Abstract
To describe the peripheral angiographic features of vasculopathy in idiopathic inflammatory myopathies (IIM) and systemic sclerosis (SSc) in comparison to polyarteritis nodosa (PAN). Angiograms of 47 extremities (24 upper and 23 lower) of 11 patients with IIM (n = 5) and SSc (n = 6), and 12 patients with PAN who presented with critical limb ischemia were retrospectively analyzed with regards to the presence of stenosis, occlusion, aneurysms and delayed distal flow, and degree of neovascularization. Diffuse narrowing was more frequent (66.1 vs. 38.0%, p = 0.001), whereas multifocal stenosis (6.5% vs. 26.8%, p = 0.002), abrupt occlusion (11.3% vs. 29.6%, p = 0.010) and aneurysm formation (1.6% vs. 11.3%, p = 0.037) were less frequent in IIM/SSc than PAN. In distal arteries, tapered occlusion (95.5% vs. 76.0%, p = ns) and delayed flow (77.3% vs. 48.0%, p = 0.039) were more common in IIM/SSc than PAN. After 1 year, auto- or surgical amputation tended to be more frequent in IIM/SSc than PAN (36.4% vs. 16.7%, p = ns). In conclusion, diffuse narrowing, tapered occlusion and delayed distal flow on conventional angiograms tend to be more frequent in IIM/SSc than PAN. Further studies are needed to verify these findings in a larger prospective cohort.
Similar content being viewed by others
Introduction
Vasculopathies are a heterogeneous group of morphologically and pathogenetically distinct vascular diseases, and can include both non-inflammatory and inflammatory vasculopathies1. A key pathologic finding in a patient with a vasculopathy is luminal stenosis and/or occlusion with resulting tissue ischemia, which clinically manifests as pain and ischemic damage of affected organs2,3.
In inflammatory vasculopathy (vasculitis) such as polyarteritis nodosa (PAN), the vascular walls are destroyed by infiltrating pro-inflammatory immune cells4. Interestingly, patients with connective tissue disease (CTD), such as idiopathic inflammatory myopathies (IIM) and systemic sclerosis (SSc), can experience a non-thrombotic proliferative vasculopathy (NTPV), a distinctive disease entity characterized by vascular wall proliferation without overt evidence of inflammation and thrombosis5,6. NTPV in IIM including dermatomyositis (DM) and polymyositis (PM) and SSc commonly affects the pulmonary and extremity arteries resulting in pulmonary arterial hypertension (PAH), secondary Raynaud’s phenomenon, and digital ulcer and gangrene7,8.
The nature of the insult to vascular endothelial and mural cells might induce different cascades of inflammatory and metabolic responses. Destruction and subsequent remodeling of affected vessels can be observed as different histologic and angiographic changes9. For example, histologic hallmarks of PAN include necrotizing vasculitis of medium-sized muscle arteries, accompanied by segmental transmural inflammation and destruction, fibrinoid necrosis and disruption of elastic lamina10. These vascular changes manifest on angiographic images as irregular constriction, occlusion and multiple aneurysms11. In addition, a complex network of inflammatory cytokines and ischemia-induced vascular growth factors such as vascular endothelial growth factor (VEGF) induce new vessel formation that manifests as tortuous, corkscrew-like elongation of arteries12. By contrast, non-inflammatory proliferative vasculopathy is mediated by an imbalance between proliferative and anti-proliferative signaling in the cells of the intimal and medial wall layers, leading to progressive obliterating thickening of the vascular walls; inflammatory infiltrates, however, are rare and the internal elastic lamina remains intact13,14.
To date, the angiographic features of IIM/SSc-vasculopathy have not been fully elucidated. The current study was designed to assess the angiographic characteristics of vasculopathies associated with IIM/SSc in patients, who presented with critical limb ischemia, in comparison to PAN.
