Abstract
Neuropeptides and neuropeptide receptors are crucial regulators to insect physiological processes. The 21.0 Gb bases were obtained from Illumina sequencing of two libraries representing the female and male heads of Phauda flammans (Walker) (Lepidoptera: Phaudidae), which is a diurnal defoliator of ficus plants and usually outbreaks in the south and south-east Asia, to identify differentially expressed genes, neuropeptides and neuropeptide receptor whose tissue expressions were also evaluated. In total, 99,386 unigenes were obtained, in which 156 up-regulated and 61 down-regulated genes were detected. Fifteen neuropeptides (i.e., F1b, Ast, NP1, IMF, Y, BbA1, CAP2b, NPLP1, SIF, CCH2, NP28, NP3, PDP3, ARF2 and SNPF) and 66 neuropeptide receptor genes (e.g., A2-1, FRL2, A32-1, A32-2, FRL3, etc.) were identified and well-clustered with other lepidopteron. This is the first sequencing, identification neuropeptides and neuropeptide receptor genes from P. flammans which provides valuable information regarding the molecular basis of P. flammans.
Similar content being viewed by others
Introduction
Insect neuropeptides as a classic signaling molecule are produced by the neurosecretory cells that are mainly located in the brain and the central nervous system, among others, to reach their distant target organs1. They are small proteins with generally about 5–80 amino acid residues, which are one of the structurally most diverse signaling molecules and most diverse group of signaling molecules in multicellular organisms2,3. Most neuropeptide receptors belong to the family of G protein-coupled receptor (GPCR), and most of the neuropeptides act via G protein coupled receptors4,5. It has been widely reported that neuropeptide and their receptors participate in intercellular information transfer from neurotransmission to intrinsic or extrinsic neuromodulation and essential signaling molecules that regulate physiological processes such as growth, development, behavior, reproduction, metabolism and muscle movement in insects2,3,4,6,7.
For now, a plethora of neuropeptides and receptors were investigated in insects, such as myoinhibiting peptides (MIPs)8,9,10,11,12,13,14, and so forth. Among these, PBAN, galanin and melanocortin are involved in the control of reproduction10,15. NPY is regulating feeding and sleep–wake behavior16. Thus, neuropeptides and their receptors could be developed as potential insecticides or targets for a novel generation of pesticides17, such as the neuropeptide CCH was proved to be regulates feeding motivation and sensory perception and olfactory behavior18,19 and the enteroendocrine peptides allatotropin (AT) and GSRYamide have feeding acceleratory effects via controlling intestinal contraction20. Therefore, identification and functional characterization of neuropeptides and their receptors from insect pests would enhance our basic understanding of neuropeptide-related signal transduction, and provide important molecular insights for pest management. Up to now, neuropeptide and receptors have been the focus of interest in many species of Lepidoptera, such as Manduca sexta21,22,23,24, which are mainly nocturnal moths. While, few researches have been reported on diurnal moth of Lepidoptera except for silkworm and butterfly25,26.
The diurnal moth Phauda flammans (Walker) (Lepidoptera: Phaudidae) is a serious defoliator which intermittent outbreaks that threaten ficus plant seriously, especially Ficus microcarpa (Miq.) and F. benjamina L.27. It not only influences the urban landscapes and ecological effects, but also affects normal growth and development of ficus plant28,29,30,31. This defoliator is abundantly distributed in south and south-east Asia and southern China32. At present, most of the researches about P. flammans focus on its morphological characteristics33,34,35,36,37,38,39,40. However, the research on neuropeptides and their receptors in P. flammans has been limited in comparison to other lepidopteran insects, due to lack of availability of genomic or transcriptomic information.
In this study, we conducted high-throughput sequencing of head, identified members of the neuropeptide and neuropeptide receptor of P. flammans, and compared them with those reported transcriptome of other lepidopteran species for the first time. We also evaluated the expression level of 12 neuropeptides in different adult tissues. Our results could provide useful information of neuropeptide and their receptor and theoretical basis for their functional analysis.
Materials and methods
Insect rearing and tissue collection for RNA-seq
The mature larvae of P. flammans were collected from July to October 2020 in Daxin County (22°50′10 N, 107°12′27E), Chongzuo City, Guangxi Province, China, and placed in plastic boxes (diameter = 25.0 cm, height = 15.0 cm) that supplied with fresh ficus leaves per day, at an indoor temperature with 26 ± 2 ℃, 70 ± 10% relative humidity (RH) with a photoperiod cycle of 14 h L/10 h D. Differentiate male and female pupae according to their ventral segments and randomly select 1-day-old healthy male and female adults for the experiment after feathering. The tissues head from adult male (n = 90) and female (n = 90) were collected. All samples were immediately frozen in liquid nitrogen and stored at − 80 °C until use.
