Abstract
Disease outbreaks have been seen as the major threat to sustainable aquaculture worldwide. Injectable vaccines have been one of the few strategies available to control the diseases, however, the adoption of this technology globally is limited. Genetic selection for disease resistance has been proposed as the alternative strategy in livestock and aquaculture. Economic analysis for such strategies is lacking and this study assesses the economic worth of using tilapia fingerlings resistant to Streptococcosis in both cage and pond production systems. The paper also assesses the profitability of paying the higher price for such fingerlings. Partial-budgeting was used to develop a stochastic simulation model that considers the benefits and costs associated with the adoption of tilapia fingerlings resistant to Streptococcosis at the farm level, in one production cycle. In both ponds and cage production systems, the use of genetically selected Streptococcus resistant tilapia fingerlings was found to be profitable where Streptococcus infection is prevalent. In the cages and ponds where Streptococcus related mortality was ≥ 10%, the Nile tilapia aquaculture was found to be profitable even if the amount paid for genetically selected Streptococcus resistant tilapia fingerlings was 100% higher than the amount paid for standard fingerlings.
Similar content being viewed by others
Introduction
Nile tilapia has become the most important freshwater aquaculture species in the last three decades with a global market value of US $7.9 billion in 20201. In these three decades, the Nile tilapia industry has gone through tremendous changes in aquaculture practices; from extensive farming to the rapid expansion in intensive commercial farming2. With the increase in the intensification of production, disease outbreaks have been seen as the major threat to sustainable tilapia production worldwide.
Of all the mortality due to infectious diseases, one of the most important causative agents is the endemic bacterial disease Streptococcosis3, with losses being reported up to 70% (Trapia, personal communication). Streptococcosis become more likely when the water temperature rises above 30 °C4,5. In the current scenario of widespread global warming, the probability of heatwaves is increasing and with it the probability of Streptococossis as well. Until now the vast majority of the tilapia industry relies on the post-infection strategy of treatment with antibiotics, with few large-scale commercial farmers shifting to a pre-infection vaccination strategy to control the infection and reduce the mortality due to Streptococcosis4.
These methods have their limitations. It is common practice to treat the entire population of fish orally using antibiotics, even though only a small percentage of the fish are infected with Streptococcosis. This makes antibiotics therapy expensive and increases the concerns for anti-microbial resistance, both in fish and humans. Similarly, vaccination by intraperitoneal injection is possible only after the fish reaches a certain size, thereby vaccines have limited effectiveness in the early stages, which can have a huge economic implication if there is any disease outbreak during this phase. Furthermore, vaccinating large groups of fish is difficult and labour intensive. Hence alternative sustainable strategies to control the major diseases must be implemented in Nile tilapia aquaculture for animal health, welfare and productivity; without compromising food safety, environment, and human health.
The development of tilapia strains resistant to Streptococcosis has been proposed as the alternative long-term sustainable strategy to control the disease6,7. However, the economic analysis to weigh the different strategies for controlling Streptococcosis infection is lacking, with only one such study available for vaccination8. These financial evaluations allow farmers to choose the models of infection control based on economic profitability. Hence, the first aim of the paper is to assess the economic worth of using tilapia fingerlings tolerant to Streptococcosis in two major production environments: cage and pond production system of Nile tilapia. The second aim of the paper is to assess the profitability of paying the higher price for such tilapia resistant strains at different levels of infection.
Methodology
We built a stochastic model to undertake a partial budget analysis of using genetically selected Nile tilapia fingerlings to control Streptococosis associated with Streptococcus agalactiae in the Nile tilapia industry. The model does not take time into account and considers the benefits and costs that are likely to occur in the new steady-state (one production cycle), as a result of the proposed intervention.
The partial budget model was developed using an Excel spreadsheet (Microsoft Corp., Redmond, WA, USA) and divided into four components, as proposed by Rushton9: (a) new revenues, (b) costs saved, (c) revenue foregone, and (d) new costs, that will be detailed in different subsections below. To assess the financial worth of such investment by farmers, we calculated the difference between benefits (a + b) and costs (c + d). If the marginal benefits exceed the marginal costs, then it would be advantageous for the farmer to invest in tilapia fingerlings resistant to Streptococcosis. The costs and benefits of using tilapia fingerlings resistant to Streptococcosis were calculated at the farm level for one tilapia production cycle. We considered two epidemiological scenarios based on the production system (cage and ponds).
