Abstract
Mullets (Mugilidae) are economically important fish in Israel. Two species of mugilids (i.e., the thinlip mullet Chelon ramada and the flathead grey mullet Mugil cephalus) have been stocked in the Sea of Galilee (Lake Kinneret) in order to increase fishermen’s income and lake water quality. These catadromous species do not reproduce in the lake, consequently, fingerlings have been introduced every year since 1958. Few additional mugilid species have been introduced unintentionally together with these two species, including C. labrosus. Following a survey of myxozoan infections in the Sea of Galilee, we described Myxobolus pupkoi n. sp. infecting the gill arches of C. labrosus, and reported Myxobolus exiguus from visceral peritoneum and gall bladder of C. ramada. Our study indicates that the parasites infecting C. ramada and C. labrosus belong to a lineage of myxozoans infecting mugilids. This result suggests that the infection took place in the Mediterranean Sea, where the fingerlings were caught, before their introduction into the Sea of Galilee. Since 2018 only farm-raised fingerlings have been introduced. We thus recommend to closely monitor the presence of these parasites in the future to determine if the presence of parasites disappear with the introduction of farm-raised fingerlings.
Similar content being viewed by others
Introduction
Mugilidae is a large fish family that includes 17 genera and 80 species. Mullets are present worldwide, mainly in coastal shallow marine and brackish waters of the tropical and temperate regions. They are an appreciated food resource with high commercial value1. In Israel, two mullet species (Mugil cephalus Linnaeus, 1758 and Chelon ramada Risso, 1827) have been artificially introduced in large numbers since 1958 in the Sea of Galilee (Lake Kinneret), the largest freshwater body in the Levant. The fish were introduced in order to lower the algal population and increase the annual fish yield2,3. Specifically, until 2018, mugilid fingerlings collected along the Mediterranean coast and in coastal streams were introduced into the Sea of Galilee after a 1–2 days stay in freshwater ponds for adaptation2,4. From 1960 to 2018, about one million fingerlings of M. cephalus and C. ramada were introduced every year into the Sea of Galilee5. Since 2018, the number of fish introduced annually has been reduced to only a few tens of thousands of M. cephalus fingerlings, originating from breeding farms (Dr. Menachem Goren, Tel-Aviv University, personal communication).
Myxozoans are one of the main parasitic lineage infecting mullets6,7,8. Myxozoans form a clade of highly-reduced, microscopic, endoparasites Cnidaria. They have complex life-cycles involving two hosts, usually a fish (the intermediate host) and an annelid (the definite host)9. Over 90 myxozoan species have been reported to infect mullets10,11. The majority of myxozoans that parasitize mullets belong to the family Myxobolidae, including 50 species of the genus Myxobolus Butschli, 188212,13. Interestingly, while myxobolids are mainly freshwater species, both marine and freshwater myxobolids are known to infect mugilids10.
During a survey of myxozoans infecting fish from the Sea of Galilee, we found myxozoan infections in the gall bladder, visceral peritoneum and gill arches of a few C. ramada specimen. A taxonomic and molecular study was performed to identify the parasites and determine whether they had a marine or freshwater origin. While Myxozoa are often host specific, a freshwater origin of the parasites could indicate a host switch. In contrast, a marine origin, would indicate that the fish and their parasites were co-introduced to the Sea of Galilee.
Results
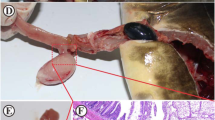
Myxozoan parasites were searched for in ten M. cephalus and twenty-three C. ramada specimens sampled in the Sea of Galilee on November 2020. While no myxozoan parasite was observed in M. cephalus, plasmodia were found in the gall bladder, in the visceral peritoneum lining the intestine and the gill arches of four C. ramada specimen. Each of the four fish was infected by a single parasite. No infection was found in other organs such as kidney, liver, eyes, brain, fins, muscles, and scales. The 18S rRNA sequences from the plasmodia from gall bladder and visceral peritoneum were identical to M. exiguus reported from Portugal (sequence accession number: MH236070)14, except for three insertions in the last 7 bp of the sequence, which most probably correspond to sequencing errors since most are located in the primer region. The parasite obtained from gill arches did not match any other myxosporean sequence available in the NCBI database.
