Abstract
This study investigated differences in colour (ΔE00) and translucency parameter (ΔTP00) of nanofilled/microhybrid composites and a glass-ionomer cement following immersion in bioflavonoid (Citrox)- or chlorhexidine-based mouth rinses. Sixty disc-shaped specimens (N = 5/group) of Filtek Supreme (3M), Gradia Anterior (GC) and Fuji IX (GC) were exposed to Citrox/0.2%CHX (Perio+0.2, Curaprox), Citrox/0.09%CHX (Perio+0.09, Curaprox), 0.2%CHX (Savacol, Colgate-Palmolive) or distilled water by 2-min agitation daily for 28 days in an orbital shaker at 200 rpm at 37 °C. Colour recordings were performed using a clinical spectrophometer to obtain CIELab coordinates. General linear model, ANOVA, Tukey test (α = 0.05) and Pearson correlation test were used to analyse data. ΔE00 ranged between 0.33 (Gradia_Savacol_T28) and 6.35 (Fuji_Savacol_T28) (p < 0.001). ΔTP00 ranged between 0.36 (Fuji_ Perio+0.2) and 1.73 (Fuji_Savacol) (p < 0.05). Savacol resulted in higher ΔE00 of Filtek and Fuji and ΔTP00 of Filtek than Perio+0.09 and Perio+0.2 (p = 0.005). Perio+0.09 and Perio+0.2 resulted in higher ΔE00 at T7 than T28 (p < 0.05). There was no correlation between ΔTP00 and ΔE00 (r = 0.445, p = 0.147). Generally, Perio+0.2 and Perio+0.09 mouth rinses produced similar or lower ΔE00 and ΔTP00 than Savacol. GIC Fuji showed higher ΔE00 and similar or higher ΔTP00 than composites Filtek and Gradia. ΔE00 in all materials decreased in Perio+0.2 and Perio+0.09 over time.
Similar content being viewed by others
Introduction
Chlorhexidine (CHX) is considered the “gold standard” antiseptic for primary and secondary prevention of gingivitis and periodontitis as an adjunct to mechanical plaque control1. Commonly, it is used as antibacterial mouth rinse at various concentrations twice a day for up to four weeks. Although CHX exhibits broad antimicrobial spectrum and has outstanding substantivity, serious adverse effects of CHX have been increasingly recognised, such as the risk of type I and IV hypersensitivity2 and increased antimicrobial resistance3,4. Another major drawback of CHX is its ability to stain teeth5,6 and aesthetic restorative materials, namely composite resins7, zirconia and feldspathic ceramics8. Attempts with anti-discolouration systems have been made to reduce the staining potential of CHX, but with limited success9.
Alternative avenues have been explored and natural substances assessed for their potential to supplement or replace CHX in dental and medical products. Bioflavonoids are phenolic compounds with recognized diverse biological and health promoting effects such as anti-inflammatory, immunomodulatory, antioxidative and antimutagenic properties10. They also may increase vascular resistance and support wound healing11. Fruits, vegetables, nuts, seeds and spices are most common sources of bioflavonoids, with the largest amounts found in citrus fruits, blueberries, blackberries, onions, peppers, oregano and parsley12.
Citrox is a soluble formulation that contains a combination of nine bioflavonoids and organic acids, and possesses strong antimicrobial, anti-inflammatory and antioxidative properties13,14. Several Citrox formulations at various inhibitory concentrations have been tested for oral application, showing that Citrox BC30 inhibited growth of the majority of oral pathogens at concentrations between 1–2% (v/v)15.
To this end, reducing concentration of CHX and supplementing it with another potential antiseptic, may result in reduced side effects, whilst maintaining antimicrobial properties. Initial in vitro studies have shown enhanced antimicrobial effect of Citrox 1% in combination with various concentrations of CHX against common oral pathogens cultured planktonically and as cariogenic and perio-pathogenic biofilms14,16.
Tooth colour is known to have a strong impact on patient’s self-esteem and quality of life17. Another aspect of colour and appearance is the impact on longevity of dental restorations as surface staining is an important clinical criterion for their evaluation18. Following primary colour attributes, translucency is viewed as the most important optical property and one of fundamental factors affecting aesthetic appearance of dental restorations19. Optical properties may be affected by extrinsic and intrinsic differences in materials, such as surface roughness20 and sorption21,22.
