Abstract
Malaria is the leading cause of morbidity and mortality in Mali. Between 2017 and 2020, the number of cases increased in the country, with 2,884,827 confirmed cases and 1454 reported deaths in 2020. We performed a malaria risk stratification at the health district level in Mali with a view to proposing targeted control interventions. Data on confirmed malaria cases were obtained from the District Health Information Software 2, data on malaria prevalence and mortality in children aged 6–59 months from the 2018 Demographic and Health Survey, entomological data from Malian research institutions working on malaria in the sentinel sites of the National Malaria Control Program (NMCP), and environmental data from the National Aeronautics and Space Administration. A stratification of malaria risk was performed. Targeted malaria control interventions were selected based on spatial heterogeneity of malaria incidence, malaria prevalence in children, vector resistance distribution, health facility usage, child mortality, and seasonality of transmission. These interventions were discussed with the NMCP and the different funding partners. In 2017–2019, median incidence across the 75 health districts was 129.34 cases per 1000 person-years (standard deviation = 86.48). Risk stratification identified 12 health districts in very low transmission areas, 19 in low transmission areas, 20 in moderate transmission areas, and 24 in high transmission areas. Low health facility usage and increased vector resistance were observed in high transmission areas. Eight intervention combinations were selected for implementation. Our work provides an updated risk stratification using advanced statistical methods to inform the targeting of malaria control interventions in Mali. This stratification can serve as a template for continuous malaria risk stratifications in Mali and other countries.
Similar content being viewed by others
Introduction
Malaria is the leading cause of morbidity and mortality among children in Mali. The number of cases increased in the country over the 2017–2020 period. In 2020, the surveillance system reported 2,884,827 confirmed cases (including 871,265 severe cases) out of 4,252,213 people tested1. That same year, health facilities reported 1454 deaths due to malaria, and the disease was the most frequent reason for health care visits (accounting for 36% of consultations)1. As these data indicate, reducing the malaria burden would considerably lower child mortality in Mali, which was still high at 101 per 1000 live births in 20182.
The main parasites responsible for malaria in Mali are Plasmodium falciparum (more than 85% of responsible parasites), Plasmodium malariae (10–15%), and Plasmodium ovale (1%)3, though Plasmodium vivax has also been observed and documented4,5. Malaria is endemic in most localities, and its transmission increases with the rainy season. The most common vector species are Anopheles gambiae s.l. (Anopheles gambiae s.s., Anopheles coluzzii, and Anopheles arabiensis) and Anopheles funestus6, which have been found to develop resistance to the insecticides used7,8. The main mechanisms of resistance to available insecticides are the Alpha esterizes, Beta esterase, Kdr, and Ace- 1 genes9.
In accordance with World Health Organization (WHO) technical guidelines10, Mali has implemented a malaria control strategy based on case management and prevention. This strategy is coordinated at different levels by the National Malaria Control Program (NMCP). Malaria case management involves early diagnostic by rapid diagnostic test (RDT) and prompt treatment. The first-line treatments used in Mali are artemether-lumefantrine combination therapy for simple malaria and artesunate injection for severe malaria1. Prevention interventions consist of vector control using long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS), seasonal chemoprevention (SMC), and intermittent preventive treatment in pregnancy (IPTp). In Mali, LLINs are distributed every 3 years in all health districts via mass campaigns and are routinely provided to pregnant women and children under 1 year of age. Indoor residual spraying was carried out in 8 health districts in 2017 and in 2 health districts in 2020. Indeed, the main vector species, An. gambiae, has developed resistance to older insecticides, and the new insecticides (pirimiphos-methyl11,12 and clothianidin13) are too costly to be used on a large scale. Seasonal malaria chemoprevention was first implemented in Mali in 2012 and has been performed in all health districts during periods of high malaria transmission since 2016. It consists in the administration of sulfadoxine-pyrimethamine (SP) and amodiaquine (AQ) to children under 5 years of age once per month from July to October (1 SP tablet and 1 AQ tablet on day 1, 1 AQ tablet on day 2, and 1 AQ tablet on day 3 of each of month). Lastly, IPTp, namely the administration of SP to pregnant women from the 13th week of pregnancy, has been implemented in all health districts since 20001.
In malaria-endemic countries, the evolution of malaria incidence strongly depends on environmental and socio-demographic characteristics and on the type of interventions used14,15,16,17,18. The WHO Framework for Malaria Elimination19 recommends that these countries adapt control interventions to suit the local epidemiology and available financial resources. This document also advises using incidence or prevalence as a measure of the malaria burden and suggests dividing the latter into 4 categories of transmission intensity (high, moderate, low, and very low). As a high incidence country, Mali has adopted the WHO’s High Burden to High Impact (HBHI) initiative10,20, a targeted malaria response to be led by the health actors of each country. This initiative, which draws on the WHO’s Global Technical Strategy for Malaria 2016–2030 and Sustainable Development Goals on Health, aims to reduce malaria mortality through the implementation of targeted interventions in high burden areas. In Mali, this initiative is also tailored to the priorities of the national malaria strategic plan.
Since 2016–2017, the WHO technical guidelines recommend using malaria risk stratification to better target malaria control interventions. Malaria risk stratification is defined as the classification of geographical areas according to epidemiological, entomological, environmental, and socio-economic factors that determine susceptibility and vulnerability to malaria transmission10. Each defined stratum is composed of health districts with similar malaria incidence patterns. The stratification of malaria risk in Mali was first performed in 1989. Five epidemiological strata were identified: (1) the Sudanese-Guinean zone, with long seasonal transmission (4–6 months); (2) the Sahelian zone, with short seasonal transmission (3–4 months); (3) the Saharan zone in the north and some localities of Koulikoro, Segou, Mopti, and Kayes regions, with sporadic or epidemic transmission; (4) dams and the inner delta of the Niger River, with bi or multimodal transmission; and (5) urban zones (especially Bamako and Mopti), with hypoendemic malaria transmission21,22. However, the distribution of malaria transmission has considerably evolved over the last 30 years due to climate change, population movement, urbanization, and the impact of malaria control interventions. Accordingly, a new stratification of malaria risk is needed.