Methods
Patients
Patients with IIM/SSc, who underwent conventional angiography of an upper and/or lower extremity for critical limb ischemia between January 2001 and May 2020, were included in this retrospective study. The symptoms of critical limb ischemia included Raynaud’s phenomenon with severe pain or numbness, irreversible digital cyanosis, ulcer or gangrene. IIM was diagnosed according to the 2017 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification criteria for probable or definite DM or PM15, whereas SSc was diagnosed according to the 2013 ACR/EULAR classification criteria16; and PAN was diagnosed according to the 1990 ACR criteria17. We excluded the patients with IIM/SSc who were clinically or histologically diagnosed with other vasculitis (such as anti-neutrophil cytoplasm antibody associated vasculitis or cryoglobulinemic vasculitis). The study was approved by the Institutional Review Board of Seoul National University Hospital (IRB # 2004-081-1117), which waived the requirement for patient informed consent due to the retrospective design of this study. The study was performed in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines.
Data collection
The baseline demographic, clinical and laboratory data at the time of angiographic studies were retrieved from the electronic medical records of Seoul National University Hospital. Data recorded include patient age, gender, symptom duration (time between the first ischemic or non-ischemic symptom, and angiography) and disease duration (time between the diagnosis of IIM/SSc or PAN and angiography), comorbidities (cardiovascular risk factors such as hypertension, diabetes, dyslipidemia and smoking status, and malignancy), treatment, and 1-year outcomes.
Image acquisition
Conventional angiography was performed with ultrasonography-guided transfemoral approach. A 5-French introducer sheath was placed in the femoral artery. A 5-Fr catheter was advanced to the proximal part of each limb under fluoroscopic guidance. The limb was divided into 3 to 4 segments and digital subtraction angiography was conducted respectively with a non-ionic contrast medium (Visipaque 270; GE Healthcare) injected at a rate of 4 mL/s. The injection duration was 3 to 5 s, and adjusted depending on the distance between the catheter tip and region of interests. The images were obtained at 1 frame/s, and recording time was prolonged in case of slow blood flow.
Angiographic descriptions of the upper and lower extremities
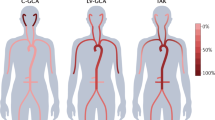
Arteries in the arm were divided into those of the shoulder, elbow and wrist/hand regions, whereas arteries in the leg were divided into those of the thigh, knee, and ankle/foot regions. Peripheral arteries above the wrist or ankle were classified as proximal arteries, whereas those at or below the wrist or ankle were classified as distal arteries. Examples of representative angiograms are shown in Fig. 1.
Angiographic features of IIM/SSc-vasculopathy and PAN. Arteries in the (A) upper extremities and (B) lower extremities of patients with IIM/SSc-vasculopathy and PAN. Diffuse narrowing is indicated by white arrowheads; tapered occlusion by white arrows; multifocal stenosis by black arrowheads; abrupt occlusion by black arrows; aneurysmal changes by empty arrows; grade 2 tortuosity by white stars; and grade 3 tortuosity by black stars. IIM idiopathic inflammatory myopathies, PAN polyarteritis nodosa, SSc systemic sclerosis.
Each region was evaluated for arterial stenosis (diffuse narrowing, and focal or multifocal stenosis), occlusion (tapered or abrupt), and aneurysmal changes according to the angiographic assessment of central nervous system vasculopathy and allograft vasculopathy18,19. The degree of neovascularization in muscle branches was analyzed using a three-point scale, with 1 representing normal to mild (kinking defined as > 60°); 2 representing a moderate degree of tortuosity (30–60°); and 3 representing a severe degree of tortuosity (hypertortuosity; < 30°), using criteria for the tortuosity of the internal carotid arteries20. Delayed blood flow of distal arteries was defined by the absence of arterial filling after prolonged recording time (> 1 s) in following arteries: digital and palmar arch arteries in wrist/hands; and pedal plantar arteries in ankle/foot images21.
All angiograms were reviewed on a picture archiving and communication system (PACS) workstation (Infinitt, Seoul, Korea) independently by two investigators, a trained rheumatologist (JY) and a radiologist (EAP) who were blinded to clinical information. Discrepancies between the two investigators were resolved by consensus.