Recently published visual thresholds define colour and translucency match/mismatch in dentistry23. CIEDE2000 visual thresholds for colour differences (ΔE00) are defined as excellent match (ΔE00 ≤ 0.8), acceptable match (0.8 < ΔE00 ≤ 1.8), mismatch type [a] (1.8 < ΔE00 ≤ 3.6), mismatch type [b] (3.6 < ΔE00 ≤ 5.4) and mismatch type [c] (ΔE00 > 5.4). Similarly, differences in translucency parameter (ΔTP00) are defined as excellent match (ΔTP00 ≤ 0.6), acceptable match (0.6 < ΔTP00 ≤ 2.6), mismatch type [a] (2.6 < ΔTP00 ≤ 5.2), mismatch type [b] (5.2 < ΔTP00 ≤ 7.8) and mismatch type [c] (ΔTP00 > 7.8)23.
Colour stability of microhybrid and nanohybrid/nanofilled composites has been tested in a variety of clinically relevant scenarios, including the effects of surface coating24, underlying dentine replacement material25, bleaching agents26, mouth rinses27,28, coloured food29 and beverages30,31,32. Similarly, glass ionomer cements (GICs) were tested for staining after exposure to coloured beverages27 but no data was found for mouth rinses. A recent meta-analysis has reported on staining potential of a number of commercially available mouthwashes on dental composites33. However, literature lacks data on the effect of CHX and, particularly, bioflavonoid complex (Citrox)-containing mouth rinses on optical properties (colour and translucency) of composites and GICs for direct restorations.
The aim of the study was to determine ΔE00 and ΔTP00 of a nanofilled and microhybrid composite and a reinforced GIC following immersion in bioflavonoid-containing (Citrox/CHX) mouth rinses or CHX control mouth rinse. The research hypotheses were that: (1) bioflavonoid-containing mouth rinses and CHX control had different effects on colour of evaluated restorative materials at several time intervals of 28-day exposure; (2) bioflavonoid-containing mouth rinses and CHX control had different effects on translucency of evaluated restorative materials after 28 days of exposure and 3) there is correlation between ΔE00 and ΔTP00 of the tested materials after immersion in mouth rinses.
Results
Table 1 shows the summary of GLM analysis for factors “mouth rinse”, “material” and “time”. Both Perio+ mouth rinses resulted in lower ΔE00 than Savacol and water, with Perio+0.09 resulting in significantly lower ΔE00 than Perio+0.2 (p < 0.05). Savacol showed the greatest ΔE00 of the tested solutions (p < 0.05). The recorded ΔE00 for the tested materials were in the following order: Fuji > Filtek > Gradia (p < 0.05). There was no significant difference in discoloration after 7, 21 and 28 days (p > 0.05). Interaction of factors “mouth rinse”, “material” and “time” was significant (p < 0.05).
Further analysis within factors is presented in Table 2. Comparing time intervals for each material-mouth rinse combination, significantly higher ΔE00 of Filtek and Gradia were recorded at T7 than T28 in all mouth rinses (p < 0.05) except for Filtek in Perio+0.2 (p = 0.221). Savacol resulted in significantly higher ΔE00 of Fuji at T28 than T7 (p = 0.008). Both Perio+ mouth rinses resulted in lower ΔE00 of Fuji at T28 than T7 but the difference was statistically significant for Perio+0.2 (p = 0.019). Water resulted in similar ΔE00 of Fuji (p = 0.269) and Gradia (p = 0.251) at T7 and T28 and lower ΔE00 of Filtek at T28 (p < 0.05).
Comparing mouth rinses at different material-time combinations, Savacol produced significantly higher ΔE00 of Filtek and Fuji than other mouth rinses (p < 0.05) for the same time interval, except Filtek at T14 and Fuji at T7 (p > 0.05). Conversely, ΔE00 of Gradia was significantly lower in Savacol compared to Perio+ mouth rinses at T7 and T28 (p < 0.05) and comparable to them at T14 and T21. Perio+0.09 resulted in significantly lower ΔE00 of Filtek and Gradia than Perio+0.2 for the same time interval (p < 0.05), except in Gradia at T14 which difference did not reach statistical significance. Perio+0.09 resulted in lower mean ΔE00 values in the Fuji group compared to Perio+0.2 but this difference did not reach statistical significance (p > 0.05). Water produced significantly higher ΔE00 of Gradia than Savacol and Perio+ mouth rinses at all times (p < 0.05) except Perio+0.2 at T7.