This study aimed to perform malaria risk stratification at the health district level in Mali with a view to proposing targeted control interventions.
Method
Study location
The Republic of Mali is located in the Sahel-Saharan strip in West Africa. In 2020, the country had 20,536,999 inhabitants and a population growth rate of 3.36% (General Population Housing Census (GPHC) 2009 data updated by the National Statistical Institute). The landscape is characterized by plains and low plateaus, with an average altitude of 500 m. The hydrographic regime is constituted by the upper Senegal and Niger Rivers basins. Mali has several dams and flooded areas used for agriculture. Flooded areas extend over an area of 41,000 km2 along the Niger River. These areas include the inner delta of the Niger River (from Segou region in the center through Mopti region to Timbuktu region in the north) as well as numerous lakes, ponds, and swamps23,24 (Fig. 1).
Mali is divided into 11 regions (including the District of Bamako) and 75 health districts. The health care system is organized in a 3-level pyramid (health district level, regional level, and national level).
Malaria, child mortality, entomological and environmental data
Data on confirmed malaria cases at the health district level were obtained from the District Health Information Software 2 (DHIS2). National data on malaria prevalence in children aged 6–59 months and mortality in children aged 6–59 months were obtained from the 2018 Demographic and Health Survey (DHS).
Entomological data were obtained from Malian research institutions6,7,9,25 that study malaria in the sentinel sites of the NMCP.
Remote sensing environmental data were obtained from the National Aeronautics and Space Administration. These data were: rainfall, relative humidity, temperature, Normalized Difference Vegetation Index (NDVI), soil moisture and wind speed.
The assembled variables are summarized in Table 1.
Data assembly
See Table 1.
Data analysis
Temporal and spatial analyses were performed using a wide range of variables to account for the complexity of malaria transmission in Mali.
Temporal analysis
Seasonality of malaria transmission
To determine the periods of low and high malaria transmission, a change point analysis of monthly incidence data was performed using the algorithm of the Power of the Pruned Exact Linear Time26,27.
Monthly malaria incidence was estimated using confirmed malaria cases obtained from the DHIS2, taking into consideration population size and time (month).
Principal component analysis of environmental variables
A principal component analysis (PCA) of the environmental variables associated with malaria transmission was performed to provide a contextual description of the epidemiological facies of malaria in Mali. These variables were: rainfall, relative humidity, soil moisture, NDVI, and wind speed. The analysis accounted for collinearities between variables and led to the creation of synthetic environmental indices (SEIs)29. The number of SEIs was selected using the elbow criterion28.
Univariate and multivariate regression analyses of the relationship between synthetic environmental indices and malaria transmission
Univariate and multivariate regression analyses of the relationship between SEIs and malaria incidence were performed using Generalized Additive Models (GAMs), taking into consideration the lag between the two30. Spline smoothing was performed to study the non-linear relationship between malaria incidence and SEIs. A negative binomial distribution was conducted to adjust for over-dispersion. The time series of monthly malaria incidence was modeled as a function of SEIs with a lag of 1–4 months.
Spatial analysis
Spatial heterogeneity of malaria incidence
Crude estimations of malaria incidence (see the Seasonality of malaria transmission sub-section above) do not fully reflect the real incidence of the disease in the population, as the following factors vary between health districts29:
-
Health facility access and usage rate.
-
Availability and use of diagnostic tests.
-
Completeness of the data.
In view of this, incidence was estimated per 1000 person-years adjusting for the health facility usage rate:
Given that the use of diagnostic tests depends on health facility access and usage, adjustment for health facility usage indirectly accounted for the testing rate. There was no need to adjust for data completeness because the completeness rate remained stable (at around 98%) over the study period30.
Based on adjusted malaria incidence, the 75 health districts of Mali were classified into 4 categories of transmission intensity (high, moderate, low, and very low) in accordance with the WHO Framework for Malaria Elimination19. The very low category was divided into two sub-categories to better reflect incidence in health districts where the risk of malaria transmission is close to zero.
Spatial distribution of rainfall
The spatial distribution of mean annual rainfall in the health districts of Mali over the period 2017–2019 was analyzed.
Spatial distribution of health facility access
The spatial distribution of health facility access in all health districts of Mali in the year 2019 was analyzed.
Spatial distribution of prevalence in children aged 6–59 months
The spatial distribution of the prevalence of malaria in children aged 6–59 months in Mali in the year 2018 was analyzed.
Spatial distribution of mortality in in children aged 6–59 months
The spatial distribution of mortality in children aged 6–59 months in Mali over the period 2012–2017 was analyzed.
Spatial distribution of vector resistance
Two parameters were examined: vector distribution and vector resistance to 10 insecticides from 5 classes: permethrin 0.75%, deltamethrin 0.05%, alpha-cypermethrin 0.05%, and lambda-cyhalothrin 0.05% (pyrethroids); DDT 4% (organochlorines); bendiocarb 0.1% and propoxur 0.1 (carbamates); pirimiphos-methyl 0.25% and 1% and fenitrothion 1% (organophosphates); and clothianidin 2% (neonicotinoids). The resistance of the main vector species, An. gambiae, was measured using bioassays as per WHO recommendations.