Myxobolus exiguus Thélohan, 1895 from the Sea of Galilee
Plasmodia: Small plasmodia (about 0.5 mm), visible with naked eye, rounded, creamish white, freely floating in the bile or present in the visceral peritoneum lining the intestine, containing 300–500 myxospores per plasmodium.
Myxospore: The morphological data are based on spores isolated from two plasmodia, one originating from the gall bladder and another from the visceral peritoneum. Few morphological differences were found between the spores of M. exiguus infecting the visceral peritoneum and the gall bladder of C. ramada.
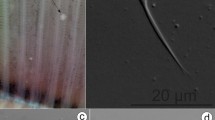
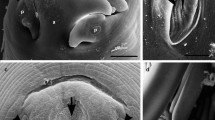
Mature gall bladder myxospores broadly oval to sub-spherical in valvular view and ellipsoidal in sutural view (Fig. 1). They measured 7.80 ± 0.34 (7.15–8.64) µm in length and 6.60 ± 0.22 (6.30–6.95) µm in width (n = 12). Those from the visceral peritoneum were smaller, measured 6.63 ± 0.22 (6.15–7.04) µm in length and 6.01 ± 0.19 (5.50–6.40) µm in width (n = 12). Inter-capsular process absent; shell valve thick, measuring 0.57 ± 0.04 µm; sporoplasm finely granular filling the inter-capsular space presenting two nuclei of slightly different size measuring 1.25 ± 0.03 and 1.10 ± 0.02 µm for the larger one and the smaller one, respectively (Fig. 1; Table 1).
Polar capsules pyriform and bottle-necked, equal in size and arranged side by side with two prominent pores at the anterior side of the myxospore measuring 3.79 ± 0.12 (3.54–3.97) µm in length and 2.54 ± 0.07 (2.41–2.67) µm in width (from gall bladder); 3.41 ± 0.11 (3.04–3.85) µm in length and 2.16 ± 0.05 (1.95–2.30) µm in width (from the visceral peritoneum). Polar tubules form 3–4 coils while inside the capsule (Fig. 1; Table 1).
Host: Chelon ramada (Risso, 1827), vern. thinlip mullet, Family: Mugilidae.
Locality: Sea of Galilee (31° 49′ N, 35° 38′ E), Israel.
Material deposited: Four slides with stained myxospores (SMNHTAU-AP-50-53) have been deposited in the parasite collection of the Steinhardt Museum of Natural History, Tel Aviv University, Israel.
Infection site: Gall bladder bile and visceral peritoneum.
Because of fish misidentification the prevalence of infection cannot be computed.
Clinical signals: Whitish patches floating inside the gall bladder and in the intestine visceral peritoneum.
Sequences deposited: Two identical sequences were deposited. GenBank accession numbers OM065835 (2017 bp—gall bladder) and OL604467 (2017 bp—visceral peritoneum).
Remarks: The present observations on M. exiguus15 show that the spore size is smaller in the Israeli specimen than previously described14. Previous work reported M. exiguus15 from the visceral peritoneum of C. ramada, C. auratus, C. saliens, C. labrosus and M. cephalus from France, Tunisia and Portugal. A new locality—the Sea of Galilee, Israel—and a new tissue—the gall bladder—are here recorded for this parasite.
Myxobolus pupkoi n. sp
Plasmodia: Small histozoic plasmodia, visible with naked eye, rounded, creamish white (about 1.0 mm in diameter), a single infected gill arch per fish with 2–4 plasmodia, 300–500 myxospores per plasmodium. Gills pale and mucous laden.
Myxospore: The morphological data are based on the observation of myxospores isolated from a single plasmodium. Mature myxospores sub-spherical in valvular view and ellipsoidal in sutural view, measuring 5.84 ± 0.13 (5.67–6.14) µm in length and 5.41 ± 0.09 (5.30–5.53) µm in width (n = 12). Polar capsules pyriform, equal in size, and located at the anterior half of the myxospore measuring 2.44 ± 0.19 (2.25–2.73) µm in length and 1.34 ± 0.18 (1.14–1.80) µm wide (n = 12). Polar tubules making 4–5 coils while inside the capsule and measuring 7–10 µm when extruded; inter-capsular process absent; 8–9 sutural folds on the ring of the myxospore; sutural line thick, measuring 0.65 ± 0.03 µm and slightly curved; sporoplasm finely granular with two nuclei of similar size measuring 0.95 ± 0.04 µm, filling most of the extracapsular cavity (Fig. 2; Table 1).