RNA-seq
Total RNA of P. flammans was extracted by TRIzol (Thermo Fisher Scientific, Waltham, MA) following the manufacturer’s instructions. The integrity of the RNA was determined with an Agilent 2100 bioanalyzer through agarose gel electrophoresis. The Nanodrop micro-spectrophotometer (Thermo Fisher, USA) was determined the purity and concentration of the RNA. After total RNA extraction, transcriptome sequencing was performed on an Illumina NovaSeq 6000 by Gene Denovo Biotechnology Co. (Guangzhou, China). To obtain high quality clean reads, reads were further filtered with fastp (version 0.18.0), mainly by removing reads containing adapters, removing reads containing more than 10% unknown nucleotides (N), and removing low quality reads with > 50% low quality reads (q value ≤ 20). Reads were then mapped to the ribosomal RNA (rRNA) database using the short reads matching tool Bowtie2 (version 2.2.8). The mapped rRNA reads were removed, and the remaining clean reads were assembled by the short read assembly program Trinity v3.030 to obtain the total unigene. The transcriptomic data were submitted to the National Center for Biotechnology Information (NCBI, USA) (http://www.ncbi.nlm.nih.gov/) with accession number of PRJNA702893.
Transcriptome data analysis
The unigene expression was calculated and normalized to RPKM (Reads Per kb per Million reads)41 and the relative expression of differential expressed genes were viewed by volcano plot.
Unigene sequences were aligned by BLASTx and TBLASTx searches against the protein database (http://blast.ncbi.nlm.nih.gov/) such as NCBI non-redundant protein (Nr) database, SwissProt database, KEGG Ontholog database (KO) and Gene Ontology (GO) for annotation information. The transcriptomic (RNA-seq) data derived from P. flammans were used for identification of the neuropeptides and receptors.
Sequence analysis and phylogenetic tree analysis
Transmembrane domains (TMDs) were calculated using the TMHMM 2.0 prediction software (http://www.cbs.dtu.dk/services/TMHMM/). The presence of signal peptide was predicted using SignalP software version 4.1 (http://www.cbs.dtu.dk/services/SignalP/). The splice sites were predicted using the Known Motif and Insect Models methods of NeuroPred (http://stagbeetle.animal.uiuc.edu/cgi-bin/neuropred.py) and were corrected based on the processing procedures of known insect neuropeptide precursors. Thesequence alignments were done using CLUSTALW, the result were implemented in MEGAv7.034 and GeneDoc software. With tBLASTn, the available sequences proteins from lepidoptera species were used as queries to identify candidate unigene involved in neuropeptides and neuropeptide receptor genes in P. flammans. To construct an evolutionary tree of neuropeptides and receptors, the amino acid sequences of the Atrijuglans hetaohei, Bombyx mori, Chilo suppressalis, H. armigera, Grapholita molesta, Ostrinia furnacalis, Papilio machaon and Pl. xylostella were downloaded from the NCBI database and performed in MEGA7 and the tree was constructed using the Neighbor-Joining method with 1000 bootstraps.
Tissue expression profile via quantitative PCR
The head (without antennae), thoraxes (without legs), abdomens were dissected from 15 virgin 1-day-old of females or males, respectively. These tissues were immediately transferred into 1.5 mL RNA-free tube, super-cooled via liquid nitrogen, and then stored at − 80 °C freezer. These tissues were used for RNA extraction with RNAiso Plus (TAKARA, 9109, Dalian, China) and then cDNA synthesis with A Prime Script RT reagent Kit with gDNA Eraser (TAKARA, RR047, Dalian, China). The quantitative PCR reactions were conducted on an ABI QuantStudioTM 6 Flex system (Thermo Fisher Scientific, Massachusetts, USA). The PCR reaction was performed with each reaction was performed with Green Premix Ex Taq II Kit (TAKARA, RR820A, Dalian, China) and prepared as introduced42. The expression level of target gene was normalized with reference gene TUB1 (α-tubulin) and GAPDH (Glyceraldehyde-3-phosphate dehydrogenase) via 2-∆∆CT method according to our previous works39,42. The primers used in this research were listed in the Table S1.
Statistical analysis
The normality and homoscedasticity of data on tissue expression of neuropeptides in female and male P. flammans adults were tested prior to analysis using Kolmogorov–Smirnov and Levene’s tests, respectively. And, they were further analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s honestly significant difference (HSD) multiple test (P < 0.05). Data analysis was performed using SPSS 25.0 (IBM Corp., Armonk, New York, USA).