In general, the Nile tilapia production system for large fish (i.e., fish weighing more than 800 g) is based on intensive farming practices and can be divided into two major categories; cage and pond farming system, based on the rearing place. In the cage farming system, tilapia are reared in cages suspended in lakes and reservoirs after the nursery period. Whereas, in a pond farming system, large ponds with aeration are used to rear the tilapia until harvest10,11. There is a major difference between these two farming systems in the adoption of vaccine technologies. While many cage farmers in Malaysia and Latin America have adopted vaccines to control Streptococcosis, there is limited adoption of vaccination by pond farmers4,12.
For each farming system, we used Pert distributions to account for the likelihood for the variability of the Streptococcus related mortality, relative percent survival, average feed conversion ratio, average weight of Streptococcus related mortality, average fish market price, the weight of treated fish, as well as genetics cost per fish.
The stochastic components of the model were handled with @Risk 7.5 (Palisade Corporation, NY, USA), an Excel add-in, using Latin Hypercube sampling, 10,000 iterations, and a random seed. The net return due to the use of the genetically selected Streptococcus resistant tilapia fingerlings was the outcome of the model. We did a sensitivity analysis of the input variables using the software’s built-in tool. Finally, we carried out a break-even (benefits ≥ costs) analysis for a combination of the cost of genetics and Streptococcus related mortality, given the two farming systems.
The values assigned to all modelled variables were based on information provided by the genetics company, hatcheries, producers, vaccine resellers, published data and personal communication from experts (Table 1) based on Malaysian tilapia farming conditions. The severity of Streptococcosis is observed to be more in cages than in the pond farming system, probably due to significantly higher density in cages3; which is modelled in the scenarios (Table 1). RPS data for pond and cage culture used to get the three Pert parameters (minimum, most likely, and maximum) is described in Table 2. One of the main reasons for the difference in the RPS values for the two-farming system is due to the difference in the practice of vaccination as stated previously.
Description of the partial budget model
New costs
Genetics-related costs comprise the unit price of Streptococcus resistant tilapia fingerlings discounted the price normally paid for standard fingerlings. The average price of the standard fingerling in Malaysia is $0.04 as reported by majors’ fingerling resellers and hatcheries. The unit cost of Streptococcus resistant tilapia fingerlings considers a variation of 10% to 30% over the amount paid for standard fingerling. This uncertainty was also modelled by a Pert distribution in the stochastic model (Table 1). The extra cost of Streptococcus resistant tilapia fingerlings was derived as:
where NFish = number of fish in batch, CResistant = cost of Streptococcus resistant tilapia fingerlings (per fish), CStandard = cost of standard tilapia fingerlings (per fish),
Revenue foregone
There is no revenue foregone because of Streptococcus resistant tilapia fingerlings.
Costs saved
Expenditure with antibiotics and feed intake are considered the only cost saved if Streptococcus resistant tilapia fingerlings are used. Genetically resistant fingerlings have the potential to contribute to reduced antibiotic usage due to the reduced prevalence of diseases13. Lower feed conversion ratio (FCR) and better growth in resistant fish populations compared to counterparts are expected. We then assumed a 50% reduction in antibacterial usage if Streptococcus resistant tilapia fingerlings are used when compared to a standard batch. The costs saved were calculated as follows:
where NTreat_Resistant = expected biomass of Streptococcus resistant tilapia to be treated with antibiotics, NTreat_Standard = expected biomass of standard to be treated with antibiotics, NTreatments = number of antibacterial treatments over the production cycle, AtbDose = dose of the antibacterial used, AtbPrice = cost of antibacterial per kg, BioHStandard = expected biomass of standard harvested (in kg), FCRStandard = feed conversion ratio for standard, BioHResistant = expected biomass of resistant harvested (in kg), and Resistant = feed conversion ratio for resistant fish. Antibacterial treatment was considered using florfenicol (20 mg/kg body weight/day) for ten consecutive days (Merck Animal Health, USA). Considering the difficulty in calculating the exact time of treatment, we calculated NTreat_Resistant assuming survival fish for each scenario (Resistant vs Standard) and multiplying it by the average weight of treated fish, which was also modelled by a Pert probability distribution (Table 1).