Type host: Chelon labrosus (Risso, 1827), vern. thicklip grey mullet, Family: Mugilidae.
Type locality: Sea of Galilee (31° 49′ N, 35° 38′ E), Israel.
Material: Two slides with stained myxospores (SMNHTAU-AP-48-49) have been deposited in the parasite collection of the Steinhardt Museum of Natural History, Tel Aviv University, Israel.
Infection site: Gill arch.
Because of fish misidentification the prevalence of infection cannot be computed.
Clinical signals: Whitish plasmodia on the gill arch.
Sequence: GenBank accession number OL605966 (2013 bp).
Etymology: The specific epithet “pupkoi” has been given in honor of Prof. Tal Pupko, an expert in molecular evolution and bioinformatics, for his endless support, and encouragements.
Remarks: For differential diagnosis, M. pupkoi n. sp. morphology, infection site and host have been compared with other Myxobolus species infecting members of the genus Chelon10,15,16,17,18,19,20,21,22,23 (Table 2).
The myxospores of M. pupkoi n. sp. are characterized by a sub-spherical to spherical shape in valvular view and an ellipsoidal shape in sutural view. Both polar capsules are equal in size and pyriform shaped, arranged side by side. The new species presents sutural folds all over the rim of the myxospore, and hence differs from M. adeli, M. exiguus, M. mugauratus, M. mugchelo, and M. ramadus spores, for which this feature is absent. The size of the novel myxospore is 5.84 ± 0.13 × 5.41 ± 0.09 µm and hence differs from M. adiposus (9.1 ± 0.3 × 9.0 ± 0.3 µm), M. cerveirensis (8.1 ± 0.2 × 6.8 ± 0.2 µm), M. labrosus (10.2 ± 0.2 × 8.1 ± 0.3 µm), M. muscularis (9.1 ± 0.6 × 7.0 ± 0.6 µm), M. parsi (9.1 × 8.1 µm), M. peritonaeum (8.1 ± 0.2 × 7.1 ± 0.2 µm), and M. pharyngobranchialis (9.3 ± 0.4 × 7.7 ± 0.4 µm), which have much larger spores. The new species is sub-spherical to spherical in shape, and hence differs from M. adeli, which is spindle shaped. The infection site of M. pupkoi n. sp. is the gill arch and hence it differs from M. episquamalis, which infects scales. It also differs from M. cerveirensis, M. exiguus, M. hepatobiliaris, and M. parvus, which infect various organs of the digestive track; and from M. renalis, which infects the kidney.
The new species is very similar in size to M. parenzani, which has been described to have round myxospores (~ 5.4 × 5.4 µm). However, it differs from the present species in having larger polar capsules. The polar capsules of M. pupkoi n. sp. are 2.4 ± 0.20 µm long and occupy about half of the myxospores. Conversely, the myxozpores of M. parenzani have been described to be ~ 2 µm long, to be positioned along the membrane (rather than side by side) and to only occupy the upper third of the spore. Although M. pupkoi n. sp. and M. parenzani infect the same host the morphological differences mentioned above justify the definition of a novel species.
Phylogenetic analysis
The phylogenetic analyses included 79 sequences of the 18S rRNA gene from the mugiliform-infecting lineage of myxobolid, as well as four outgroup sequences. The trees reconstructed based on maximum likelihood and Bayesian criteria only differ in the position of a few low-supported branches (Fig. 3). These trees are in agreement with previous studies on myxozoans infecting mugilids10,11,14.
Phylogenetic relationships within the mugiliform-infecting lineage inferred from 18S rRNA sequences under the ML criterion (TVM + F + R3 model). The new sequences of M. exiguus (OL604467 and OM065835) and M. pupkoi (OL605966) are indicated in bold and with a yellow background. Branch supports (i.e. ML bootstrap percentages [BP] above 50/posterior probabilities [PP] above 0.5) are indicated near the corresponding nodes. Maximal support values (BP = 100/PP = 1.0) are indicated by an asterisk. A dash indicates BP < 50 or PP < 0.5. The sequences identified as M. muelleri in NCBI, but recognized to be M. exiguus10,43, are indicated within brackets.