Results
Overview of cephalic transcriptomes
The cDNA libraries were constructed from P. flammans tissue samples of male and female heads to next-generation sequencing analysis by using Illumina HiSeq (TM) 4000 platform. A total of 21.0 G of clean bases were obtained, Q20 and Q30 values were all > 93%, and GC content was 39.82 ~ 40.87%. The combined unigene of P. flammans were functionally annotated by BLASTx according to six functional public databases: NCBI non-redundant protein (Nr), the Kyoto Encyclopedia of Genes and Genomes (KEGG) database, Swiss-Prot, Cluster of Orthologous Groups (COG) and gene ontology (GO) (e value < 0.00001). A total of 99,386 unigene (average length 911 bp) were obtained with 37,602, 28,494, 17,458, 19,910 annotations to the Nr, KEGG, KOG, SwissProt databases, respectively. A total of 40,131 annotations, account for 40.38% of the total unigene (Table 1).
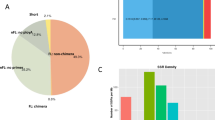
The Nr databases comprise all non-redundant protein sequences in GenBank, EMBL, DDBJ and PDB that belong to phylogenies of more than 70,000 species. Based on Nr annotation, unigene sequences of P. flammans can be mapped with sequences from 10 top species (Fig. 1). The number of homologous sequences sorted from most to least is Eumeta japonica (7.56%), B. mori (5.37%), Galleria mellonella (4.34%), O. furnacalis (4.15%), Hyposmocoma kahamanoa (3.65%), Amyelois transitella (3.32%), H. armigera (3.28%), Danaus plexippus (3.26%), Pa. machaon (3.05%), and Pa. xuthus (2.98%).
Differentially expressed genes (DEGs) between female and male heads
The results of differential expression analysis of genes in the heads of male and female adult P. flammans showed that a total of 217 differentially expressed genes were screened, with 156 genes up-regulated and 61 genes down-regulated, using FDR < 0.05 and |log2FC|> 1 as screening criteria (Fig. 2). The detailed information about these DGEs were listed in the supporting information 1.
Identification of neuropeptides and their receptors
The neuropeptides in P. flammans were identified (Table 2). The neuropeptides F1b, Ast, NP1, IMF, Y, BbA1, CAP2b, NPLP1, SIF, CCH2, NP28, NP3, PDP3, ARF2, and SNPF were identified from the data sets with the length between 331 and 2947 bp. Except for NP1 and BbA1 have 3′ non-coding regions, and the others had complete open reading coding frames (ORFs), including F1b, Ast, IMP, Y, NP2, NPLP1, SIF, CCH2, NPLP28, NPLP3, PDP3, ARF2 and SNPF. Fifteen neuropeptides except for ARNPFT2 had signal peptide, and their signal peptide most likely cleavage site between 16 to 28.
The PfSNPF precursor had an N-terminal signal peptide of 19 amino acids and 3 mature SNPF were generated by sulfidation modifications. The PfSNPF precursor contained the –RLRF sequence, which belongs to the C-terminal motif unique to the SNPF family. Thereafter followed an amidation site (G) and a dibasic cleavage site (RR). The multiple alignments also showed that the SNPF of P. flammans had a higher similarity with other lepidopteron (Fig. 3).
Of the data sets, 67 neuropeptide receptors were identified (Table 3). Thirty-six neuropeptide receptors have completed ORFs. A2-1, FRL2, A32-1, A32-2, FRL3, CCH1R-1, B1-2 FRL5, A10-1, CPR3, A21-2, FRL6, A19, RYES2L1, RYES2L2, A16, CC1R1 and CRLIX2 comprise 3′ non-coding region. Versus those sequences were published such as A. hetaohei, B. mori, C. suppressalis and H. armigera, the number of neuropeptides in P. flammans is much lower than those in other insects.
Phylogenetic analyses
Neuropeptide sequences of P. flammans were used to construct maximum likelihood phylogenetic trees with 137 published neuropeptide sequences from lepidoptera including A. hetaohei, B. mori, C. suppressalis, H. armigera, G. molesta, Pa. machaon and O. furnacalis (Fig. 4). Among all neuropeptides, F1b, Ast, NP1, IMF, Y, BbA1, CAP2b, NPLP1, SIF, CCH2, NP28, NP3, PDP3, ARF2, and SNPF were clustered together with the orthologs from other lepidoptera insects in the same clade. On the contrary, ARF2 and NP28 in a single clade, they are considered as owning special function in the P. flammans.
Phylogenetic analysis of lepidopterous neuropeptides. Ah: A. hetaohei; Bm: B. mori; Cs: C. suppressalis; Ha: H. armigera; Gm: G. molesta; Pf: P. flammans, Pm: Pa. machaon; Of: O. furnacalis. The P. flammans neuropeptide are labeled with red, and the colors of other species are shown in the icon. The tree was conducted with MEGA 7.0, using the Maximum-Likelihood method and the bootstrap analysis with 1000 replicates.