Statistical analysis
Continuous variables were reported as mean and standard deviation (SD) or as median and interquartile range (IQR), as appropriate. Categorical variables were reported in absolute numbers and percentages. Continuous variables were compared using Student’s t-tests or Mann–Whitney tests, whereas categorical variables were compared using chi-square tests or Fisher’s exact tests, as appropriate. P-values ≤ 0.05 were considered statistically significant. All statistical analyses were performed with SPSS (IBM SPSS version 26.0, IBM Corp., USA).
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of Seoul National University Hospital (IRB # 2004-081-1117). The study was performed in accordance with the principles of the Declaration of Helsinki.
Consent for publication
This study has previously been published as an abstract (POS1386) in EULAR 2021 conference.
Results
Baseline characteristics of patients
This study included 11 patients with IIM/SSc, consisting of four with DM, one with PM, and six with SSc, and 12 patients with PAN. Baseline demographic and clinical characteristics of patients at angiographic studies are presented in Table 1. The mean age was 58.0 ± 13.0 years in the IIM/SSc group, and 52.4 ± 17.8 years in the PAN group (p = ns). 90.9% of the IIM/SSc group and 50.0% of the PAN group (p = ns) were women. The median disease duration was 5.7 (2.0–10.0) years in the IIM/SSc group and 3.7 (0.8–10.0) years in the PAN group (p = ns). IIM/SSc patients (5.7 (1.0–9.4) years) had a longer symptom duration of digital ischemia than PAN patients (0.4 (0.1–1.7) years, p = 0.002). Digital pain or numbness were reported in 2 (18.2%) of the IIM/SSc group, and 6 (50.0%) of the PAN group (p = ns). There were 5 (45.5%) patients with digital gangrene in the IIM/SSc group, as compared to none in the PAN group (p = 0.014). Cardiovascular risk factors, such as hypertension, diabetes mellitus, and dyslipidemia, did not differ in these two groups. The average daily glucocorticoid doses at baseline were similar in the two groups (0.3 (0.0–0.3) mg/kg/day in the IIM/SSc group vs. 0.2 (0.0–1.6) mg/kg/day in the PAN group, p = ns). Anti-nuclear antibodies of titer ≥ 1:80 were observed in seven (63.6%) patients with IIM/SSc but in none with PAN.
Angiographic characteristics of vasculopathy associated with IIM/SSc
Sixty-two angiographic images, 40 of the proximal portion and 22 of the distal portion, were analyzed in the patients with IIM/SSc, whereas 71 images, 46 of the proximal portion and 25 of the distal portion, were analyzed in the patients with PAN (Table 2 and Supplementary Table S1). Diffuse narrowing was significantly more frequent (66.1% vs. 38.0%, p = 0.001), whereas multifocal stenosis was significantly less common (6.5% vs. 26.8%, p = 0.002) in the IIM/SSc-vasculopathy than in the PAN. The proximal arteries showed similar findings; diffuse narrowing (47.5% vs. 19.6%, p = 0.006) was more common and multifocal stenosis (2.5% vs. 19.6%, p = 0.017) was less common in the IIM/SSc than in the PAN group (Fig. 2A). Notably, all patients in the IIM/SSc group, compared with 72.0% in the PAN group, had diffuse narrowing in distal arteries (p = 0.010) (Fig. 2B).
Angiographic parameters in the extremities of patients with IIM/SSc-vasculopathy and PAN. Angiographic features in arteries of the (A) proximal and (B) distal extremities of patients with IIM/SSc-vasculopathy and PAN. (C) Frequencies of neovascularization (hypertortuosity, grade 3) between the IIM/SSc-vasculopathy and PAN. IIM idiopathic inflammatory myopathies, PAN polyarteritis nodosa, SSc systemic sclerosis. Y-axis is the percentage (%) of total images.