Comparing materials within each mouth rinse at T28, Savacol resulted in significant differences in ΔE00 in the following order: Fuji > Filtek > Gradia (p < 0.001). Perio+0.2 showed no differences in ΔE00 of the tested materials (p = 0.510). Perio+0.09 produced significantly higher ΔE00 of Fuji than Gradia (p = 0.049) with Filtek being in between the other two materials. No significant differences in ΔE00 of the tested materials were found in water, even with ln and sqrt transformations, despite Fuji showing considerably higher mean ΔE00 than Filtek and Gradia (p = 0.053).
Figure 1 presents final ΔE00 data, corresponding to T0-T28, compared to CIEDE2000 visual thresholds. Perio+0.09 induced ΔE00 of Filtek and Gradia in the excellent match range and acceptable match in the Fuji group. Perio+0.2 resulted in ΔE00 of all materials that corresponded to acceptable match. Conversely, CHX-based control (Savacol) produced ΔE00 of Gradia in the excellent match range whilst ΔE00 of Filtek and Fuji corresponded to mismatch [b] and mismatch [c], respectively.
CIEDE2000 colour differences (ΔE00) for tested materials23. Excellent match (ΔE00 ≤ 0.8), acceptable match (0.8 < ΔE00 ≤ 1.8), mismatch type [a] (1.8 < ΔE00 ≤ 3.6), mismatch type [b] (3.6 < ΔE00 ≤ 5.4) and mismatch type [c] (ΔE00 > 5.4).
GLM analysis of ΔTP00 showed significant differences for factor “mouth rinse” (p = 0.006) but no differences for factor “material” (p = 0.060). However, interaction of the two factors was significant (p = 0.013).
Further analysis within factors is presented in Table 3. Comparing mouth rinses within each material, Savacol resulted in significantly higher ΔTP00 of Filtek than other mouth rinses (p = 0.005). There were no significant differences between mouth rinses in ΔTP00 of Gradia (p = 0.073) and Fuji (p = 0.086). Comparing materials within each mouth rinse, there were no significant differences in ΔTP00 among the tested materials after exposure to Savacol (p = 0.554) and water (p = 0.732). Fuji showed significantly higher ΔTP00 than other materials after exposure to Perio+0.09 (p = 0.006) whilst the same was true for Gradia after exposure to Perio+0.2 (p = 0.003).
Figure 2 presents ΔTP00 data compared to CIEDE2000 visual thresholds. All groups showed ΔTP00 values that were excellent or acceptable match. CHX-based control (Savacol) produced acceptable match of ΔTP00 in all tested materials. Perio+0.2 resulted in excellent match ΔTP00 in Filtek and Fuji groups and acceptable match in Gradia. Perio+0.09 was associated with excellent match ΔTP00 in Filtek and acceptable match in Gradia and Fuji.
CIEDE2000 translucency parameter differences (ΔTP00) compared with literature data for visual thresholds for tooth-coloured restorative materials26. Excellent match (ΔTP00 ≤ 0.6), acceptable match (0.6 < ΔTP00 ≤ 2.6), mismatch type [a] (2.6 < ΔTP00 ≤ 5.2).
No correlation was found between ΔE00 and ΔTP00 after 28 days of exposure to mouth rinses (Pearson’s r = 0.445, p = 0.147).
Discussion
Statistically significant colour differences were found among the tested materials following exposure to tested mouth rinses at different time intervals. Statistically significant differences in translucency among the materials were observed after 28 days of exposure. Therefore, both the first and second hypotheses were accepted. No significant correlation was found between ΔE00 and ΔTP00 of the tested materials after immersion in mouth rinses, hence the third hypothesis was rejected.