The 152 reported neuropeptide receptor sequences of B. mori, C. suppressalis, H. armigera, G. molesta and Pl. xylostella from lepidopteran and the identified neuropeptide receptors of P. flammans were used to construct an interspecies phylogenetic tree (Fig. 5). The results showed that A26 of P. flammans was clustered together with the A26 of B. mori, C. suppressalis and Pl. xylostella in 100; B3-1 of P. flammans was clustered together with the B3 of B. mori and C. suppressalis; A6-b of P. flammans was clustered together with the A6 of H. armigera. A19-2-, A10-1, A32-2, CCH1R2, B3-2 and FRL2 were individually clustered together. A21-1, A21-2, A21-3 and A21-4 were individually clustered together, and it’s the same with CCHIR-1, CC1R1, CPR2 and CPR3. It showed that neuropeptide receptor emerged highly differentiation in P. flammans. The remaining receptors were clustered together with the orthologs from other lepidopteran insects in the same clade.
Phylogenetic tree analysis of lepidopterous neuropeptide receptors. Bm: B. mori; Cs: C. suppressalis; Ha: H. armigera; Gm: G. molesta; Pf: P. flammans, Px: Pl. xylostella. The P. flammans neuropeptide receptors are labeled with red, and the colors of other species are shown in the icon. The tree was conducted with MEGA 7.0, using the Maximum-Likelihood method and the bootstrap analysis with 1000 replicates.
Tissue expression profile in female and male adults
The expression profiles of 12 neuropeptides of P. flammans in heads, thoraxes, and abdomens of male and female adults were showed in Fig. 6. The expression of CA, LM, Ast, F1b, and NPLP1 were significantly higher in heads than other two body parts in both female and male. While the expression level of AR, DP3, and NP28 showed no significant difference in these three body parts in both sexes. All these neuropeptides showed no difference in female and male heads except for CCH2.
Discussion
Neuropeptides and receptors regulate a wide range of physiological processes in insects. Transcriptome sequencing is fundamental to dentification of genes, and identification of neuropeptides and their receptors is the first and foremost step of deep function depth studies in physiological processes. However, the types and expression of neuropeptides and their receptors in P. flammans are unavailable. Therefore, a sequencing analysis was performed of head in P. flammans. After high-throughput sequencing, among the 99,386 unigene acquired by the assembly program Trinity, 40.38% could be annotated through NR, KEGG, Swiss-Prot, KOG and GO databases, implies that not all unigene contain annotated genes. Some unigene may be non-coding which do not BLAST with the non-redundant protein/nucleotide database. Compared with the transcriptome data of head from Mythimna separata43 and H. armigera44, the P. flammans had a similar result. Q20 and Q30 values were all > 93%, and GC content was similar, indicating that the data was accurate and reliable. In Nr databases, the number of homologous sequences most with P. flammans include E. japonica, B. mori, G. mellonella, which all order of Lepidoptera, suggesting that the transcriptome was commendably sequenced and annotated. Overall, the assembly quality of transcriptome was adequate.
Basically, the number of achieved target gene should be closely related to the sample resource and expression abundance in addition to sequencing depth with species specificity. The same was true for neuropeptides and neuropeptide receptors in P. flammans. Totally, 15 neuropeptides and 66 neuropeptide receptors were identified from head of adult P. flammans, which was different with other lepidopteran species44,45,46,47 and should partly be relevant with their differences in sample physiological status. For example, in B. mori, 32 neuropeptide genes and 6 neuropeptide-like precursor genes were identified from larval and pupal brain45. In C. suppressalis, 43 neuropeptide precursors and 51 putative neuropeptide G protein-coupled receptors were identified the fifth instar larval central nervous system including brain, suboeophageal ganglion, thoracic ganglion, and the abdominal ganglion46. In H. armigera, 34 neuropeptides and peptide hormones, 17 neurotransmitter precursor processing enzymes, and 58 neurotransmitter receptors were identified from mixed pupa and adult head44. It seems that more sophisticated sampling would yield a larger number of neuropeptides and receptor genes. In addition, the number of identified genes might also have species specificity. The number of identified neuropeptides of P. flammans was less than the number of some other lepidopteran species such as from the transcriptome data of head, such as A. hetaohei47.
There are several factors that may account for the difference in the number of identified genes of specific functions which has been discussed48,49. Firstly, the head used as the sequenced samples did not cover complete the individual and all stages of life cycle. Secondly, some genes with small expression levels made it impossible to quantitatively measure the gene expressions in samples presented a not expression state, or them may not have been expressed at all. And then, due to does not involve the modification of corresponding protein-coding regions, many genes lack strong sequence conservation, their clear orthologs could not be found in P. flammans based on homology searches. The neuropeptides may truly present with small quantity in P. flammans because of highly species specificity which needs further investigation.