New revenue
An increase in fish survival is the only consequence of using genetically resistant fingerlings that yields an additional return. It was calculated as:
Considering:
where MortalityStandard = total mortality due to Streptococcosis in a farm rearing standard fingerlings (number of fish), MortalityResistant = total mortality due to disease in a farm rearing genetically Streptococcus resistant fingerlings (number of fish), MortStrepStandard = expected mortality due to S. agalactiae if standard fingerlings (in %), MortStrepResistant = expected mortality due to S. agalactiae if genetically Streptococcus resistant fingerlings (in %), RPS = relative percent survival provided by genetically Streptococcus resistant fingerlings (in %), WHarvested = fish weight during harvesting and FishPrice = fish market price. Streptococcus related mortality and RPS were modelled using Pert probability distribution (Table 2).
Results
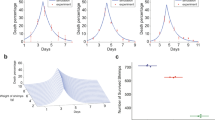
The results for the two different production system scenarios where we used probability distributions to model variability and uncertainty are shown in Fig. 1. Figure 1 shows the range of possible outcomes (x-axis) and their relative likelihood of occurrence (y-axis). Because a simulation yields many possible values for the outcome, the summary statistics is used to summarize the range of net results per kilogram of biomass harvested when using tilapia strains resistant to Streptococcosis at the farm level. For tilapia pond farms, the average net result is US$ 0.07 (± 0.021) but it can range from US$ 0.01 to US$ 0.13 depending on several inputs (and their uncertainty) that interact to produce the outcome. Similarly, for tilapia cage farms, the average net result is US$ 0.075 (± 0.023) but it can range from US$ 0.01 to US$ 0.17.
Table 3 displays the layout of the baseline partial budget model used to estimate profitability of using genetically Streptococcus resistant fingerlings in a modal pond tilapia farm in Malaysia, for one production cycle starting with 30,000 fingerlings, 15% mortality over the whole period, improvement in FCR (1.60 for standard and 1.52 for resistant), which is a very realistic scenario, genetics-related costs (price normally paid for standard fingerlings + 20%) and an RPS of 32%. The net change in income (benefits – costs) was US$ 0.12 per kilogram of biomass harvested. A similar deterministic approach (Table 3) was used for a modal cage tilapia farm in Malaysia, for one production cycle starting with 60,000 fingerlings, 20% mortality over the whole period, a similar improvement in FCR (1.60 for standard and 1.52 for resistant), genetics-related costs (price normally paid for standard fingerlings + 20%) and an RPS of 20%. The net change in income (benefits – costs) was US$ 0.11 per kilogram of biomass harvested.
The results for the two different scenarios where we used probability distributions to model variability and uncertainty are shown in Table 4 and displayed as a break-even analysis (i.e. the probability of benefits being equal or greater than costs for each combination of variables). The extra cost of genetically selected Streptococcus resistant fingerlings and cumulative Streptococcus related mortality are the only parameters that can be realistically assessed by the farmer before deciding whether to buy or not genetically selected Streptococcus resistant tilapia fingerlings and therefore were the parameters chosen for the break-even analysis. In the pond scenario, when Streptococcus related mortality is over 20% the net return would break even in 100% of iterations, indicating that using Streptococcus resistant fingerlings is profitable, even when the extra cost paid is 100%. In scenarios with lower expected mortality, profitability is more dependent on the extra amount paid. For example, if the expected Streptococcus related mortality is 1% and the extra cost paid for genetics is 50%, the probability of break-even drops to 46.2%. For the cage scenario, the break-even probability was more dependent on expected Streptococcus related mortality. Either way, if the extra amount paid is up to 30%, the profitability is very likely to occur even in low Streptococcus related mortality (≥ 5%).
Figure 2 shows the results from the regression sensitivity analysis for pond and cage farms, respectively, displaying a ranking of the parameters that impact the output: net results per kg of biomass harvested. Parameters with the largest impact on the distribution of the output have the longest bars in the graph and are ordered from top-down. A positive coefficient indicates that this input has a positive impact: increasing this input will increase the output. A negative coefficient indicates that this input has a negative impact: increasing this input will decrease the output. For both production systems, the results suggest that “Average FCR” had the greatest impact (negative) in the net results per kg of biomass harvested, being slightly higher for pond (− 0.95) when compared to cages (− 0.89). For pond farms, the second most influential input variable was “Average weight of treated fish by florfenicol” followed by “Average weight of Streptococcus related mortality”. On the other hand, for cage farms, the second most influential input variable was “Streptococcus related mortality” followed by “Average weight of treated fish by florfenicol”, and “Average weight of Streptococcus related mortality”. The inputs “Genetically selected fingerlings extra cost”, “RPS”, and “Average fish market price” had minor effects on the output, in both scenarios.