Selection of interventions
In accordance with the WHO Global Technical Strategy for Malaria 2016–20309, the WHO HBHI initiative20, the WHO Framework for Malaria Elimination19, the national health priorities for malaria control, and the national malaria strategic plan, a package of interventions was discussed and qualitatively selected with the NMCP and the different funding partners. Depending on the type of intervention, health districts were targeted based on the most relevant variables, the impact of each intervention on the burden of malaria (HBHI), the cost of the intervention, and the resources available for the intervention.
For the purpose of intervention selection, variables were categorized as follows:
-
Malaria incidence and malaria prevalence were classified into 4 categories of transmission intensity (high, moderate, low, and very low) in accordance with the WHO Framework for Malaria Elimination.
-
Mortality in children aged 6–59 months was dichotomized into high or low. The threshold value was 101 per 1000 live births, which corresponds to the national average estimated in 2019.
-
Health districts were classified as vector resistant or not, depending on whether they showed confirmed An. gambiae resistance to pyrethroids.
-
Health facility usage rate was dichotomized into high or low. The threshold value was 58%, which corresponds to the national average estimated in 2019.
The resources available for interventions consisted in grants from funding partners and funds allocated in the NMCP national budget for malaria control over the period 2021–2023.
The targeting of interventions is detailed in the Selected interventions section below.
Software and packages
Statistical analyses were carried out with R software version 3.4 (R Development Core Team, R Foundation for Statistical Computing, Vienna, Austria). The following packages were used: {mgcv}{caschrono}{FactoMineR}{forecast}{ggplot2}{party}{changepoint}.
Mapping was performed using ArcGIS Desktop version 10.1 software (Environmental Systems Research Institute (ESRI), 380 New York Street, Redlands, CA 92373-8100, USA).
The Paint.net software version 4.2.13 (Rick Brewster and al., Washington State University, USA) was used for image treatment.
Consent to publication
Not applicable.
Results
Temporal analysis
Seasonality of malaria transmission
The descriptive analysis of monthly malaria incidence time series showed typical seasonality at the national level, with a lag between cases and rainfall (Fig. 2).
Principal component analysis of environmental variables
The PCA analysis yielded 2 SEIs (Fig. 3).
Synthetic indices resulting from the Principal Component Analysis of the different environmental variables. (Panel a) Two Synthetic Environmental Indices with 87.7% inertia. (Panel b) Synthetic Environmental Index 1 (rainfall, relative humidity, soil moisture, Normalized Difference Vegetation Index, and wind speed). (Panel c) Synthetic Environmental Index 2 (temperature).
Univariate and multivariate regression analyses of the relationship between synthetic environmental indices and malaria transmission
Univariate regression analysis using GAM found a 1-month lag between SEI 1 (rainfall, relative humidity, soil moisture, NDVI, and wind speed) and malaria incidence. No lag was found between SEI 2 (temperature) and malaria incidence.
Multivariate regression analysis using GAM (Fig. 4) showed a positive linear relationship between SEI 1 (rainfall, relative humidity, soil moisture, NDVI, and wind speed) and malaria incidence (p value < 0.001) (Fig. 4, panel a) and a negative non-linear relationship between SEI 2 (temperature) and malaria incidence (p value < 0.01) (Fig. 4, panel b).
Changes in malaria incidence as a function of synthetic environmental indices; multivariate regression analysis using a Generalized Additive Model. (Panel a) Positive linear relationship between SEI 1 (rainfall, relative humidity, soil moisture, NDVI, and wind speed) and malaria incidence (p value < 0.001). (Panel b) Negative non-linear relationship between SEI 2 (temperature) and malaria incidence (p value < 0.01).
Spatial analysis
Spatial heterogeneity of malaria incidence
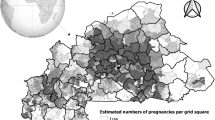
Adjusted median incidence for the year 2019 was 125.34 cases per 1000 person-years with a standard deviation of 86.48.
In accordance with WHO recommendations, adjusted malaria incidence per 1000 person-years for the year 2019 was classified into 4 categories of transmission intensity (Fig. 5):
-
12 health districts were located in areas of very low malaria transmission (less than 100 cases per 1000 person-years), including 7 health districts with 5–50 cases per 1000 person-years (in the northern regions) and 5 health districts with 50–100 cases per 1000 person-years (3 in the northern regions, 1 in the western part of the country, 1 in the District of Bamako);
-
19 health districts were located in areas of low malaria transmission (between 100 and 250 cases per 1000 person-years);
-
20 health districts were located in areas of moderate malaria transmission (between 250 and 450 cases per 1000 person-years).
-
24 health districts were located in areas of high malaria transmission (450 or more cases per 1000 person-years) (mainly in the southern and central-western regions).
Annual malaria incidence adjusted for health facility usage rate in 75 health districts Mali, 2019. ArcGIS Desktop version 10.8 software (Environmental Systems Research Institute (ESRI), 380 New York Street, Redlands, CA 92373-8100, USA, https://www.esri.com).
Spatial distribution of rainfall
Average annual rainfall over the 2017–2019 period ranged from less than 200 mm in the Saharan zone to more than 1100 mm in the pre-Guinean zone, with a clear trend from north to south. Of the 75 health districts, 17 had an average annual rainfall of less than 200 mm (Saharan zone), 20 had an average annual rainfall between 200 and 600 mm (Sahelian zone), 31 had an average annual rainfall between 600 and 1100 mm (Sudanese zone), and 7 had an average annual rainfall > 1100 mm (pre-Guinean zone) (Fig. 6).
Map of average annual rainfall by health district, Mali, 2017–2019. ArcGIS Desktop version 10.8 software (Environmental Systems Research Institute (ESRI), 380 New York Street, Redlands, CA 92373-8100, USA, https://www.esri.com).