Compared to Savacol (control), Perio+0.09 and Perio+0.2 induced similar or lower ΔE00 in nanofilled composite Filtek Supreme and reinforced, conventional glass ionomer Fuji IX. In addition to bioflavonoids, citric and ascorbic acid, Perio+0.09 and Perio+0.2 contain polyvinylpyrrolidone/vinyl acetate (PVP-VA) copolymer, known for creating transparent films which adhere to various substrates, including glass and plastics. Lower staining capacity of Perio+0.09 and Perio+0.2 mouth rinses could be associated with PVP-VA copolymer protective film on material specimens as well as lower concentration of CHX (Perio+0.09). It was previously shown that surface protection of composite/glass ionomers with nanofilled coating reduces water sorption and internal staining24. PVP-VA copolymer in Citrox-containing mouth rinses could have a similar protective effect. It is unknown whether citrus bioflavonoids have an anti-staining effect on restorative materials.
The present findings showed initial ΔE00 at T7 to be higher than at T28 for Perio+0.09 and Perio+0.2 mouth rinses. Protective PVP-VA coating may also be the reason for a different staining pattern in composites (and glass ionomer), i.e. the lack of progressive increase in staining over time, which was previously reported in other staining scenarios30,31. It is generally known that staining of dental restorative materials has an extrinsic and intrinsic component, the former occurring due to pigment adsorption on material surface and the latter due to absorption of pigments and internal structural changes in the polymer. Increased development of the protective PVP-VA coating with subsequent specimen immersions in Perio+0.09 and Perio+0.2 may prevent further ingress of the staining medium. Another potential mechanism is the wash-out of loosely bound surface pigments during subsequent shaking cycles.
The ability of Savacol to limit or reduce staining over time was not as pronounced as Perio+0.09 and Perio+0.2 mouth rinses in Filtek and Gradia, and completely lacked in Fuji, which showed progressive increase in ΔE00 with the highest values detected at the final observation period. A similar effect of other CHX-based mouth rinses was previously reported for 7 and 14 days of exposure of microhybrid/nanofilled composites from the Filtek group7. Savacol contains glycerine, suggesting a limited film forming capacity than PVP-VA in Perio+0.09 and Perio+0.2. The idea that a surface coating may have a protective effect against staining is supported by a recent meta-analysis showing that the addition of an anti-discoloration system to CHX mouth rinses reduces tooth surface discoloration in non-brushing studies whilst the same effect was not observed in brushing studies9.
The effect of Savacol was strongly dependent on the material, Gradia showing substantial resistance to staining in Savacol than Perio+0.09 and Perio+0.2 mouth rinses. Gradia was generally less susceptible for staining in the tested mouth rinses than Filtek and Fuji. Higher staining of Filtek than Gradia in the present study could be due to greater pigment absorption of Filtek than Gradia. Greater volumetric changes were previously reported for Filtek than Gradia over 7 weeks of storage in water34 indicating greater susceptibility of Filtek for ingress of water and water-based media such as mouth rinses.
The present results showed higher staining for glass ionomer Fuji than composites Filtek and Gradia. This finding may be explained by generally greater sorption of glass ionomers compared to composites, as was previously reported22. Increased sorption leads to intrinsic staining due to pigment absorption and hydrolytical changes in the polyacid matrix. No GC coat was used in the present study to simulate conditions in which the material is exposed to the oral environment after the protective coating, initially placed to prevent water disbalance, is worn off. Another contributing factor to higher staining of glass ionomer Fuji compared to composites Filtek and Gradia is lower polishability of glass ionomers than composites. Glass ionomers tend to have higher surface roughness and more generalized surface irregularities than composites20. Glass ionomer matrix is based on loosely bound cation cross-linked polyacid molecules and is more likely to have larger filler particles dislodged during polishing compared to more efficient silane binding of resin matrix and filler particles in composites. Surface irregularities serve as pigment adsorption nuclei leading to increased surface staining.
Differences in ΔTP00 of the tested materials following exposure to mouth rinses were both material- and medium-dependent. ΔTP00 did not follow the same pattern as ΔE00 and no correlation between the two properties was established. TP is expressed as the relative amount of light passing through material and obtained by calculating colour difference of specimen against the white and black backgrounds35. Translucency is related to two aspects of light interaction with material (absorption and scattering) and factors such as surface topography, filler type and volume and refractive index mismatch between fillers and resin matrix have a significant effect19. Refractive index mismatch is known to change during polymerization as monomers convert to polymers but also after aging due to hydrolytical changes16. The present results indicate unpredictable effects of mouth rinses on ΔTP00 of restorative materials, that are both medium- and material-dependent.