The phylogenetic tree divides the mugiliform-infecting lineage into two clades. The first harbors only Myxobolus ramadus and Myxobolus cochinensis (ML bootstrap support BP = 99; Bayesian posterior probability PP = 0.83). The second, which includes most species (ML = 75; PP = 0.98), is composed of three subclades. One subclade includes myxobolid lineages, infecting mainly fish hosts from the genus Chelon, and is where M. exiguus and the newly described species Myxobolus pupkoi n. sp. branch. The second subclade includes myxobolid lineages infecting mainly fish hosts from the genus Mugil. Both subclades include myxozoans from annelid hosts, for which the fish host is unknown (Fig. 3). The third subclade includes M. suppamattayai and Tetraspora discoidea (ML = 80; PP = 0.99).
Discussion
The present study describes M. pupkoi n. sp. and presents a novel tissue of infection, the gall bladder, for M. exiguus. It also reveals their presence in the Sea of Galilee. Morphologic and morphometric differences were observed between the myxospores of M. exiguus found in the visceral peritoneum and in the gall bladder, and their taxonomic identity could only be confirmed based on the 18S rRNA analysis. These data illustrate, once more, the importance of 18S rRNA sequences to complement the morphological identification of myxozoan species9,10,19, especially in the case of mugilids, which are infected with closely related myxobolid parasites10.
Tissue specificity is a characteristic of many histozoic myxozoans and an important taxonomical character24,25,26,27. Unfortunately, because of the small number of infected fish observed, the infected tissues were used for molecular identifications rather than histological preparation. Future work should determine, which tissue of the gill arch is the site of M. pupkoi infection (e.g., blood vessels, cartilaginous matrix or the connective tissue), following Molnár et al.24,27. Myxobolus exiguus is a histozoic parasite described from the visceral peritoneum14. Its presence in the gall bladder bile, which is a coelozoic localization, is thus surprising. However, it is possible that the plasmodia found in the bile originated from the gall bladder wall or the hepatic bile ducts and thus is of a histozoic origin25,28.
Both M. exiguus and M. pupkoi n. sp. belong to a well-defined clade of mugilid infecting myxozoans14. Members of this clade have also been found in marine, brackish and freshwater annelids present in estuaries1,10,29. Since mugilids are not native species from the Sea of Galilee and Jordan Valley system, it is most likely that these myxozoans were introduced to the Sea of Galilee with their mugilid hosts during stocking5,30, being the infection originated in the Mediterranean or the coastal plain estuaries of Israel from where the fingerlings originated before their introduction to the lake.
The fact that mugilid fingerlings are hosting numerous myxozoan parasites, sometimes even within a single individual, has been noted by Sharon et al.11. It was further suggested that the introduction of wild caught mugilid to new growing area may lead to the spread of their myxozoan parasites11. We here show that this prediction likely reflects the course of events that occurred in Israel where the transfer of infected fingerlings from the coastal environment led the presence of alien parasites in the Sea of Galilee.
Myxozoans need two hosts to complete their life cycle, it may thus seem that myxozoans are not a serious threat to the Sea of Galilee since the annelid hosts were not transferred with the fish hosts. However, the establishment of a transmission cycle in that environment using local annelids species cannot be ruled out.
The annelid fauna of the Sea of Galilee is poorly known. Previous studies have indicated the presence of Psammoryctides albicola as well as members of the genus Potamothrix (previously identified as Euilyodrilus)30. These freshwater annelids are known to harbor myxozoans closely related to the ones described in this work10 (see Fig. 3). This suggests that the repeated introduction of infected mugillid fingerlings to the Sea of Galilee could have led to the establishment of myxozoan parasites to this new environment. While we did not find myxozoan parasites on the M. cephalus specimen studied during this work, their past introductions from wild-caught fingerlings pose the same threats than C. ramada2,3,4,5. Indeed, M. cephalus fingerlings from the north of Israel were found to be infected by another myxobolid species (sequence MF118765, Fig. 3)11, which has been considered to be M. adiposus10. This observation suggests that more than two myxobolid species may have been introduced to the Sea of Galilee with their mugilid hosts. Since 2018, only farm raised fingerlings of M. cephalus have been introduced to the Sea of Galilee. These fingerlings are expected to be parasite-free. If the introduced myxozoans cannot reproduce due to the lack of annelid hosts, we expect to stop observing myxobolid infected mugilids in the future. We thus recommend to closely monitor the presence of these parasites in the next 5 years to determine if these myxozoan parasites disappear.