Abrupt occlusion (11.3% vs. 29.6%, p = 0.010) and aneurysm formation (1.6% vs. 11.3%, p = 0.037) were significantly less frequent in the IIM/SSc-vasculopathy than in the PAN group. Interestingly, both abrupt occlusion and aneurysm formation did not involve proximal arteries in patients with IIM/SSc-vasculopathy (Fig. 2A). In addition, a higher degree of tortuosity (grade 3) was significantly less frequent in the IIM/SSc-vasculopathy than in the PAN group (9.7% vs. 37.0%, p = 0.002) (Fig. 2C). In most IIM/SSc vasculopathy showed tapered occlusion in distal arteries, while PAN showed lower frequency, albeit not significantly (95.5% vs. 76.0%, p = ns) (Fig. 2B). Strikingly, poor overall distal blood flow was more frequent in patients with IIM/SSc-vasculopathy than PAN (77.3% vs. 48.0%, p = 0.039) (Fig. 2B). In the subgroup analysis of the IIM/SSc-vasculopathy, angiographic characteristics showed no significant difference between patients with IIM and those with SSc (Table 3).
Treatment of digital ischemia and clinical outcomes
Treatment and 1-year outcome of limb ischemia are summarized in Table 4. Of the patients with IIM/SSc, eight (72.7%) and nine (81.8%) were treated with calcium channel blockers and prostanoids, respectively. The frequency of treatment with antithrombotic drugs did not differ significantly in the IIM/SSc and PAN groups (p = ns). After 1 year, three (27.3%) patients in the IIM/SSc group and seven (58.3%) in the PAN group showed improvements in digital ischemia. Auto- or surgical amputation tended to be more frequent in the IIM/SSc than in the PAN groups (36.4% vs. 16.7%, p = ns).
Discussion
To our knowledge, this study is the first to compare the angiographic involvement of arteries in the extremities of IIM/SSc patients with severe limb ischemia and PAN patients. This study found that diffuse narrowing, tapered occlusion and delayed distal blood flow tended to be more common in IIM/SSc patients, whereas multifocal stenosis, abrupt occlusions, aneurysmal changes and tortuous hypervascularization were more common in patients with PAN. In addition, angiographic findings did not differ between IIM and SSc.
The angiographic characteristics of IIM/SSc-vasculopathy have not been fully elucidated. As a secondary Raynaud's phenomenon with endothelial dysfunction was thought to account for digital ischemia and ulcer/gangrene in patients with IIM/SSc, these patients do not routinely undergo peripheral angiography22. We and others reported previously that patients with SSc had structural changes involving the digital arteries, as well as the distal ulnar and radial arteries23,24,25,26. The current study unequivocally showed that also IIM/SSc-vasculopathy involves more proximal arteries as well. The angiographic features of IIM/SSc-vasculopathy included diffuse, segmental narrowing with distal tapering, with multifocal stenosis and abrupt occlusion being less frequent in IIM/SSc than in PAN. This angiographic pattern of vasculopathy in IIM/SSc-vasculopathy are consistent with the abnormal vascularity, smaller arterial lumens, and thickened artery walls observed on color-Doppler ultrasound in patients with secondary Raynaud's phenomenon27,28. The diffuse and extensive vasculopathy in proximal and distal arteries might account for the delayed or absent flow in the distal arteries, including the palmar and plantar arches. This may also explain the greater susceptibility of patients with IIM/SSc to severe “watershed” ischemia/damage during Raynaud’s attacks29,30. Angiographic findings did not differ between groups of patients with IIM and SSc, suggesting that the mechanisms of vasculopathy are similar in these diseases. Interestingly, PAN was frequently accompanied by a high degree of tortuosity of muscle arteries, suggesting that the production of angiogenic cytokines during active vasculitis drives new vessel formation in synergy with VEGF12.