Potential mechanisms of CHX staining of teeth have been suggested: (1) the Maillard reaction between sugars and proteins in the biofilm catalysed by CHX resulting in the formation of coloured pigments melanoidins; (2) Protein denaturation by CHX and the formation of organic yellow–brown ferric sulphides and (3) CHX and pigment interaction from coloured food and beverages6,9.
CHX staining mechanisms of composites and glass ionomers have not been elucidated. The same mechanisms previously suggested for tooth staining may be involved in material staining by CHX in the oral environment. However, in the present study, neither of the three mechanisms could be associated with material staining as no biofilm was used, there was no source of protein, and no colour food/beverages were used. Staining of restorative materials could also be related to the formation of pigments following chelation of CHX and inorganic elements in the materials. Namely, high staining of glass ionomer Fuji could be related to the interaction between CHX and Fuji’s iron oxides.
ΔE00 observed in the present study was interpreted based on literature data on CIEDE2000 visual thresholds23. Perio+0.09 resulted more frequently in ΔE00 of tested materials in the excellent match range than Perio+0.2, which resulted in acceptable match. Conversely, Savacol produced ΔE00 of Gradia in the excellent match range whilst ΔE00 of Filtek and Fuji corresponded to mismatch [b] and mismatch [c], respectively. A previous study on composites from the Filtek group 7 exposed to various CHX-based mouth rinses showed ΔE00 to be mostly an acceptable match, however the exposure of 14 days was shorter than in the present study. Even shorter exposure of ceramics to CHX-based mouth rinse in another study showed ΔE00 to be an excellent match8, likely due to material type and shorter exposure compared to the present study. ΔE00 of Gradia and Filtek in water were on the threshold between acceptable match and mismatch [a], respectively, and well in the mismatch [a] region in the Fuji group. The present results indicate that Perio+0.2 and Perio+0.09 induce minor to moderate changes in colour of restorative materials that are below the perceptibility threshold or within the acceptability limit. There is a dose-dependent relationship between the CHX concentration in these mouth rinses, the higher CHX concentration the greater ΔE00 of restorative materials.
Similarly, ΔTP00 observed in the present study were interpreted based on literature data on CIEDE2000 visual thresholds23. All groups showed ΔTP00 values that were excellent or acceptable match. The present results indicate that exposing restorative materials to mouth rinses, in general, results in minor changes in translucency that are below the perceptibility threshold or within the acceptability limit.
Based on the present results, it is concluded that:
-
The effects of mouth rinses on ΔE00 were medium-, material- and time-dependent. Perio+0.09 and Perio+0.2 produced lower ΔE00 of Filtek Supreme and Fuji IX than Savacol. Savacol induced the lowest ΔE00 of Gradia among the tested media.
-
Glass ionomer Fuji IX showed greaterΔE00 than BisGMA-based nanofilled composite Filtek Supreme and UDMA-based microhybrid composite Gradia Anterior following exposure to mouth rinses.
-
Perio+0.09 and Perio+0.2 showed similar or lower ΔE00 from 7 to 28 days of exposure.
-
The effects of mouth rinses on ΔTP00 were medium- and material-dependent. Perio+0.09 and Perio+0.2 produced lower ΔTP00 of Filtek than Savacol whilst no differences were observed between mouth rinses in relation to ΔTP00 of Gradia and Fuji. Fuji IX showed significantly higher ΔTP00 than other materials after exposure to Perio+0.09 whilst the same was true for Gradia after exposure to Perio+0.2.
-
No correlation was found between ΔTP00 and ΔE00 after 28 days of exposure to mouth rinses.
Methods
Specimen preparation
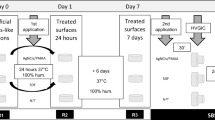
Sixty disc-shaped specimens (N = 5/group) were fabricated using the aesthetic dental restorative materials listed in Table 4. All materials were shade A2.