Methods
Parasite material and morphological identification
Mugil cephalus specimens (n = 10) with a length of 18–20 cm, and Chelon ramada specimens (n = 23) with a length of 15–20 cm freshly collected from the Sea of Galilee, were obtained from a local fisherman and immediately transported to the lab. The fish sampling was performed under the authorization of the Fisheries and Aquaculture Department of the Israeli Ministry of Agriculture and Rural Development (authorization provided on 11.11.2020). The fish specimens were carefully examined externally for the presence of plasmodia followed by a dissection of each fish sample. Gills, scales, brain, kidney, visceral peritoneum, and other body parts were removed and examined under a stereo-microscope. When plasmodia were identified, fresh myxospores were photographed under a compound microscope with a DS-Ri2 Nikon photographic unit. The myxospores were stained with freshly prepared Ziehl–Neelsen and Giemsa solutions31. Spore measurements were done using a calibrated ocular micrometer.
DNA isolation and amplification
The samples for molecular work were fixed in absolute alcohol and stored at −20 °C. The extraction of genomic DNA was performed using the Qiagen DNeasy Blood and Tissue Kit according to the manufacturer’s instructions. PCR amplification of the 18S rRNA was performed using both universal and myxosporean specific primers32,33,34 (Table 3). The 25 µL of PCR mix consisted of 1 μL DNA template (9.9 ng/μL of M. exiguus and 34.7 ng/μL of M. pupkoi), 2.5 μL of 10X Taq buffer (Takara Bio Inc., Japan), 0.2 μL of Ex Taq polymerase (5 units/μL; Takara Bio Inc., Japan), 2 μL of 20 µM dNTP mix (Takara Bio Inc., Japan), 2.5 μL of 5 pmol/μL of each primer (Merck, Germany), 0.2 μL of DMSO (100%, Sigma Aldrich, US), 5 μL of 5 M Betaine (Bio-Lab Ltd., Jerusalem), and 9.1 μL of molecular grade water. Amplification was performed using the following protocol: 1—initial denaturation at 94 °C for 5 min; 2—35 cycles of denaturation at 94 °C for 45 s, annealing of primers at 58 °C for 45 s, and extension at 72 °C for 2 min; 3—a final extension at 72 °C for 10 min. The obtained PCR products were purified using the ExoSAP method35.
Sequencing was performed using the external primers ER1B1 and ER1B10 together with six additional primers (Table 3) at the DNA Sequencing Unit at Tel-Aviv University on an ABI 3500xl Genetic Analyzer (Applied Biosystems™). The obtained sequences were visualized, assembled and edited using Geneious 11.1.5.
Phylogenetic reconstructions
To reconstruct the phylogenetic relationships of the Israeli species, we first blasted the obtained 18S rRNA sequences, against the NCBI nr databased (https://blast.ncbi.nlm.nih.gov/) on 05.09.2021. This allowed us to determine that both sequences belong to a distinct lineage of myxobolid infecting mugilids in the Histozoic III lineage sensus14,36. We downloaded all sequence hits from this clade that were longer than 800 bp. The sequences of Henneguya shaharini (EU643630), H. rhinogobii (AB447992), H. cynoscioni (JN017203), and Myxobolus khaliji (KC711053) were used as outgroups.
Because the start and end of sequences can include sequencing errors, we excluded the first and last 10 base pairs of all sequences, except for sequence MH183025 Myxobolus bragantinus for which we removed the first 20 bp based on a manual examination. The webserver Guidance237 (last accessed 15.12.2021) was used to align the edited sequences and to remove ambiguously aligned positions. Specifically, the sequences were aligned with the MAFFT algorithm under the options “Max-Iterate: 1000” and “Pairwise Alignment Method: localpair”. Positions with a score below 0.93 were removed as well as positions with more than 50% of missing data. Finally, we also removed positions corresponding to the annealing regions of the primers 18Se (5′-CTGGTTGATCCTGCCAGT-3′)38 and 18Sr (5′-CTACGGAAACCTTGTTACG-3′)39, the primers used to amplify most sequences in our sequence alignment10,14,40. The final dataset included 83 taxa and 1781 positions (Supplementary Files 1 and 2).