Discussion
Streptococcosis has become the major bacterial disease affecting commercial tilapia farming worldwide and various prevention and control measures are being applied affecting the profitability of the farms4. Using genetics to control disease in aquaculture has become a common practice14, which is because breeders were able to reduce the incidence of a major viral disease, infectious pancreatic necrosis (IPN), to near zero in salmon farming via selective breeding15. The power of genetics to control Streptococcosis is increasingly being recognised6,7 with various breeding programs incorporating this trait in their selective breeding program16 and Streptococcus resistant tilapia fingerlings being made available to the farmers17.
Our results indicate that using genetically selected fingerlings is likely to be profitable in Nile tilapia farms where Streptococosis is a production constrain. For either pond or cage tilapia farms, genetics proved to be profitable in scenarios where Streptococcus related mortality are higher (≥ 15%), even though the cost to be paid was close to 100% higher than the cost of a standard fingerling.
In the pond scenario, the net return was positive (benefits ≥ costs) in 99.7% of iterations, indicating that genetically selected Streptococcus resistant fingerlings are profitable, even in the absence of any improvement in feed conversion, which is a very conservative assumption. However, the profitability of genetically selected Streptococcus resistant fingerlings can be lower when cumulative Streptococcus related mortality is lower (< 10%), in which case it would be more dependent on the extra cost to be paid for the genetically selected Streptococcus resistant fingerlings. For example, genetically selected Streptococcus resistant fingerlings is likely to yield economic gains for pond production when the extra cost of genetically selected Streptococcus resistant fingerlings is up to 30% of the price paid for the standard fingerling, regardless of Streptococcus related mortality (in at least 94.6% of iterations). Whereas in ≥ 10% Streptococcus related mortality genetically selected Streptococcus resistant fingerlings is very likely (at least 91.2% of iterations) to be lucrative even if the amount paid for genetically selected Streptococcus resistant fingerlings was 100% higher than the amount paid for standard fingerlings.
In the cage scenario, despite likely being profitable, Streptococcus related mortality had a greater impact on the net results compared to pond farms, as shown by the sensitivity analysis. Thus, the profitability of genetically selected Streptococcus resistant fingerlings is lower when cumulative mortality was lower, in which case it would be more dependent on the genetics related costs. Likewise, Thorarinsson and Powell18 and Delphino et al.8 described disease risk level (mortality pre-intervention) to have a profound influence on the profitability of salmon and tilapia vaccination, respectively. In addition, in cage farms where Streptococcus related mortality is very low, the profitability of the use of genetically selected Streptococcus resistant fingerlings would be very dependent on better “FCR” and “genetics related cost”. The inputs “fish market price” and “RPS” had minor effects on the output, in production system scenarios.
This model considered marginal benefits and costs that are directly associated with the use of genetics to control Streptococcosis in tilapia farms and was not designed to be a farm budget. Although our results indicate that genetically selected Streptococcus resistant fingerling is likely to yield economic gains, tilapia farmers should be aware that the profitability of the intervention is a combination of parameters. Therefore, the baseline partial budget model must be updated to reflect changes in economic (e.g., market price, genetics price) or biological factors (e.g., RPS and FCR). Although this model is based on conservative values and considers uncertainty about the modelled parameters, we conclude that the use of genetically selected Streptococcus resistant fingerlings is likely to be profitable in Nile tilapia farms, under similar economic and biological factors assessed.
Conclusions
In both ponds and cage production systems of Nile tilapia, the use of genetically selected Streptococcus resistant tilapia fingerlings was found to be profitable where Streptococcus infection is prevalent. Higher the mortality due to Streptococcus infection, higher the economic profitability of using such Streptococcus resistant tilapia fingerlings. In the cages and ponds where Streptococcus related mortality was ≥ 10%, the Nile tilapia aquaculture was found to be profitable even if the amount paid for genetically selected Streptococcus resistant tilapia fingerlings was 100% higher than the amount paid for standard fingerlings.
Data availability
All the parameters and data used for the analysis are listed in the manuscript.
References
IMARC Group. Tilapia Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2021–2026 [WWW Document] (2021). https://www.imarcgroup.com/tilapia-market (Accessed 25 Aug 2021).