Spatial distribution of health facility access
The percentage of the population living outside a radius of 5 km (Euclidian distance) from a health facility was high (especially in the northern regions), justifying the implementation of specific strategies to improve health facility access (Fig. 7).
Percentage of the population living outside a radius of 5 km from a health facility access, Mali, 2019. ArcGIS Desktop version 10.8 software (Environmental Systems Research Institute (ESRI), 380 New York Street, Redlands, CA 92373-8100, USA, https://www.esri.com).
Health facility access was low in health districts with high malaria transmission. One strategy for improving access to malaria diagnosis is the conduct of RDTs at the community level via integrated community case management (iCCM).
Spatial distribution of prevalence of malaria in children aged 6–59 months
According to the Mali DHS VI, the prevalence of malaria in children aged 6–59 months was 18.9% at the national level in 2018. This prevalence was lower in the northern regions of Kidal, Taoudenit, and Tombouctou (between 1 and 10%) and in the District of Bamako (less than 1%). The regions of Sikasso, Segou, and Mopti had the highest prevalence (29.7%, 25.9%, and 24.9%, respectively) (Fig. 8).
Map of the prevalence of malaria in children aged 6–59 months, Mali, 2018. ArcGIS Desktop version 10.8 software (Environmental Systems Research Institute (ESRI), 380 New York Street, Redlands, CA 92373-8100, USA, https://www.esri.com).
Spatial distribution of mortality in children aged 6–59 months
Kidal region, Gao region, and the District of Bamako had the lowest child mortality (20, 78, and 55 deaths per 1000 live births, respectively) (Fig. 9).
Spatial distribution of vector resistance
The main malaria vector species—An. gambiae, An. coluzzii, and to a lesser extent An. Arabiensis—were found in all sentinel sites using morphological analysis. Only An. gambiae was detected in the northern regions of Gao, Kidal, and Tombouctou. An. funestus was identified mainly in the regions of Niono and Mopti (inner delta of the Niger River) (Fig. 10).
Map of malaria vector distribution, Mali, 2010–2019. The sector diagrams show the presence of different vector species. ArcGIS Desktop version 10.8 software (Environmental Systems Research Institute (ESRI), 380 New York Street, Redlands, CA 92373-8100, USA, https://www.esri.com).
The main vector species, An. gambiae, showed high resistance to pyrethroids and organochlorines in most sentinel sites. Resistance to carbamates (bendiocarb and propoxur) was also noted, although it was less extensive than resistance to pyrethroids. Anopheles gambiae was sensitive to pirimiphos-methyl and clothianidin in all sentinel sites. Depending on the site, it showed sensitivity or resistance to fenitrothion (Fig. 11).
Map of distribution of vector resistance in sentinel sites, Mali, 2010–2019. Red dots represent vector resistance; yellow dots show moderate sensitivity (below 90%); and green dots show high sensitivity (above 90%). ArcGIS Desktop version 10.8 software (Environmental Systems Research Institute (ESRI), 380 New York Street, Redlands, CA 92373-8100, USA, https://www.esri.com).
Selected interventions
Potential control interventions were discussed with the NMCP and the different funding partners. Eight intervention combinations were qualitatively selected for implementation in the different health districts based on the following variables: spatial heterogeneity of malaria incidence, prevalence of malaria in children aged 6–59 months, distribution of vector resistance, health facility usage, mortality in children aged 6–59 months, and seasonality of transmission. The impact of each intervention on the burden of malaria (HBHI), the cost of the intervention, and the resources available for the intervention were also taken into consideration. Environmental variables were not considered in discussions with the NMCP but were indirectly accounted for as they are correlated with malaria incidence in Mali (see Figs. 4, 5 and 6).
The 8 intervention combinations are made up of the following interventions:
Long-lasting insecticidal net distribution will be implemented on a large scale based on the relatively low cost of the intervention and the endemicity of malaria in Mali. This intervention will be carried out using 2 methods. In the first method, LLINs will be routinely distributed in all 75 health districts to 9-month-old children vaccinated against measles via the Expanded Programme on Immunization and to pregnant women during prenatal consultations. In the second method, LLINs will be distributed in 69 health districts as part of the 2023 universal coverage campaign. Six health districts located in the urban area of Bamako will be excluded because of low malaria prevalence in children aged 6–59 months2. The distribution of LLINs in these 69 health districts will be renewed every 3 years as part of the universal coverage campaign31.
Different types of LLINs will be distributed based on malaria incidence and vector resistance to pyrethroids. Interceptor G2 (IG2) nets will be distributed in 4 health districts with high malaria incidence and confirmed vector resistance to pyrethroids (Yorosso, Yanfolila, Selingue, and Kadiolo) both routinely and as part of the 2023 universal coverage campaign. Note that these 4 health districts already received IG2 nets in 2020 (pilot phase). Long-lasting piperonyl butoxide-treated insecticidal nets (PBO-LLINs) will be distributed in 14 health districts with high malaria incidence (Bandiagara, Bankass, Bareouli, Bla, Bougouni, Djenne, Fana, Kati, Kayes, Kita, Koulikoro, Mopti, Niono, and Tominian) both routinely and as part of the 2023 universal coverage campaign. In 13 of these health districts, the main vector species, An. gambiae, was found to be resistant to pyrethroids. The last health district, Tominian, will receive PBO-LLINs despite the lack of data on vector resistance because it has a high level of malaria incidence and because it is surrounded by health districts that will receive IG2 nets and PBO-LLINs. In the other 57 health districts, for which there is no data on vector resistance, standard LLINs will be distributed routinely; 51 of these health districts (all but the 6 health districts of Bamako) will also receive standard LLINs as part of the universal coverage campaign2.