Phylogenetic relationships were reconstructed both under the maximum likelihood and the Bayesian criteria. Maximum likelihood analyses were performed with the program IQ-TREE version 1.6.1241. The analyses were run with the options –m MFP –b 1000 (i.e., ModelFinder + tree reconstruction + 1000 non-parametric bootstrap replicates). The model selected based on the BIC criterion was the TVM + F + R3 model. Bayesian reconstructions were performed with PhyloBayes MPI version 1.842 under the CAT + GTR + GAMMA 4 model. Four chains ran for 55,000 points. The first 5000 trees from each chain were discarded as burn-in, and the consensus was built from trees sampled every 10 points (i.e., 5000 trees per chain). At the end of the run, the bpcomp maxdiff value was 0.051, indicating a proper convergence. We also verified that, for all parameters, the tracecomp eff size values were above 5000 and the rel_diff below 0.05, as recommended by the PhyloBayes manual.
Data availability
All the uncropped images of M. exiguus and M. pupkoi n. sp. are available in the Figshare repository (https://doi.org/10.6084/m9.figshare.17705105). Novel sequences obtained in this study were deposited to GenBank under accession numbers OM065835 and OL604467 for M. exiguus and OL605966 for M. pupkoi.
Change history
20 March 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41598-023-31318-z
References
Froese, R. & Pauly, D. FishBase. World Wide Web Electronic Publication. www.fishbase.org (2021).
Bar-Ilan, M. Stocking of Mugil capito and Mugil cephalus and their commercial catch in Lake Kinneret. Aquaculture 5, 85–89 (1975).
Ben-Tuvia, A., Davidoff, E. B., Shapiro, J. & Shefler, D. Biology and management of Lake Kinneret fisheries. Isr. J. Aquac. 44, 48–65 (1992).
Gophen, M. Fisheries management in Lake Kinneret (Israel). Lake Reserv. Manag. 2, 327–332 (1986).
Gophen, M. & Snovsky, G. Mugilids (Mugil cepalus, Linnaeus, 1758; Liza ramada, Risso, 1810) Stocking in Lake Kinneret (Israel). Open J. Ecol. 5, 389–399 (2015).
Cardim, J., Silva, D., Hamoy, I., Matos, E. & Abrunhosa, F. Myxobolus bragantinus n. sp. (Cnidaria: Myxosporea) from the gill filaments of the redeye mullet, Mugil rubrioculus (Mugiliformes: Mugilidae), on the eastern Amazon coast. Zootaxa 4482, 177–187 (2018).
Kim, W. S., Kim, J. H. & Oh, M. J. Morphologic and genetic evidence for mixed infection with two Myxobolus species (Myxozoa: Myxobolidae) in gray mullets, Mugil cephalus, from Korean waters. Korean J. Parasitol. 51, 369–373 (2013).
Yurakhno, V. M. & Ovcharenko, M. O. Study of Myxosporea (Myxozoa), infecting worldwide mullets with description of a new species. Parasitol. Res. 113, 3661–3674 (2014).
Lom, J. & Dyková, I. Myxosporidia (phylum Myxozoa). In Protozoan parasites of fishes. Developments in aquaculture and fisheries science Vol. 26 (eds Lom, J. & Dyková, I.) 159–235 (Elsevier, 1992).
Rocha, S. et al. Myxozoan biodiversity in mullets (Teleostei, Mugilidae) unravels hyperdiversification of Myxobolus (Cnidaria, Myxosporea). Parasitol. Res. 118, 3279–3305 (2019).
Sharon, G., Ucko, M., Tamir, B. & Diamant, A. Co-existence of Myxobolus spp. (Myxozoa) in gray mullet (Mugil cephalus) juveniles from the Mediterranean Sea. Parasitol. Res. 118, 159–167 (2019).
Ovcharenko, M. Microparasites of worldwide mullets. Ann. Parasitol. 61, 229–239 (2015).
Ozer, A. et al. Molecular characterization and morphological aspects of Myxobolus parvus (Myxozoa) from Liza saliens (Mugilidae) off the Turkish Black Sea coasts. Parasitol. Res. 115, 3513–3518 (2016).