Composite resin and GIC specimens were prepared by filling standardized polyvinylsiloxane moulds (Affinis heavy body 6520), 8 mm in diameter and 2 mm thick, held on a glass slide. Composite specimens were covered with a transparent polyethylene terephthalate strip (Mylar, Henry Schein, Melville, NY, USA), pressed with another glass slide to extrude excess materials and light-cured through the strip with a LED light-curing unit (Elipar™ DeepCure-S, 3M, St. Paul, MN), operating at an intensity of 1400–1500 mW/cm2 and wavelength of 430–480 nm. GIC specimens were pressed with a glass slide to extrude excess material and allowed to set for 6 min. Each disc-shaped specimen was wet-polished using resin finishers and polishers (Dentsply Sirona Enhance Finishing and Polishing System Kit) for 30 s. Prior to staring the experiment, specimens were immersed in distilled water and incubated at 37 °C for 24 h in a closed container to simulate the oral cavity.
Subsequently, specimens of each aesthetic material were randomly allocated to one of four groups according to staining solutions. Mouth rinses containing Citrox/0.2%CHX and Citrox/0.09% CHX were test solutions (Perio+0.09 and Perio+0.2, respectively), mouth rinse with 0.2% CHX with no additives (Savacol) was used as a positive control and distilled water as a negative control (Table 4).
Prior to the experiment, pH values of each of the solutions were measured using a pH meter (PHM 83 AUTOCAL pH METER). Average pH of PerioPlus mouth rinses was 7.22, Savacol 8.24 and distilled water 7.28, respectively.
Containers with specimens immersed in 50 ml of their respective solutions were placed on an orbital shaker (Infors AG CH-4103 Bottmingen) at 200 rpm at 37 °C and agitated for two minutes to mimic the effect of one-minute rinsing two times per day, according to manufacturer’s recommendations. Staining solutions were renewed daily. Following each cycle of rinsing procedure, specimens were incubated in distilled water at 37 °C for the rest of the day and overnight. The rinsing cycle was repeated daily for four weeks to simulate the use of mouth rinses as commonly prescribed during periodontal treatment.
Colour measurements
CIELab colour coordinates were recorded to determine colour and translucency differences before and after specimen exposure to solutions. Following initial immersion in distilled water, baseline colour values (L*, a*, b*) were measured against a white and black background using a calibrated clinical spectrophotometer (VITA EasyShade V, Zahnfabrik, Bad Säckingen, Germany). Colour measurements against the white background were recorded at baseline and after each full cycle corresponding of 7, 14, 21 and 28 days of clinical exposure (T7, T14, T21 and T28, respectively). The black background measurements were recorded at baseline and after 28 days (T28).
Colour differences (ΔE00) were calculated using CIEDE2000 formula36:
Translucency values (TP00) were calculated using the following formula35:
where ∆L′, ∆C′ and ∆H′ are metric differences computed on the basis of the uniform colour space used in CIEDE2000 and L’, C’ and H’ denote lightness, chroma and hue, respectively, against white (*W) and black (*B) backgrounds. SL, SC, SH adjust the total colour difference for variation in the location of the colour difference sample over the B and W backgrounds in L′, a′, b′ coordinates. The empirical terms KLSL, KCSC and KHSH are used for correcting (weighting) the metric differences to the CIEDE2000 differences for each coordinate. Parametric factors KL, KC and KH were set at 1. RT accounts for the interaction between chroma and hue differences in the blue region.
ΔE00 was determined for each measurement interval (T0-T7, T0-T14, T0-T21 and T0-T28). ΔTP00 was calculated as the difference between TP00 values measured initially (T0) and after final immersion period (T28).
Statistical analysis
Data were statistically analysed in the software package Minitab 16 (Minitab Inc., State College, PA). Data were first checked for normality and equal variances as preconditions for parametric testing. If necessary, the appropriate data transformation was performed, e.g. log or sqrt to achieve normality and/or equal variances. Data were tested using general linear model (GLM) for factors “material”, “mouth rinse” and “time” with included factor interaction. In case of significant factor interaction, further analysis of variance (ANOVA) were performed within each factor. Tukey's post-hoc test was used for intergroup comparison. Pearson correlation was used to test the relationship between ΔE00 and ΔTP00. The level of significance was set at 0.05.
Data availability
All data are available from the corresponding author upon request.