Seasonal malaria chemoprevention will be implemented in all but 17 health districts based on malaria incidence, malaria prevalence in children aged 6–59 months (in the case of Bamako), and seasonality of transmission. The 6 health districts of Bamako will be excluded from the intervention due to low malaria prevalence (Fig. S1, Panel b)2. The 6 health districts of Taoudenit region along with 3 health districts in Kidal region (Tin Essako, Abeibara, and Tessalit) and 2 health districts in Menaka region (Inekar and Anderamboukane) will be excluded due to very low malaria incidence (less than 100 cases per 1000 person-years) and to the absence of seasonality throughout the year (Fig. S1, Panel a). Based on the duration of the high malaria transmission season, the intervention will be carried out in 4 rounds (over a period of 4 months) in the health districts located in the southern regions (Fig. S1, Panels c, d, e) and in 3 rounds (over a period of 3 months) in the health districts located in the northern regions (Fig. S1, Panel f).
Intermittent preventive treatment in pregnancy will be continued in all 75 health districts based on the relatively low cost of the intervention and the endemicity of malaria in Mali32, in accordance with WHO recommendations33.
Indoor residual spraying will be implemented based on available resources, the cost of insecticides, and vector resistance to pyrethroids in the different health districts. The intervention was initially planned in 11 health districts with high malaria incidence (Bandiangara, Bougouni, Dioila, Douentza, Fana, Kalabancoro, Nara, Niono, Segou, Yanfolila, and Youwarou). However, due to insufficient resources, only the health districts of Bandiagara, Mopti, and Djenne will receive IRS in 20227,8,34. These 3 health districts were selected because they are the most accessible and because they have the highest population density.
Malaria case management will be implemented in all 75 health districts based on the relatively low cost of the intervention and the endemicity of malaria in Mali. This intervention will be performed in accordance with national guidelines and protocols, themselves based on WHO recommendations.
Integrated community case management will be implemented in 51 health districts based on mortality in children aged 6–59 months and health facility access (Fig. 7). Most of the targeted health districts have high or moderate malaria incidence. Existing iCCM sites will be used and new ones created, and all iCCM sites will be provided with additional diagnostic tools. A pilot study of SP distribution to pregnant women will be conducted in 3 health districts.
Lastly, weekly and monthly epidemiological surveillance will be continued in all health districts and will be reinforced in the 27 health districts with epidemic potential.
Selected intervention combinations at the health district level are shown in Fig. 12. The main interventions (SMC, IG2-LLIN, PBO-LLIN, IRS, IPT, CM) will be implemented in all health districts with high and moderate transmission.
Intervention combinations at the health district level in Mali. CM: Malaria case management; IG2-LLIN: Interceptor G2 Net; R: Routine distribution; C: Universal campaign; IPTp: Intermittent preventive treatment in pregnancy; iCCM: Integrated community case management; SMC: Seasonal malaria chemoprevention; Surv: Weekly and monthly epidemiological surveillance; PBO-LLIN: Long-lasting piperonyl butoxide-treated insecticidal net; IRS: Indoor residual spraying; Standard LLIN: Standard long-lasting insecticidal net. ArcGIS Desktop version 10.8 software (Environmental Systems Research Institute (ESRI), 380 New York Street, Redlands, CA 92373-8100, USA, https://www.esri.com).
The population targeted by the 8 intervention combinations is shown in Table 2. Note that the colors associated with the different intervention combinations are the same as those used in Fig. 12.
The selected intervention combinations will target more than 78% of the total population of Mali.
Discussion
In accordance with the WHO Global Technical Strategy for Malaria 2016–2030, the WHO HBHI initiative, the WHO Framework for Malaria Elimination, the national health priorities for malaria control, and the national malaria strategic plan, a package of interventions was discussed and selected with the NMCP and the different funding partners. Interventions were qualitatively selected based on the following variables: spatial heterogeneity of malaria incidence, prevalence of malaria in children aged 6–59 months, distribution of vector resistance, health facility usage, mortality in children aged 6–59 months, and seasonality of transmission. The impact of the intervention on the burden of malaria, the cost of the intervention, and the resources available for the intervention were also taken into consideration. Eight intervention combinations were selected for implementation in the different health districts. The stratification will be revised every 5 years depending on the evolution of malaria incidence and the availability of new data on parasite and vector resistance.
In the absence of prevalence data at the health district level, incidence data were used for the stratification of malaria risk. Because access to care varies according to the number of health facilities and the availability of community health workers, these incidence data were adjusted for the health facility usage rate to estimate the number of effective cases in the population29,35. It should be noted that these incidence data were of high quality thanks to the DHIS2 data collection platform and the availability of malaria diagnostic tools, particularly RDTs, at the health district level. Incidence data were relevant for our stratification as they are available for free and allow for measuring the temporal evolution of malaria transmission.