Rocha, S. et al. Phylogeny and comprehensive revision of mugiliform-infecting myxobolids (Myxozoa, Myxobolidae), with the morphological and molecular redescription of the cryptic species Myxobolus exiguus. Parasitology 146, 479–496 (2019).
Thélohan, P. Recherches sur les myxosporidies. Bull. Sci. Fr. Belg. 5, 100–394 (1895).
Parenzan, P. Myxobolus mugilis e Myxobolus branchialis, due nuovi missosporidi parassiti di Mugil chelo dello Jonio. Boll. Soc. Nat. Napoli 75, 3–8 (1966).
Sarkar, N. K. Myxobolus anili sp. nov. (Myxozoa: Myxosporea) from a marine teleost fish Rhinomugil corsula Hamilton. Proc. Zool. Soc. Calcutta 42, 71–74 (1989).
Egusa, S., Maeno, Y. & Sorimachi, M. A new species of Myxozoa, Myxobolus episquamalis sp. nov. infecting the scales of the mullet, Mugil cephalus L. Fish Pathol. 25, 87–391 (1990).
Landsberg, J. H. & Lom, J. Taxonomy of the genera of the Myxobolus/Myxosoma group (Myxobolidae: Myxosporea), current listing of species and revision of synonyms. Syst. Parasitol. 18, 165–186 (1991).
Dorothy, K. P. & Kalavati, C. Description of four species of Myxobolus Bütschli, 1882 from the mullet, Liza macrolepis, in the backwater regions of Visakhapatnam, East Coast of India. Proc. Zool. Soc. Calcutta 45, 197–204 (1992).
Das, M. K. Myxozoan and urceolarid ciliate parasites of wild and cultured Liza parsia in deltaic West Bengal. J. Inland Fish. Soc. India 28, 46–56 (1996).
Haldar, D. P., Samal, K. K. & Mukhopadhyaya, D. Studies on the protozoan parasites of fishes in Orissa: eight species of Myxobolus Bütschli (Myxozoa: Bivalvulida). J. Bengal Nat. Hist. Soc. 16, 3–24 (1996).
Ovcharenko, M., Sayyaf, D. B., Castaldelli, G., Lanzoni, M. & Giari, L. Histological and ultrastructural study of Myxobolus mugchelo (Parenzan, 1966) with initial histopathology survey of the Liza ramada host intestine. Parasitol. Res. 116, 1713–1721 (2017).
Molnár, K. Site preference of fish myxosporeans in the gill. Dis. Aquat. Organ. 48, 197–207 (2002).
Molnár, K. & Eszterbauer, E. Specificity of infection sites in vertebrate hosts. In Myxozoan Evolution, Ecology and Development (eds Okamura, B. et al.) 295–323 (Springer, 2015).
Molnár, K. & Székely, C. Tissue preference of some myxobolids (Myxozoa: Myxosporea) from the musculature of European freshwater fishes. Dis. Aquat. Organ. 107, 191–198 (2014).
Molnár, K., Varga, Á. & Székely, C. Cross section of gill filaments in histological preparations helps better identification of the location of myxosporean plasmodia in gill tissues. Acta Vet. Hung. 66, 241–249 (2018).
Dyková, I. & Lom, J. Chloromyxum reticulatum (Myxozoa, Myxosporea) in the liver of burbot (Lota lota L.) and its migration to the final site of infection. Eur. J. Protistol. 23, 258–261 (1988).
Golani, D. & Mires, D. Introduction of fishes to the freshwater system of Israel. Isr. J. Aquac. 52, 47–60 (2000).
Por, F. D. The invertebrate zoobenthos of Lake Tiberias: I Qualitative aspects. Isr. J. Zool. 17, 51–79 (1968).
Bruno, D. W., Nowak, B. & Elliott, D. G. Guide to the identification of fish protozoan and metazoan parasites in stained tissue sections. Dis. Aquat. Organ. 70, 1–36 (2006).
Rocha, S. et al. Morphology and phylogeny of Thelohanellus marginatus n. sp. (Myxozoa: Myxosporea), a parasite infecting the gills of the fish Hypophthalmus marginatus (Teleostei: Pimelodidae) in the Amazon River. J. Eukaryot. Microbiol. 61, 586–593 (2014).