References
Eley, B. M. Antibacterial agents in the control of supragingival plaque: a review. Br Dent J. 186, 286–296 (1999).
Opstrup, M. S., Jemec, G. B. E. & Garvey, L. H. Chlorhexidine allergy: on the rise and often overlooked. Curr Allergy Asthma Rep. 19, 23 (2019).
Wand, M. E., Bock, L. J., Bonney, L. C. & Sutton, J. M. Mechanisms of increased resistance to chlorhexidine and cross-resistance to colistin following exposure of klebsiella pneumoniae clinical isolates to chlorhexidine. Antimicrob Agents Chemother. 61, e01162-e1116 (2017).
Cieplik, F. et al. Antimicrobial efficacy of alternative compounds for use in oral care toward biofilms from caries-associated bacteria in vitro. Microbiologyopen. 8, e00695 (2019).
Supranoto, S. C., Slot, D. E., Addy, M. & Van der Weijden, G. A. The effect of chlorhexidine dentifrice or gel versus chlorhexidine mouthwash on plaque, gingivitis, bleeding and tooth discoloration: a systematic review. Int J Dent Hyg. 13, 83–92 (2015).
Zanatta, F. B., Antoniazzi, R. P., Rosing, C. K. Staining and calculus formation after 0.12% chlorhexidine rinses in plaque-free and plaque covered surfaces: a randomized trial. J Appl Oral Sci. 18, 515–521 (2010).
Khosravi, M., Esmaeili, B., Nikzad, F. & Khafri, S. Color stability of nanofilled and microhybrid resin-based composites following exposure to chlorhexidine mouthrinses: an in vitro study. J Dent (Tehran). 13, 116–125 (2016).
Derafshi, R., Khorshidi, H., Kalantari, M. & Ghaffarlou, I. Effect of mouthrinses on color stability of monolithic zirconia and feldspathic ceramic: an in vitro study. BMC Oral Health 17, 129 (2017).
Van Swaaij, B. W. M., van der Weijden, G. A. F., Bakker, E. W. P., Graziani, F. & Slot, D. E. Does chlorhexidine mouthwash, with an anti-discoloration system, reduce tooth surface discoloration without losing its efficacy? A systematic review and meta-analysis. Int J Dent Hyg. 18, 27–43 (2020).
Kumar, S. & Pandey, A. K. Chemistry and biological activities of flavonoids: an overview. TheScientificWorldJOURNAL 2013, 162750 (2013).
Mulvihill, E. E. & Huff, M. W. Citrus flavonoids and the prevention of atherosclerosis. Cardiovasc Hematol Disord Drug Targets. 12, 84–91 (2012).
Kozłowska, A. & Szostak-Wegierek, D. Flavonoids–food sources and health benefits. Rocz Panstw Zakl Hig. 65, 79–85 (2014).
Stohs, S. J. Safety, Efficacy, and mechanistic studies regarding citrus aurantium (Bitter Orange) extract and p-Synephrine. Phytother Res. 31, 1463–1474 (2017).
Malic, S., Emanuel, C., Lewis, M. A. O. & Williams, D. W. Antimicrobial activity of novel mouthrinses against planktonic cells and biofilms of pathogenic microorganisms. Microbiol. Discov. 1, 11 (2013).
Hooper, S. J., Lewis, M. A., Wilson, M. J. & Williams, D. W. Antimicrobial activity of Citrox bioflavonoid preparations against oral microorganisms. Br Dent J. 210, E22 (2011).
Jeyakumar, J., Sculean, A. & Eick, S. Anti-biofilm activity of oral health-care products containing chlorhexidine digluconate and citrox. Oral Health Prev Dent. 18, 981–990 (2020).
Pavicic, D. K. et al. Changes in quality of life induced by tooth whitening are moderated by perfectionism: a randomized, double-blind, placebo-controlled trial. Int J Prosthodont. 31, 394–396 (2018).
Hickel, R. et al. FDI World Dental Federation - clinical criteria for the evaluation of direct and indirect restorations. Update and clinical examples. J Adhes Dent. 12, 259–272 (2010).
Lee, Y.-K. Translucency of human teeth and dental restorative materials and its clinical relevance. J. Biomed. Opt. 20, 045002 (2015).