In this analysis, female and male head transcriptome in P. flammans was performed with focus on the feeding behavior regulation and sexual difference. Only a total of 217 differentially expressed genes were screened, with 156 genes up-regulated and 61 genes down-regulated. Approximately 12% of these DGEs were olfactory association related genes (Supporting information 1), while no neuropeptide or neuropeptide receptor were found. Moreover, some neuropeptide and neuropeptide receptors have reported to induce sex pheromone biosynthesis and feeding behaviors50,51. Therefore, the small number of neuropeptides and neuropeptide receptors from head in P. flammans might lead to those gene tightly to olfactory regulation and reduce workload in targeting behavior regulation gene. For instance, there were 19 unigenes which located in the Ko00981, the insect hormone biosynthesis pathway, where only unigene0063695 and unigene0024395 were significantly differential expressed and annotated as gene cytochrome P450 18a1 (CYP18A1) and farnesol dehydrogenase-like (FoLDH), respectively (Fig. S1). CYP18A1 played a controlling role in 20-hydroxyecdysone inactivation in B. mori52, and were reported to function in development, especially to regulate dimorphic metamorphosis via by insect hormones53,54. FoLDH could induce oxidation of farnesol to farnesal and produce the second branch of JH III in Pl. xylostella55. In addition, DGE Unigene0010507 was annotated as juvenile hormone binding-like protein (Supporting information 1) and how the relationship between it and insect hormone biosynthesis pathway attracted our attention. Therefore, the functions of these DGEs require further analysis and validation in P. flammans.
Neuropeptides and neuropeptide receptors identified from the head of P. flammans showed no significant difference between male and female adults, however, they are crucial in regulating a range of physiological functions, including development, reproduction and feeding56. Therefore, identification and analysis neuropeptides and their receptors are still necessary and meaningful. In the aspects of feeding behavior, for example, short neuropeptide F peptide is expressed in the nervous system and it regulates food intake and body size by overexpression of SNPF with regulate expression of insulin-like peptides in Drosophila57. Another example, NPF as a pleiotropic factor, is well known for its role in the regulation of feeding58, through activating neuropeptide G protein-coupled receptor to regulate feeding and growth in B. mori59,60, which is also a daily oligophagous species that might provide some references for P. flammans. In the aspects of sexual difference, the release of SIFamide in the brain could inhibit sexual behavior until the flies encounter the right physiological conditions61, which might also function in sexual differences. All these deductions need further confirmation far and away via quantitative PCR, tissue localization, function inhibition and so on.
Neuropeptides were less abundant in this study and easier to target their expression in different tissues. From a general view, all the measured neuropeptides were expressed highly or moderately in heads where they were identified from transcriptome (Fig. 6). As mentioned above, the neuropeptide CCH2 and the neuropeptide receptor CCH1R-1 could be identified, but them were no significantly different expressed in the head of females and males (Supporting information 1), while quantitative PCR results showed a slightly significant difference in CHH2 (Fig. 6C). Similar results were also found in CCHamide 1 and CCHamide 2 which were significantly different expressed in the head of females and males of A. hetaohei47. Fold changes in CHH2 expression in female and male heads by QPCR was minor, and therefore the conflicting point shall result from the sensitiveness of QPCR and RNA-Seq methods. In addition, SIFamide a highly conserved neuropeptide and has been reported to modulate courtship behavior differently in female and male Drosophila61,62, which making SIF a gene of interest in P. flammans.
The drawbacks of the adopted second generation sequencing were undoubted. However, we did obtain a mass of valuable genetic data for P. flammans with a tight fund, especially in neuropeptides and their receptors. Novel neuropeptides could be supplemented via Genomics- and peptidomics -based discovery in the future63. Moreover, association of multiple omics, such as full-length transcriptome, proteome and metabolome might be needed64, which would contribute to the feeding and sexual behavior regulation researches in this diurnal moth P. flammans by outlining a chain with cascaded neuropeptide, neuropeptide receptor, pheromone metabolism and behavior.
Conclusion
In this study, 15 neuropeptides and 66 neuropeptide receptors were identified from P. flammans, and the genes exhibited no significantly different expression in head between female and male. Phylogenetic analyses tree with neuropeptides and receptors of other lepidopteran species illustrated clear interspecies relationships and contributed to further function understanding. Our findings enriched neuropeptides and neuropeptide receptor gene database, which provide a theoretical support for pest management strategies and physiological and biochemical researches in P. flammans.
References
Toprak, U. The role of peptide hormones in insect lipid metabolism. Front. Physiol. 11, 434 (2020).