In our study, SEIs were created to measure the impact of environmental factors on malaria transmission, and to thus provide a contextual description of the epidemiological facies of malaria in Mali. These SEIs were not considered in the targeting of interventions but were indirectly accounted for because the spatial heterogeneity of malaria incidence and the seasonality of transmission, which were taken into consideration, are correlated with environmental factors in Mali following a north–south gradient. Indeed, Fig. 4 shows that malaria incidence changes as a function of SEIs, while Figs. 5 and 6 indicate that health districts with the highest incidence of malaria are also those with the highest annual rainfall. These findings, however, must be qualified. Different environmental factors do not have the same impact on malaria transmission. They are also correlated with each other: for instance, a rainstorm simultaneously leads to a decrease in temperature and to an increase in humidity and vegetation growth; a spike in temperature results in a reduction or drying up of water sources; etc. This is supported by our analysis of the relationship between malaria transmission and environmental variables, which found that transmission increases with rainfall, humidity and vegetation growth (positive linear relationship with SEI1) but decreases with temperature (negative non-linear relationship with SEI2). Another issue is that the relationship between malaria transmission and environmental factors varies across and between scales (health districts, regions, country). While our study and the study by Ouedraogo et al.36 found a linear relationship between malaria transmission and rainfall/humidity at the national scale, this same relationship was found to be non-linear in Malian studies conducted at the health district scale16,37. These observations suggest the importance of accounting for the differentiated and interrelated impact of environmental factors on malaria transmission in future stratifications of malaria risk. Our study can serve as a template for such stratifications in Mali or other malaria endemic countries.
As our findings indicate, 36.4% of the Malian population lives in areas of high malaria transmission, 34.61% in areas of moderate malaria transmission, and 24.3% in areas of low malaria transmission. Only 4.7% of the population lives in areas of very low malaria transmission. However, malaria transmission has evolved considerably over time due to climate change38. A study conducted in Dire, a health district of Mali located in the Sahelian zone, found that malaria incidence decreased from 120 to 20 cases per 1000 person-years between 2013 and 201737, suggesting that malaria control interventions also have an impact on the intensity of malaria transmission. Because these factors affect the spatial heterogeneity of malaria incidence, the stratification of malaria risk needs to be updated on a regular basis.
Our study found that the health districts located in the Saharan zone have very low malaria transmission and present few risk factors (Figs. 5 and 6). In these health districts, the main intervention will consist in reinforcing epidemiological surveillance. In the future, mass drug administration and molecular surveillance could also be implemented in these districts39,40,41. Given the resurgence of Plasmodium vivax and Plasmodium malariae in the Saharan zone42,43, diagnosis and treatment could be improved by introducing multi-species RDTs44,45. These various strategies could help to achieve malaria pre-elimination. Algeria and Morocco, two countries that border the north of Mali, have succeeded in eliminating malaria using such strategies46,47.
In most health districts, the percentage of the population living outside a radius of 5 km from a health facility is very high (Fig. 7). This explains why the malaria diagnosis rate (number of people tested/number of people with malaria symptoms) was only 88% over the period of data collection (2017–2019). To improve the diagnosis and treatment of malaria, iCCM will be implemented in health districts with high child mortality and low health facility usage48. Existing iCCM sites will be used and new ones created, and all iCCM sites will be provided with additional diagnostic tools. Since iCCM has shown its efficacy in improving general health and fighting malnutrition in low-income countries like Mali35,49, we can expect it to be effective against malaria as well. A study conducted in Zambia has shown that the implementation of iCCM for the diagnosis and treatment of simple malaria improves malaria control50.
In both the Saharan zone and the District of Bamako, child mortality is low (under 100 deaths per 1000 population)2, as is the prevalence of malaria in children aged 6–59 months (2–10% in the Saharan zone and under 1% in Bamako, Figs. 8 and 9). The low prevalence of malaria in children observed in most West African cities51 is explained by urbanization and by the low density of malaria vectors, itself due to low rainfall. In 2018, the NMCP decided to exclude the District of Bamako from SMC interventions and the universal campaign of LLIN distribution because of this low prevalence. However, this decision did not take into consideration variations in malaria transmission levels between urban and peri-urban areas16. Peri-urban areas, which are more conducive to malaria transmission, may need to be reintegrated given the higher malaria incidence in Bamako observed in our study.
One of the major interventions selected in our study is the distribution of LLINs, which is recommended by the WHO to further reduce the burden of malaria. A study conducted in Uganda found that LLINs were responsible for a considerable reduction in malaria cases52. In Mali, however, insecticide resistance appears to be widespread and could compromise the effectiveness of vector control. To further reduce the parasite load, we initially planned to distribute PBO-LLINs and IG2 nets (instead of standard LLINs) in health districts with high malaria incidence and high vector resistance13,53. Yet, because of limited resources and the high cost of these new LLINs, this intervention will be restricted to health districts with proven pyrethroid resistance (25% of all health districts), leaving out neighboring districts with similar climatic characteristics. It should be noted that data on vector resistance are lacking for health districts located in the Saharan zone due to the non-functionality of some sentinel sites (Figs. 10 and 11). The next step will be to map parasite and vector resistance in all health districts to improve the targeting of control interventions.
The IRS intervention was initially planned to be performed in the 11 health districts with the highest malaria incidence. However, due to insufficient financial resources, only 3 health districts located in areas of high transmission will receive IRS in 2022. These 3 districts were selected for two reasons. First, they are located in Mopti region, which has the highest malaria prevalence of the country according to the 2012 DHS. Second, many internally displaced persons (IDPs) have settled in these districts as a result of the security crisis in the north and center of the country. According to the Mali Ministry of Health and a 2021 report by the UN refugee agency, this population alone accounts for 40% of Mali’s IDPs54. Given the high vulnerability of IDPs to malaria, this population should also be targeted by high impact interventions.
Malaria case management and IPTp will continue unchanged in all health districts. Free healthcare will be maintained for standard target groups (children aged 6–59 months, pregnant women) and will be provided to new target groups (IDPs).
As the third pillar of the Global Technical Strategy for Malaria 2016–2030, weekly and monthly surveillance of malaria is currently implemented in all health districts regardless of the risk of transmission. This surveillance will be reinforced in the 27 health districts with epidemic potential via the monitoring and training of health care personnel.