Komalsingsakul, A., Srisatjaluk, R. L. & Senawongse, P. Effect of brushing on surface roughness, fluoride release, and biofilm formation with different tooth-colored materials. J Dent Sci. 17, 389–398 (2022).
Salvador, M. V. et al. Physicochemical properties of dental resins formulated with amine-free photoinitiation systems. Dent Mater. 37, 1358–1365 (2021).
Cefaly, D. F. et al. Water sorption of resin-modified glass-ionomer cements photoactivated with LED. Braz Oral Res. 20, 342–346 (2006).
Paravina, R. D., Perez, M. M. & Ghinea, R. Acceptability and perceptibility thresholds in dentistry: a comprehensive review of clinical and research applications. J Esthet Restor Dent. 31, 103–112 (2019).
Comba, A. et al. Influence of surface coating sealer on resin composite water absorption and discoloration: an in vitro study. Am J Dent. 31, 24–28 (2018).
Miletic, V., Marjanovic, J., Veljovic, D. N., Stasic, J. N. & Petrovic, V. Color stability of bulk-fill and universal composite restorations with dissimilar dentin replacement materials. J Esthet Restor Dent. 31, 520–528 (2019).
Vidal, M. L., Pecho, O. E., Collares, K., Brandeburski, S. & Della Bona, A. Color change of resin-based composites after in vitro bleaching protocols: a systematic review and meta-analysis. Oper Dent. (2022).
Bayraktar, Y., Karaduman, K., Ayhan, B. & Karsiyaka Hendek, M. The effect of SARS-CoV-2 effective mouthwashes on the staining, translucency and surface roughness of a nanofill resin composite. Am J Dent. 34, 166–170 (2021).
Festuccia, M. S., Garcia Lda, F., Cruvinel, D. R. & Pires-De-Souza Fde, C. Color stability, surface roughness and microhardness of composites submitted to mouthrinsing action. J Appl Oral Sci. 20, 200–205 (2012).
Yew, H. Z., Berekally, T. L. & Richards, L. C. A laboratory investigation of colour changes in two contemporary resin composites on exposure to spices. Aust Dent J. 58, 468–477 (2013).
Antonov, M. et al. Changes of color and fluorescence of resin composites immersed in beer. J Esthet Restor Dent. 28, 330–338 (2016).
Miletic, V., Stasic, J. N., Komlenic, V. & Petrovic, R. Multifactorial analysis of optical properties, sorption, and solubility of sculptable universal composites for enamel layering upon staining in colored beverages. J Esthet Restor Dent. 33, 943–952 (2021).
Domingos, P. A., Garcia, P. P., Oliveira, A. L. & Palma-Dibb, R. G. Composite resin color stability: influence of light sources and immersion media. J Appl Oral Sci. 19, 204–211 (2011).
Morais Sampaio, G. A., Rangel Peixoto, L., Vasconcelos Neves, G. & Nascimento Barbosa, D. D. Effect of mouthwashes on color stability of composite resins: A systematic review. J Prosthet Dent. 126, 386–392 (2021).
Kangwankai, K. et al. Monomer conversion, dimensional stability, strength, modulus, surface apatite precipitation and wear of novel, reactive calcium phosphate and polylysine-containing dental composites. PLoS ONE 12, e0187757 (2017).
Johnston, W. M., Ma, T. & Kienle, B. H. Translucency parameter of colorants for maxillofacial prostheses. Int J Prosthodont. 8, 79–86 (1995).
Luo, M., Cui, G. & Rigg, B. The development of the CIE 2000 colour-difference formula: CIEDE2000. Color. Res. Appl. 26, 340–350 (2001).
Acknowledgements
The authors are grateful to Dr. Filip Vujovic for his technical help with lab work.
Author information
Authors and Affiliations
Contributions
T.D.R.—study design, data analysis, writing draft manuscript; J.J.S., J.V.T.N., D.W.L.—specimen preparation, data collection and analysis; V.M.—supervised research, study design, review draft paper and finalized the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Divnic-Resnik, T., Shen, J.J., Nguyen, J.V.T. et al. Effects of bioflavonoid-containing mouth rinses on optical properties of tooth-coloured dental restorative materials. Sci Rep 12, 9944 (2022). https://doi.org/10.1038/s41598-022-14254-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-14254-2
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.