Schoofs, L., De Loof, A. & Van Hiel, M. B. Neuropeptides as regulators of behavior in insects. Annu. Rev. Entomol. 62, 35–53 (2017).
Abou-Ei, A. R., Cools, D. & Vanden, B. J. Role of peptide hormones in insect gut physiology. Curr. Opin. Insect Sci. 41, 71–78 (2020).
Caers, J. et al. More than two decades of research on insect neuropeptide GPCRs: an overview. Front. Endocrinol. 30, 151 (2012).
Audsley, N. & Down, R. E. G protein coupled receptors as targets for next generation pesticides. Insect Biochem. Molec. 67, 27–37 (2015).
Nässel, D. R. & Homberg, U. Neuropeptides in interneurons of the insect brain. Cell Tissue Res. 326, 1–24 (2006).
Audsley, N. & Weaver, R. J. Neuropeptides associated with the regulation of feeding in insects. Gen. Comp. Endocrinol. 162, 93–104 (2009).
Vandersmissen, H. P., Nachman, R. J. & Broeck, J. V. Sex peptides and MIPs can activate the same G protein-coupled receptor. Gen. Comp. Endocrinol. 188, 137–143 (2013).
Suggs, J. M., Jones, T. H., Murphree, S. C. & Hillyer, J. F. CCAP and FMRFamide-like peptides accelerate the contraction rate of the antennal accessory pulsatile organs (auxiliary hearts) of mosquitoes. J. Exp. Biol. 219, 2388–2395 (2016).
Cha, W. H., Jung, J. K., Kim, Y. & Lee, D. W. Identification and pheromonotropic activity of pheromone biosynthesis activating neuropeptide in Maruca vitrata. J. Asia-Pac. Entomol. 21, 156–160 (2018).
Nakazato, M. et al. A role for ghrelin in the central regulation of feeding. Nature 409, 194–198 (2001).
Raina, A. K., Kingan, T. G. & Kochansky, J. P. A pheromonotropic peptide of Helicoverpa zea, with melanizing activity, interaction with PBAN, and distribution of immunoreactivity. Arch. Insect Biochem. 53, 147–157 (2003).
Huang, Y., Crim, J. W., Nuss, A. B. & Brown, M. R. Neuropeptide F and the corn earworm, Helicoverpa zea: a midgut peptide revisited. Peptides 32, 483–492 (2011).
Weaver, R. J. et al. Adipokinetic hormones (AKHs) of sphingid Lepidoptera, including the identification of a second M. sexta AKH. Peptides 34, 44–50 (2012).
Senthilkumar, R. & Srinivasan, R. Sex-specific spatial and temporal gene expressions of pheromone biosynthesis activating neuropeptide (PBAN) and binding proteins (PBP/OBP) in Spoladea recurvalis. Sci. Rep. 9, 3515 (2019).
Chung, B. Y. et al. Drosophila neuropeptide F signaling independently regulates feeding and sleep-wake behavior. Cell Rep. 19, 2441–2450 (2017).
Verlinden, H. et al. Receptors for neuronal or endocrine signalling molecules as potential targets for the control of insect pests. Adv. Insect Physiol. 46, 167–303 (2014).
Ida, T. et al. Isolation of the bioactive peptides CCHamide-1 and CCHamide-2 from Drosophila and their putative role in appetite regulation as ligands for G protein-coupled receptors. Front. Endocrinol. 3, 177 (2012).
Farhan, A., Gulati, J. & Groβe-Wilde, E. The CCHamide 1 receptor modulates sensory perception and olfactory behavior in starved Drosophila. Sci. Rep. 3, 1–6 (2013).
Matsumoto, S. et al. Enteroendocrine peptides regulate feeding behavior via controlling intestinal contraction of the silkworm Bombyx mori. PLoS ONE 14, e0219050 (2019).
Gammie, S. C. & Truman, J. W. Neneuropeptide ierarchies and the activation of sequential motor behaviors in the hawkmoth, Manduca sexta. J. Neurosci. 17, 4389–4397 (1997).
Duve, H., Audsley, N., Weaver, R. J. & Thorpe, A. Triple co-localisation of two types of allatostatin and an allatotropin in the frontal ganglion of the lepidopteran Lacanobia oleracea (Noctuidae): innervation and action on the foregut. Cell Tissue Res. 300, 153–163 (2000).
Zhang, T. Y. et al. The diapause hormone-pheromone biosynthesis activating neuropeptide gene of Helicoverpa armigera encodes multiple peptides that break, rather than induce, diapause. J. Insect Physiol. 50, 547–554 (2004).
Lee, D. W. & Boo, K. S. Molecular characterization of pheromone biosynthesis activating neuropeptide from the diamondback moth, Plutella xylostella (L.). Peptides 26, 2404–2411 (2005).