Histologically, IIM/SSc-associated vasculopathy is characterized by luminal stenosis/occlusion with hyperplasia of mural cells31, which may explain the long-segmental narrowing without aneurysm formation observed on angiograms of patients with IIM/SSc. By contrast, the vascular walls in patients with PAN were destroyed by infiltrating immune cells, which can be observed on angiograms as abrupt occlusion and aneurysm formation.
Patients with IIM/SSc-vasculopathy tended to have poorer outcomes than those with PAN, as shown by a numerically higher rate of auto- and surgical amputation (36.4% vs. 16.7%, p = ns) during 1-year follow-up. It is not clear if IIM/SSc-vasculopathy would respond to more aggressive anti-inflammatory treatment, since there is little histologic evidence of inflammation. Therefore, IIM/SSc-vasculopathy may require a treatment targeting “pauci-inflammatory” vascular remodeling. Studies are needed to address the therapeutic roles of anti-proliferative drugs, such as endothelin receptor antagonist (used in the treatment of PAH) and paclitaxel (used to prevent restenosis of coronary artery after percutaneous coronary intervention)32,33,34. Since different immunosuppressants were used in IIM/SSc and PAN groups, it is difficult to evaluate the effect of immunosuppressive therapies on angiographic changes in two groups.
Patients with systemic lupus erythematosus (SLE) were intentionally excluded in this study since they are more likely to develop both inflammatory and thrombotic (due to antiphospholipid syndrome) vasculopathies35. Therefore, evaluation of SLE vasculopathy is needed in further studies.
This study had several limitations, due in part to its retrospective design. First, the relatively small number of patients limits the ability to generalize the current findings to all patients with IIM/SSc-vasculopathy. Especially, the relationship between vasculopathy and myositis-specific autoantibodies including anti-NXP2 and anti-MDA5, which may confer risk of vasculopathy36, and underlying diseases such as PAH and malignancy needs to be determined. Second, all the included patients had more severe ischemic symptoms requiring angiographic evaluation, leading to a selection bias. Third, conventional angiography cannot accurately differentiate NTPV from other forms of vascular abnormalities including vasculitis, atherosclerosis or thrombosis. Fourth, follow-up angiograms would help to determine whether the angiographic findings in the present study are reversible or progress with or without treatment. Finally, biopsy of affected arteries are needed to correlate histopathologic findings with angiographic abnormalities. Studies in larger patient cohorts are needed to determine the prevalence of and risk factors for IIM/SSc-vasculopathy, as well as long-term outcomes.
Conclusions
The present study showed that diffuse narrowing, tapered occlusion and delayed distal blood flow on conventional angiograms tend to be more frequent in patients with IIM/SSc-vasculopathy than with PAN. Larger studies are needed to validate the current findings.
Data availability
The data in this article are available from the corresponding authors upon reasonable request.
References
Jayne, D. The diagnosis of vasculitis. Best Pract. Res. Clin. Rheumatol. 23, 445–453. https://doi.org/10.1016/j.berh.2009.03.001 (2009).
Jennette, J. C. et al. 2012 revised International Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 65, 1–11. https://doi.org/10.1002/art.37715 (2013).
Weyand, C. M. & Goronzy, J. J. Medium- and large-vessel vasculitis. N. Engl. J. Med. 349, 160–169. https://doi.org/10.1056/NEJMra022694 (2003).
De Virgilio, A. et al. Polyarteritis nodosa: A contemporary overview. Autoimmun. Rev. 15, 564–570. https://doi.org/10.1016/j.autrev.2016.02.015 (2016).
Kahaleh, M. B. Vascular involvement in systemic sclerosis (SSc). Clin. Exp. Rheumatol. 22, S19-23 (2004).
Youse, J. S., Harris, S. & Naina, H. V. Clinical images: Polymyositis and vasculitis. Arthritis Rheum. 56, 3270. https://doi.org/10.1002/art.22944 (2007).
Tjiu, J. W. et al. Digital infarcts showing microangiopathy in adult dermatomyositis suggest severe pulmonary involvement and poor prognosis. Br. J. Dermatol. 150, 1214–1216. https://doi.org/10.1111/j.1365-2133.2004.05979.x (2004).