A major strength of our study is that the variables used for the selection of interventions are major determinants of transmission in malaria endemic countries, which ensures the reproducibility of our risk stratification. Another strength is that the targeting of interventions takes into consideration the resources that can be mobilized from all actors (government and donors), unlike what is the case in other countries. Indeed, a 2013 review of NMCPs found that most countries target malaria control interventions on the basis of malaria risk maps that use prevalence or incidence data alone55. A stratification performed in 2020 in the Harari Region of Ethiopia used only malaria incidence data56, while the malaria control interventions implemented in Tanzania in 2020 were based on a stratification of malaria risk that was restricted to incidence data, prevalence data and fever test positivity rates57. Compared to these approaches, our strategy allows for a more cost-effective utilization of resources for malaria control.
The main limitation of our study is the insufficiency of data on vector and parasite resistance and prevalence of malaria in children older than 5 years. Indeed, WHO 2017 Malaria Elimination Framework guidelines recommend using such data when conducting malaria risk stratification. Despite this limitation, our work provides an updated risk stratification using advanced statistical methods to inform the targeting of malaria control interventions that can serve as a template for continuous malaria risk stratifications in Mali and in other countries.
To improve the stratification of malaria risk in Mali, the prevalence of malaria at the health district level should be evaluated in future studies. Moreover, studies should be conducted to update the mapping of Plasmodium species in areas with very low transmission, particularly in the northern part of the country where an increase in the prevalence of Plasmodium malariae and Plasmodium vivax has recently been observed42,43. Indeed, cases may be missed in measures of malaria transmission due to the fact that current diagnostic tools can only detect Plasmodium falciparum.
Conclusion
In this work, a new stratification of malaria risk using advanced statistical methods was performed to inform the targeting of malaria control interventions in Mali. Eight intervention combinations were discussed and qualitatively selected with the NMCP and the different funding partners for implementation in the country’s different health districts. The targeting of interventions was based on the following variables: spatial heterogeneity of malaria incidence, prevalence of malaria in children aged 6–59 months, distribution of vector resistance, health facility usage, mortality in children aged 6–59 months, and seasonality of transmission. The impact of the intervention on the burden of malaria, the cost of the intervention, and the resources available for the intervention were also taken into consideration. Our work can serve as a template for continuous malaria risk stratifications in Mali and in other countries.
Data availability
The data and background maps are available on request from the authors and from the NMCP of Mali.
References
MNCP Mali. Plan Stratégique National de lutte contre le paludisme du Mali 2018–2022 (2018).
CPS/SSDSPF, Institut National de la Statistique (INSTAT), Centre d’Études et d’Information Statistiques (INFO-STAT) & Bamako, Mali. Demographic and Health Survey (DHS) in Mali, 2018 (2019).
Tandina, F. et al. Mosquitoes (Diptera: Culicidae) and mosquito-borne diseases in Mali, West Africa. Parasit Vectors 11, 467 (2018).
Niangaly, A. et al. Plasmodium vivax infections over 3 years in Duffy blood group negative Malians in Bandiagara, Mali. Am. J. Trop. Med. Hyg. 97, 744–752 (2017).
Bernabeu, M. et al. Plasmodium vivax malaria in Mali: A study from three different regions. Malar. J. 11, 405 (2012).
Coetzee, M. et al. Anopheles coluzzii and Anopheles amharicus, new members of the Anopheles gambiae complex. Zootaxa 3619, 246–274 (2013).
Keïta, M. et al. Susceptibilité d’Anopheles gambiae sensu lato aux insecticides communément utilisés dans la lutte antivectorielle au Mali. Bull. Soc. Pathol. Exot. 109, 39–45 (2016).
Cisse, M. B. M. et al. A village level cluster-randomized entomological evaluation of combination long-lasting insecticidal nets containing pyrethroid plus PBO synergist in Southern Mali. Malar. J. 16, 477 (2017).
Cisse, M. B. M. et al. Characterizing the insecticide resistance of Anopheles gambiae in Mali. Malar. J. 14, 327 (2015).
World Health Organization. Global Technical Strategy for Malaria 2016–2030. https://www.who.int/malaria/publications/atoz/9789241564991/en/ (2015).
Shaffer, J. G. et al. Clustering of asymptomatic Plasmodium falciparum infection and the effectiveness of targeted malaria control measures. Malar. J. 19, 33 (2020).
Coulibaly, B. et al. Malaria vector populations across ecological zones in Guinea Conakry and Mali, West Africa. Malar. J. 15, 191 (2016).
Wagman, J. et al. Combining next-generation indoor residual spraying and drug-based malaria control strategies: Observational evidence of a combined effect in Mali. Malar. J. 19, 293 (2020).
Ateba, F. F. et al. Spatio-temporal dynamic of malaria incidence: A comparison of two ecological zones in Mali. Int. J. Environ. Res. Public Health 17, 4698 (2020).
Coulibaly, D. et al. Spatio-temporal dynamics of asymptomatic malaria: Bridging the gap between annual malaria resurgences in a sahelian environment. Am. J. Trop. Med. Hyg. 97, 1761–1769 (2017).
Sissoko, M. S. et al. Temporal dynamic of malaria in a suburban area along the Niger River. Malar. J. 16, 1–10 (2017).
Keïta, M. et al. Transmission vectorielle du paludisme dans un village du bord du fleuve Niger et son hameau de pêche (Kéniéroba et Fourda, Mali). Bull. Soc. Pathol. Exot. 107, 356–368 (2014).
Saugeon, C., Baldet, T., Akogbeto, M. & Henry, M. C. Will climate and demography have a major impact on malaria in sub-Saharan Africa in the next 20 years. Med. Trop. (Mars.) 69, 203–207 (2009).
World Health Organization. A Framework for Malaria Elimination (Organisation Mondiale de la Santé, 2017).