Yang, J. et al. Specific activation of the G protein-coupled receptor BNGR-A21 by the neuropeptide corazonin from the silkworm, Bombyx mori, dually couples to the Gq and Gs signaling cascades. J. Biol. Chem. 288, 11662–11675 (2013).
Marco, H. G. & Gäde, G. Structure and function of adipokinetic hormones of the large white butterfly Pieris brassicae. Physiol. Entomol. 42, 103–112 (2017).
Liu, J. Y. et al. Studies on the feeding preferences of Phauda flammans Walker (Lepidoptera: Zygaenidae). J. Environ. Entomol. 38, 924–930 (2016) (in Chinese with English abstract).
Nageshchandra, B. K., Rajagopal, B. K. & Balasubramanian, R. Occurrence of slug caterpillar Phauda flammans (Walker) (Lepidoptera: Zygaenidae) on Ficus racemosa L. in South India. Mysore J. Agr. Sci. 6, 186–189 (1972).
Verma, T. D. & Dogra, G. S. Occurrence of Phauda flammans (Walker) (Lepidoptera: Zygaenidae) on Ficus species in Himachal Pradesh. J. Tree Sci. 1, 130–132 (1982).
Huang, Z. J., Mao, Y. T., Zhu, Y., Sun, Z. H. & Wen, X. J. An Investigation on leaf devastation caused by Phauda flammans in 4 cities in Guangdong Province. J. Hebei Forest. Sci. Technol. 3, 47–49 (2018) (in Chinese with English abstract).
Lin, Q. T. et al. Occurrence of Phauda flammans Walker in Xiamen. Subtrop. Plant Sci. 49, 307–311 (2020) (in Chinese with English abstract).
Lu, X. Y. et al. Study on potential geographical distribution of Phauda flammans Walker in China based on the MaxEnt Model. J. Environ. Entomol. 41, 1268–1275 (2019) (in Chinese with English abstract).
Mao, Y. T. et al. Identification of sexing and observation on reproductive system of Phauda flammans. J. Zhejiang Forest. Sci. Technol. 37, 87–92 (2017) (in Chinese with English abstract).
Liu, J. Y. et al. Biological characteristics of Phauda flammans (Lepidoptera: Zygaenidae). Plant Protect. 41, 188–192 (2015) (in Chinese with English abstract).
Zheng, X. L., Liu, J. Y., Zhang, Z. L., Wang, P. & Lu, W. Diel rhythms of sexual behavior and pheromone responses in Phauda flammans Walker (Lepidoptera: Zygaenidae). Pest Manag. Sci. 75, 3070–3075 (2019).
Liu, Q. L., Wu, R. G. & Zeng, C. X. Nuclear polyhedrosis virus of Phauda flammans Walker. Forest. Environ. Sci. 4, 29–30 (1985) (in Chinese).
Yang, Y. R. Preliminary study on biology and prevention measures of Phauda flammans. Sci. Technol. Qinghai Agr. Forest. 2, 87–90 (2018) (in Chinese).
Chen, X. M., Wang, X. Y., Lu, W. & Zheng, X. L. Toxicity of four entomopathogenic fungus Phauda flammans larvae. Guangxi Plant Protect. 33, 1–5 (2020) (in Chinese).
Chen, L. et al. Screening of reference genes for quantitative real-time PCR in Phauda flammans (Walker) (Lepidoptera: Phaudidae). J. Environ. Entomol. 43, 15–24 (2021) (in Chinese with English abstract).
Chen, X. M., Wang, X. Y., Lu, W. & Zheng, X. L. Use of Beauveria bassiana in combination with commercial insecticides to manage Phauda flammans (Walker) (Lepidoptera: Phaudidae): Testing for compatibility and synergy. J. Asia-Pac. Entomol. 24, 272–278 (2021).
Mortazavi, A., Williams, B. A., McCue, K., Schaeffer, L. & Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods. 5, 621–628 (2008).
Su, R. R. et al. Evaluation of reference genes in Glenea cantor (Fabricius) by using qRT-PCR. Genes 12, 1984 (2021).
Liu, Z. X., Wang, X. Y., Lei, C. L. & Zhu, F. Sensory genes identification with head transcriptome of the migratory armyworm, Mythimna separata. Sci. Rep. 7, 1–14 (2017).
Wang, L. et al. Members of the neuropeptide transcriptional network in Helicoverpa armigera and their expression in response to light stress. Gene 671, 67–77 (2018).
Gan, L., Liu, X. L., Xiang, Z. H. & He, N. J. Microarray-based gene expression profiles of silkworm brains. BMC Neurosci. 12, 1–14 (2011).
Xu, G. et al. Identification and expression profiles of neuropeptides and their G protein-coupled receptors in the rice stem borer Chilo suppressalis. Sci. Rep. 6, 1–15 (2016).
