie, D. R. Relative Wulst volume is correlated with orbit orientation and binocular visual field in birds. J. Comp. Physiol. A. 194(3), 267–282 (2008).
Corfield, J. R., Kolominsky, J., Craciun, I., Mulvany-Robbins, B. E. & Wylie, D. R. Is cerebellar architecture shaped by sensory ecology in the New Zealand Kiwi (Apteryx mantelli)?. Brain Behav. Evol. 87(2), 88–104 (2016).
Iwaniuk, A. N., Olson, S. L. & James, H. F. Extraordinary cranial specialization in a new genus of extinct duck (Aves: Anseriformes) from Kauai, Hawaiian Islands. Zootaxa 2296(1), 47–67 (2009).
Witmer, L. M., Ridgely, R. C., James, H. F., Olson, S. L. & Iwaniuk, A. N. The remarkable, recently extinct “mole-duck” Talpanas lippa (Aves: Anseriformes) from Kauai, Hawaii: Behavioral implications of its neuroanatomy and skull morphology. FASEB J. 31, 251–256 (2017).
Chiappe, L. M. & Bertelli, S. Skull morphology of giant terror birds. Nature 443(7114), 929–929 (2006).
Gussekloo, S. W. & Cubo, J. Flightlessness affects cranial morphology in birds. Zoology 116(2), 75–84 (2013).
Maxwell, E. E. Comparative ossification and development of the skull in palaeognathous birds (Aves: Palaeognathae). Zool. J. Linn. Soc. 156(1), 184–200 (2009).
Gaspar, J., Gibb, G. C. & Trewick, S. A. Convergent morphological responses to loss of flight in rails (Aves: Rallidae). Ecol. Evol. 10(13), 6186–6207 (2020).
Wylie, D. R., Gutierrez-Ibanez, C., Pakan, J. M. & Iwaniuk, A. N. The optic tectum of birds: Mapping our way to understanding visual processing. Can. J. Exp. Psychol. 63(4), 328 (2009).
Hunt, S. P. & Webster, K. E. The projection of the retina upon the optic tectum of the pigeon. J. Comp. Neurol. 162(4), 433–445 (1975).
Remy, M. & Güntürkün, O. Retinal afferents to the tectum opticum and the nucleus opticus principalis thalami in the pigeon. J. Comp. Neurol. 305(1), 57–70 (1991).
Mpodozis, J., Letelier, J. C., Concha, M. L. & Maturana, H. Conduction velocity groups in the retino-tectal and retino-thalamic visual pathways of the pigeon (Columba livia). Int. J. Neurosci. 81(3–4), 123–136 (1995).
Martin, G. R. et al. Kiwi forego vision in the guidance of their nocturnal activities. PLoS ONE 2(2), e198 (2007).
Iwaniuk, A. N. et al. The endocast of the Night Parrot (Pezoporus occidentalis) reveals insights into its sensory ecology and the evolution of nocturnality in birds. Sci. Rep. 10(1), 1–9 (2020).
Johnston, P. & Mitchell, K. J. Contrasting patterns of sensory adaptation in living and extinct flightless birds. Diversity 13, 538 (2021).
Iwaniuk, A. N. & Wylie, D. R. The evolution of stereopsis and the Wulst in caprimulgiform birds: a comparative analysis. J. Comp. Physiol. A. 192(12), 1313–1326 (2006).
Wiman, C. & Edinger, T. Sur les cranes et les encephales d’Aepyornis et de Mullerornis. Bull. Acad. Malgache 24, 1 (1942).
Corfield, J., Kubke, M. F., Parsons, S., Wild, J. M. & Köppl, C. Evidence for an auditory fovea in the New Zealand kiwi (Apteryx mantelli). PLoS ONE 6(8), e23771 (2011).
Corfield, J. R., Eisthen, H. L., Iwaniuk, A. N. & Parsons, S. Anatomical specializations for enhanced olfactory sensitivity in kiwi, Apteryx mantelli. Brain Behav. Evol. 84(3), 214–226 (2014).
Cunningham, S. J. et al. The anatomy of the bill tip of kiwi and associated somatosensory regions of the brain: Comparisons with shorebirds. PLoS ONE 8(11), e80036 (2013).
Karten, H. J., Hodos, W., Nauta, W. J. & Revzin, A. M. Neural connections of the “visual wulst” of the avian telencephalon: Experimental studies in the pigeon (Columba livia) and owl (Speotyto cunicularia). J. Comp. Neurol. 150(3), 253–277 (1973).
Wild, J. M. Evolution of the Wulst. In Encyclopedia Neuroscience (eds Binder, M. D. et al.) (Springer, 2009).
Michael, N., Löwel, S. & Bischof, H.-J. Features of the retinotopic representation in the visual wulst of a laterally eyed bird, the zebra finch (Taeniopygia guttata). PLoS ONE 10(4), e0124917 (2015).
Budzynski, C. A. & Bingman, V. P. Participation of the thalamofugal visual pathway in a coarse pattern discrimination task in an open arena. Behav. Brain Res. 153(2), 543–556 (2004).
Steadman, D. W. Extinction and Biogeography of Tropical Pacific Birds (University of Chicago Press, 2006).
Kimball, R. T. et al. A phylogenomic supertree of birds. Diversity 11(7), 109 (2019).
Kuhl, H. et al. An unbiased molecular approach using 3′-UTRs resolves the avian family-level tree of life. Mol. Biol. Evol. 38(1), 108–127 (2021).
Corfield, J. R., Wild, J. M., Parsons, S. & Kubke, M. F. Morphometric analysis of telencephalic structure in a variety of neognath and paleognath bird species reveals regional differences associated with specific behavioral traits. Brain Behav. Evol. 80(3), 181–195 (2012).
Baumel, J. J. Handbook of Avian Anatomy: Nomina Anatomica Avium Vol. 23 (Publications of the Nuttall Ornithological Club, 1993).
Jarvis, E. D. et al. Avian brains and a new understanding of vertebrate brain evolution. Nat. Rev. Neurosci. 6(2), 151–159 (2005).
Acknowledgements
We thank R. Allain and C. Sagne (MNHN, Paris, France) for access to the fossil S. neocaledoniae material, and C. Lefèvre (MNHN, Paris, France) and E. Robert (UCBL, Lyon, France) for the extant comparative specimens. We also thank M. Bouchet-Combe for her assistance and expertise in the acquisition of CT scans data. JSS thanks the CNRS for its regular annual credits. We dedicate this work to the regretted J.-C. Balouet who worked with us on this project and collected the fossils decades ago. We thank Martin Pickford for correcting the English.
Author information
Authors and Affiliations
Contributions
A.L. conceived the study. J.C.B. found and collected the fossil material from New Caledonia. S.R., J.R.G. and C.S. performed the analyses. S.R., A.L. and J.S.S. developed and discussed interpretations, and prepared the manuscript. All authors read and modified the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Riamon, S., Balouet, JC., Rolland-Guillard, J. et al. The endocast of the insular and extinct Sylviornis neocaledoniae (Aves, Galliformes), reveals insights into its sensory specializations and its twilight ecology. Sci Rep 12, 21185 (2022). https://doi.org/10.1038/s41598-022-14829-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-14829-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.