Diamant, A., Ucko, M., Paperna, I., Colorni, A. & Lipshitz, A. Kudoa iwatai (Myxosporea: Multivalvulida) in wild and cultured fish in the Red Sea: redescription and molecular phylogeny. J. Parasitol. 91, 1175–1189 (2005).
Hallett, S. L. & Diamant, A. Ultrastructure and small-subunit ribosomal DNA sequence of Henneguya lesteri n. sp. (Myxosporea), a parasite of sand whiting Sillago analis (Sillaginidae) from the coast of Queensland Australia. Dis. Aquat. Organ. 46, 197–212 (2001).
Bell, J. R. A simple way to treat PCR products prior to sequencing using ExoSAP-IT. Biotechniques 44, 834 (2008).
Holzer, A. S. et al. The joint evolution of the Myxozoa and their alternate hosts: a cnidarian recipe for success and vast biodiversity. Mol. Ecol. 27, 1651–1666 (2018).
Sela, I., Ashkenazy, H., Katoh, K. & Pupko, T. GUIDANCE2: Accurate detection of unreliable alignment regions accounting for the uncertainty of multiple parameters. Nucleic Acids Res. 43, W7-14 (2015).
Hillis, D. M. & Dixon, M. T. Ribosomal DNA: Molecular evolution and phylogenetic inference. Q. Rev. Biol. 66, 411–453 (1991).
Whipps, C. M. et al. First report of three Kudoa species from eastern Australia: Kudoa thyrsites from mahi mahi (Coryphaena hippurus), Kudoa amamiensis and Kudoa minithyrsites n. sp. from sweeper (Pempheris ypsilychnus). J. Eukaryot. Microbiol. 50, 215–219 (2003).
Rocha, S. et al. Involvement of sphaeractinomyxon in the life cycle of mugiliform-infecting Myxobolus (Cnidaria, Myxosporea) reveals high functionality of actinospore morphotype in promoting transmission. Parasitology 147, 1320–1329 (2020).
Nguyen, L. T., Schmidt, H. A., von Haeseler, A. & Minh, B. Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32, 268–274 (2015).
Lartillot, N., Rodrigue, N., Stubbs, D. & Richer, J. PhyloBayes MPI: Phylogenetic reconstruction with infinite mixtures of profiles in a parallel environment. Syst. Biol. 62, 611–615 (2013).
Molnár, K. Remarks to the validity of Genbank sequences of Myxobolus spp. (Myxozoa, Myxosporidae) infecting Eurasian fishes. Acta Parasitol. 56, 263–269 (2011).
Andree, K. B., Székely, C., Molnár, K., Gresoviac, S. J. & Hedrick, R. P. Relationships among members of the genus Myxobolus (Myxozoa: Bilvalvidae) based on small subunit ribosomal DNA sequences. J. Parasitol. 85, 68–74 (1999).
Acknowledgements
The authors acknowledge the support of Israel Science Foundation (Grant Number 652/20 to DH). The authors thanks Captain Menachen Lev (Kibbutz Ein Gev fishing vessel) for providing the fish samples; Prof. Noa Shenkar (Tel-Aviv University) for sharing her microscope apparatus and providing technical help; Prof. Menachem Goren (Tel-Aviv University) for his advises and for sharing his endless knowledge of fish; and Dr. Shevy Rothman (Steinhardt Natural History Museum) for her help with the sample submission.
Author information
Authors and Affiliations
Contributions
A.G. and D.H. conceived the study. K.G. performed taxonomic identifications of the fish hosts. A.G. performed the taxonomic identifications of the Myxozoa and the sequencing work. M.H.-S. provided assistance at the bench. D.H. supervised the phylogenetic analyses. A.G. and D.H. drafted the manuscript. All authors assisted in revising the manuscript and approved the final version of the text.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained errors in the Abstract and Results section, as well as in Figure 3 and Table 2. Full information regarding the corrections made can be found in the correction for this Article.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gupta, A., Haddas-Sasson, M., Gayer, K. et al. Myxozoan infection in thinlip mullet Chelon ramada (Mugiliformes: Mugilidae) in the Sea of Galilee. Sci Rep 12, 10049 (2022). https://doi.org/10.1038/s41598-022-13215-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-13215-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.