Li, F. F. et al. Identification and expression profiling of neuropeptides and neuropeptide receptor genes in Atrijuglans hetaohei. Gene 743, 144605 (2020).
Wang, X. Y., Xiong, M., Lei, C. L. & Zhu, F. The developmental transcriptome of the synanthropic fly Chrysomya megacephala and insights into olfactory proteins. BMC Genomics 16, 20 (2015).
Huang, Z. Y., Wang, X. Y., Lu, W. & Zheng, X. L. Sensory gene identification in the transcriptome of the ectoparasitoid Quadrastichus mendeli. Sci. Rep. 11, 9726 (2021).
Du, M. F. et al. Calcineurin-mediated dephosphorylation of acetyl-coA carboxylase is required for PBAN-induced sex pheromone biosynthesis in Helicoverpa armigera. Mol. Cell. Proteomics 16, 2138–2152 (2017).
Jurenka, R. Regulation of pheromone biosynthesis in moths. Curr. Opin. Insect Sci. 24, 29–35 (2017).
You, L. et al. Two dehydroecdysone reductases act as fat body-specific 20E catalyzers in Bombyx mori. Insect Sci. 29, 100–110 (2022).
Liu, P. et al. Regulation of hormone-related genes in Ericerus pela (Hemiptera: Coccidae) for dimorphic metamorphosis. J. Insect Sci. 19, 16 (2019).
Liu, S. et al. Molecular characterization and functional analysis of the Halloween genes and CYP18A1 in Bemisia tabaci MED. Pestic. Biochem. Phys. 167, 104602 (2020).
Zifruddin, A. N., Mohamad-Khalid, K. A., Suhaimi, S. A. & Mohamed-Hussein, Z. A. Molecular characterization and enzyme inhibition studies of NADP+- farnesol dehydrogenase from diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Biosci. Biotech. Bioch. 85, 1628–1638 (2021).
Nässel, D. R. & Winther, Å. Drosophila neuropeptides in regulation of physiology and behavior. Prog. Neurobiol. 92, 42–104 (2010).
Lee, K. S., You, K. H., Choo, J. K., Han, Y. M. & Yu, K. Drosophila short neuropeptide F regulates food intake and body size. J. Biol. Chem. 279, 50781–50789 (2004).
Holzer, P., Reichmann, F. & Farzi, A. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut-brain axis. Neuropeptides 46, 261–274 (2012).
Deng, X. et al. Activation of Bombyx neuropeptide G protein-coupled receptor A4 via a Gai-dependent signaling pathway by direct interaction with neuropeptide F from silkworm, Bombyx mori. Insect Biochem. Mol. 45, 77–88 (2014).
Ni, M. et al. Nanoparticulate anatase TiO2 (TiO2 NPs) upregulates the expression of silkworm (Bombyx mori) neuropeptide receptor and promotes silkworm feeding, growth, and silking. Peptides 68, 64–71 (2015).
Terhzaz, S., Rosay, P., Goodwin, S. F. & Veenstra, J. A. The neuropeptide SIFamide modulates sexual behavior in Drosophila. Biochem. Biophys. Res. Comm. 352, 305–310 (2007).
Verleyen, P. et al. SIFamide is a highly conserved neuropeptide: a comparative study in different insect species. Biochem. Biophys. Res. Comm. 320, 334–341 (2004).
Zeng, H. et al. Genomics- and peptidomics-based discovery of conserved and novel neuropeptides in the American cockroach. J. Proteome Res. 20, 1217–1228 (2021).
Yang, P., Wang, D., Guo, W. & Kang, L. FAWMine: An integrated database and analysis platform for fall armyworm genomics. Insect Sci. 28, 590–601 (2021).
Acknowledgements
This work was funded by National Natural Science Foundation of China (31660206) and Guangxi Scholarship Fund of Guangxi Education Department.
Author information
Authors and Affiliations
Contributions
The conception and the design of the work were formulated by H.P.W., X.Y.W. and X.L.Z. Bioinformatics analyses were done by H.P.W., X.Y.W. and J.H. The laboratory experiments were performed by H.P.W., J.H. and R.R.S. The statistics analysis was carried out by H.P.W., X.Y.W. and J.H. The analyses of experimental data and interpretation of data were done by H.P.W., X.Y.W., J.H., W.L. and X.L.Z. The manuscript was written, validated and revised by H.P.W., X.Y.W., J.H., W.L. and X.L.Z.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, HP., Wang, XY., Hu, J. et al. Identification of neuropeptides and neuropeptide receptor genes in Phauda flammans (Walker). Sci Rep 12, 9892 (2022). https://doi.org/10.1038/s41598-022-13590-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-13590-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.