Hughes, M. et al. Raynaud phenomenon and digital ulcers in systemic sclerosis. Nat. Rev. Rheumatol. 16, 208–221. https://doi.org/10.1038/s41584-020-0386-4 (2020).
Carmeliet, P. & Jain, R. K. Molecular mechanisms and clinical applications of angiogenesis. Nature 473, 298–307. https://doi.org/10.1038/nature10144 (2011).
Hernandez-Rodriguez, J., Alba, M. A., Prieto-Gonzalez, S. & Cid, M. C. Diagnosis and classification of polyarteritis nodosa. J. Autoimmun. 48–49, 84–89. https://doi.org/10.1016/j.jaut.2014.01.029 (2014).
Stanson, A. W. et al. Polyarteritis nodosa: Spectrum of angiographic findings. Radiographics 21, 151–159. https://doi.org/10.1148/radiographics.21.1.g01ja16151 (2001).
Kikuchi, K., Hoashi, T., Kanazawa, S. & Tamaki, K. Angiogenic cytokines in serum and cutaneous lesions of patients with polyarteritis nodosa. J Am Acad Dermatol 53, 57–61. https://doi.org/10.1016/j.jaad.2005.02.018 (2005).
Fleming, J. N., Nash, R. A., Mahoney, W. M. Jr. & Schwartz, S. M. Is scleroderma a vasculopathy?. Curr Rheumatol Rep 11, 103–110. https://doi.org/10.1007/s11926-009-0015-3 (2009).
Radic, M. & Radic, J. Updates in the Diagnosis and Treatment of Vasculitis (Intech, 2013).
Lundberg, I. E. et al. 2017 European League against rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Arthritis Rheumatol. 69, 2271–2282. https://doi.org/10.1002/art.40320 (2017).
van den Hoogen, F. et al. 2013 classification criteria for systemic sclerosis: An American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 65, 2737–2747. https://doi.org/10.1002/art.38098 (2013).
Lightfoot, R. W. Jr. et al. The American College of Rheumatology 1990 criteria for the classification of polyarteritis nodosa. Arthritis Rheum. 33, 1088–1093. https://doi.org/10.1002/art.1780330805 (1990).
Avery, R. K. Cardiac-allograft vasculopathy. N. Engl. J. Med. 349, 829–830. https://doi.org/10.1056/NEJMp038124 (2003).
Edgell, R. C. et al. The role of catheter angiography in the diagnosis of central nervous system vasculitis. Interv. Neurol. 5, 194–208. https://doi.org/10.1159/000445255 (2016).
Weibel, J. & Fields, W. S. Tortuosity, coiling, and kinking of the internal carotid artery. I. Etiology and radiographic anatomy. Neurology 15, 7–18. https://doi.org/10.1212/wnl.15.1.7 (1965).
Manzi, M. et al. Vascular imaging of the foot: The first step toward endovascular recanalization. Radiographics 31, 1623–1636. https://doi.org/10.1148/rg.316115511 (2011).
Wigley, F. M. & Flavahan, N. A. Raynaud’s phenomenon. N. Engl. J. Med. 375, 556–565. https://doi.org/10.1056/NEJMra1507638 (2016).
Park, J. K. et al. Baseline MRA predicts the treatment response to vasodilator udenafil in patients wit. Clin. Exp. Rheumatol. 32, S167–S170 (2014).
Emad, Y. et al. Arterial vasculopathy in systemic sclerosis: Computerized tomography (CT) angiographic features of macrovascular and microvascular upper limbs arteries. Joint Bone Spine 81, 433–437. https://doi.org/10.1016/j.jbspin.2014.03.014 (2014).