FAO. Tilapia Trade: Global and Regional Trends [WWW Document] (2018). http://www.fao.org/fi/static-media/MeetingDocuments/TiLV/dec2018/p13.pdf.
Kayansamruaj, P., Areechon, N. & Unajak, S. Development of fish vaccine in Southeast Asia: A challenge for the sustainability of SE Asia aquaculture. Fish Shellfish Immunol. 103, 73–87 (2020).
Maulu, S., Hasimuna, O. J., Mphande, J. & Munangandu, H. M. Prevention and control of streptococcosis in Tilapia culture: A systematic review. J. Aquat. Anim. Health. 33(3), 162–177 (2021).
Rodkhum, C. et al. Effect of water temperature on susceptibility to Streptococcus agalactiae serotype Ia infection in Nile tilapia (Oreochromis niloticus). Thai J. Vet. Med. 41, 309 (2011).
Joshi, R., Skaaurd, A. & Tola Alvarez, A. Experimental validation of genetic selection for resistance against Streptococcus agalactiae via different routes of infection in the commercial Nile tilapia breeding programme. J. Anim. Breed. Genet. 138(3), 338–348 (2020).
Suebsong, W. et al. Selection response for Streptococcus agalactiae resistance in Nile tilapia Oreochromis niloticus. J. Fish Dis. 42(11), 1553–1562 (2019).
Delphino, M. K. V. C. et al. Economic appraisal of vaccination against Streptoccocus agalactiae in Nile tilapia farms in Brazil. Prev. Vet. Med. 162, 131–135 (2019).
Rushton, J. The Economics of Animal Health and Production (Cabi, 2009).
Bhujel, R. C., Klinbunga, S., Karoonuthaisiri, N. & Piyapattanakorn, S. Cage Culture of Monosex Tilapia for Food and Financial Security. In (Sirawut Klinbunga Nitsara Karoonuthaisiri Sanit Piyapattanakorn eds) 19 (2019).
Mengistu, S. B., Mulder, H. A., Benzie, J. A. H. & Komen, H. A systematic literature review of the major factors causing yield gap by affecting growth, feed conversion ratio and survival in Nile tilapia (Oreochromis niloticus). Rev. Aquac. 12, 524–541 (2020).
Zamri-Saad, M., Amal, M. N. A., Siti-Zahrah, A. & Zulkafli, A. R. Control and prevention of Streptococcosis in cultured tilapia in Malaysia: A review. Pertanika J. Trop. Agric. Sci. 37, 389–410 (2014).
Hjeltnes, B., Bang-Jensen, B., Bornø, G., Haukaas, A. & Walde, C. S. The health situation in Norwegian aquaculture 2016. Nor. Vet. Inst. 127, 1–126 (2017).
Houston, R. D. et al. Harnessing genomics to fast-track genetic improvement in aquaculture. Nat. Rev. Genet. 21(7), 389–409 (2020).
Norris, A. Application of genomics in salmon aquaculture breeding programs by Ashie Norris: Who knows where the genomic revolution will lead us?. Mar. Genomics 36, 13–15 (2017).
Yáñez, J. M., Joshi, R. & Yoshida, G. M. Genomics to accelerate genetic improvement in tilapia. Anim. Genet. 51(5), 658–674 (2020).
GenoMar Genetics AS. Selection for Streptococcus resistance. Genomar Genet. AS Intern. Quaterly Newsl. 2, 1–2 (2019).
Thorarinsson, R. & Powell, D. B. Effects of disease risk, vaccine efficacy, and market price on the economics of fish vaccination. Aquaculture 256, 42–49. https://doi.org/10.1016/j.aquaculture.2006.01.033 (2006).
Sukhavachana, S., Poompuang, S., Onming, S. & Luengnaruemitchai, A. Heritability estimates and selection response for resistance to Streptococcus agalactiae in red tilapia Oreochromis spp. Aquaculture 502, 384–390 (2019).
Author information
Authors and Affiliations
Contributions
M.D., R.J. and A.T.A. conceived the idea of the paper; M.D. performed the modelling; M.D. and R.J. wrote the initial draft of the paper; and all authors contributed to finalising the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Delphino, M., Joshi, R. & Alvarez, A.T. Economic appraisal of using genetics to control Streptococcus agalactiae in Nile tilapia under cage and pond farming system in Malaysia. Sci Rep 12, 8754 (2022). https://doi.org/10.1038/s41598-022-12649-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-12649-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.