Leyden, J. et al. Upper extremity angiographic patterns in systemic sclerosis: Implications for surgical treatment. J Hand Surg Am 44, 900e991-900e997. https://doi.org/10.1016/j.jhsa.2019.01.004 (2019).
van Vugt, R. M. et al. The outcome of angiography in patients with Raynaud’s phenomenon: An unexpected role for atherosclerosis and hypercholesterolemia. Clin. Exp. Rheumatol. 21, 445–450 (2003).
Keberle, M. et al. Assessment of microvascular changes in Raynaud’s phenomenon and connective tissue disease using colour doppler ultrasound. Rheumatology (Oxford) 39, 1206–1213. https://doi.org/10.1093/rheumatology/39.11.1206 (2000).
Schmidt, W. A., Wernicke, D., Kiefer, E. & Gromnica-Ihle, E. Colour duplex sonography of finger arteries in vasculitis and in systemic sclerosis. Ann. Rheum. Dis. 65, 265–267. https://doi.org/10.1136/ard.2005.039149 (2006).
Singer, A. J., Tassiopoulos, A. & Kirsner, R. S. Evaluation and management of lower-extremity ulcers. N. Engl. J. Med. 377, 1559–1567. https://doi.org/10.1056/NEJMra1615243 (2017).
Zhang, W., Xu, J. R., Lu, Q., Ye, S. & Liu, X. S. High-resolution magnetic resonance angiography of digital arteries in SSc patients on 3 Tesla: Preliminary study. Rheumatology 50, 1712–1719. https://doi.org/10.1093/rheumatology/keq453 (2011).
Lee, J. S., Kim, H., Lee, E. B., Song, Y. W. & Park, J. K. Nonthrombotic proliferative vasculopathy associated with antiphospholipid antibodies: A case report and literature review. Mod. Rheumatol. 29, 388–392. https://doi.org/10.1080/14397595.2016.1218596 (2019).
Hui, A., Min, W. X., Tang, J. & Cruz, T. F. Inhibition of activator protein 1 activity by paclitaxel suppresses interleukin-1-induced collagenase and stromelysin expression by bovine chondrocytes. Arthritis Rheum. 41, 869–876. https://doi.org/10.1002/1529-0131(199805)41:5%3c869::AID-ART15%3e3.0.CO;2-3 (1998).
Herdeg, C. et al. Local paclitaxel delivery for the prevention of restenosis: Biological effects and efficacy in vivo. J. Am. Coll. Cardiol. 35, 1969–1976. https://doi.org/10.1016/s0735-1097(00)00614-8 (2000).
Channick, R. N., Sitbon, O., Barst, R. J., Manes, A. & Rubin, L. J. Endothelin receptor antagonists in pulmonary arterial hypertension. J. Am. Coll. Cardiol. 43, 62S-67S. https://doi.org/10.1016/j.jacc.2004.02.042 (2004).
Leone, P. et al. Lupus vasculitis: An overview. Biomedicines https://doi.org/10.3390/biomedicines9111626 (2021).
Betteridge, Z. & McHugh, N. Myositis-specific autoantibodies: An important tool to support diagnosis of myositis. J. Intern. Med. 280, 8–23. https://doi.org/10.1111/joim.12451 (2016).
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science, ICT and Future Planning, Republic of Korea (grant number: 2019R1G1A1100477).
Author information
Authors and Affiliations
Contributions
J.Y and E.A.P shared equal contribution as co-first authors. Conceptualization and supervision: J.K.P. Data curation and formal analysis: J.Y, E.A.P, J.K.P. Investigation and methodology: J.Y, E.A.P, E.B.L. Funding acquisition: J.K.P. Writing draft, review and editing: J.Y, E.A.P, E.B.L, J.K.P. All authors approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
EBL has acted as a consultant to Pfizer, and received research grants from Green Cross Corp and Hanmi Pharm Company. The other authors declare no potential conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yeo, J., Park, EA., Lee, E.B. et al. Angiographic characteristics of vasculopathy in patients with idiopathic inflammatory myopathies and systemic sclerosis. Sci Rep 12, 9051 (2022). https://doi.org/10.1038/s41598-022-12991-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-12991-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.