World Health Organization. High Burden to High Impact: A Targeted Malaria Response (World Health Organization, 2018).
Diakalia, K., Drissa, C. & Ogobara, D. PNLP, MRTC and INFORM (2015). An Epidemiological Profile of Malaria in Mali. A Report Prepared for the Ministry of Health, Mali, the Roll Back Malaria Partnership and the Department for International Development, UK. http://www.inform-malaria.org/wp-content/uploads/2015/03/Mali-Malaria-Epi-Profile-Report_030315.pdf (2015).
Dombo, O.; Sankare, O.; Toure, Y. Malaria in the Sahel: The example of Mali. Maladies Tropicales transmissibles, Ed. John Libbey Eurotext 11–32 (1989).
Wetlands International Afrique. Rapport annuel 2019. Wetlands International Afrique. https://africa.wetlands.org/publications/rapport-annuel-2019/.
Justice, C. O. & Hiernaux, P. H. Y. Monitoring the grasslands of the Sahel using NOAA AVHRR data: Niger 1983. Int. J. Remote Sens. 7, 1475–1497 (1986).
Abdoulaye Dao. Surveillance de la résistance des vecteurs du paludisme aux insecticides dans cinq sites sentinelles au Mali, Financé par PSI-Mali—Thèse de Pharmacie. (2020).
Killick, R., Fearnhead, P. & Eckley, I. A. Optimal detection of changepoints with a linear computational cost. J. Am. Stat. Assoc. 107, 1590–1598 (2012).
Chen, J. & Gupta, A. K. Parametric Statistical Change Point Analysis: With Applications to Genetics, Medicine, and Finance (Birkhäuser, 2012). https://doi.org/10.1007/978-0-8176-4801-5.
Husson, F., Lê, S. & Pagès, J. Analyse De Données Avec R. Presses universitaires de Rennes. (2009).
Thwing, J. et al. A robust estimator of malaria incidence from routine health facility data. Am. J. Trop. Med. Hyg. 102, 811–820 (2020).
DGSHP - Mali. Annuaire Statistique du Système Local d’Information Sanitaire du Mali (2020).
Ghimire, P. et al. The durability of long-lasting insecticidal nets distributed to the households between 2009 and 2013 in Nepal. Trop. Med. Health 48, 36 (2020).
Hill, J. et al. Effectiveness of antenatal clinics to deliver intermittent preventive treatment and insecticide treated nets for the control of malaria in pregnancy in Mali: A household survey. PLoS ONE 9, e92102 (2014).
World Health Organization. WHO policy brief for the implementation of intermittent preventive treatment of malaria in pregnancy using sulfadoxine-pyrimethamine (IPTp-SP). https://apps.who.int/iris/handle/10665/338350 (2014).
Bah, M. et al. Combining next-generation indoor residual spraying and drug-based malaria control strategies: Observational evidence of a combined effect in Mali. Malar. J. https://doi.org/10.1186/s12936-020-03361-y (2020).
Rowe, A. K. et al. Caution is required when using health facility-based data to evaluate the health impact of malaria control efforts in Africa. Malar. J. 8, 209 (2009).
Ouedraogo, B. et al. Spatio-temporal dynamic of malaria in Ouagadougou, Burkina Faso, 2011–2015. Malar. J. 17, 1–12 (2018).
Cissoko, M. et al. Geo-epidemiology of malaria at the health area level, dire health district, Mali, 2013–2017. Int. J. Environ. Res. Public Health 17, 3982 (2020).
World Health Organization. World Malaria Report 2018. https://www.who.int/malaria/publications/world-malaria-report-2018/report/en/ (2018).
Dayananda, K. K., Achur, R. N. & Gowda, D. C. Epidemiology, drug resistance, and pathophysiology of Plasmodium vivax malaria. J. Vector Borne Dis. 55, 1–8 (2018).
Erdman, L. K. & Kain, K. C. Molecular diagnostic and surveillance tools for global malaria control. Travel Med. Infect. Dis. 6, 82–99 (2008).
World Health Organization. Mass Drug Administration for Falciparum Malaria: A Practical Field Manual. 112. https://apps.who.int/iris/handle/10665/259367 (2017).
Koita, O. A. et al. Effect of seasonality and ecological factors on the prevalence of the four malaria parasite species in northern Mali. J. Trop. Med. 2012, 367160 (2012).
Bernabeu, M. et al. Plasmodium vivax malaria in Mali: A study from three different regions. Mala. J. 11, 405 (2012).
Mukkala, A. N. et al. An update on malaria rapid diagnostic tests. Curr. Infect. Dis. Rep. 20, 49 (2018).
Mosquera-Romero, M., Zuluaga-Idárraga, L. & Tobón-Castaño, A. Challenges for the diagnosis and treatment of malaria in low transmission settings in San Lorenzo, Esmeraldas, Ecuador. Malar. J. 17, 440 (2018).
de Jesus, E. I. G. Algeria and Argentina declared malaria-free. Nature https://doi.org/10.1038/d41586-019-01684-8 (2019).
Ba, O., Ouldabdallahi, M., Koïta, M., Sy, O. & Dahdi, S. A. Epidemiology of malaria and elimination prospects in Maghreb Countries. Tunis. Med. 96, 590–598 (2018).
Malaria Consortium. Malaria Consortium—Integrated Community Case Management (iCCM). https://www.malariaconsortium.org/pages/integrated_community_case_management.htm.
Charle-Cuéllar, P. et al. Impact of different levels of supervision on the recovery of severely malnourished children treated by community health workers in Mali. Nutrients 13, 367 (2021).
Hamer, D. H. et al. Quality and safety of integrated community case management of malaria using rapid diagnostic tests and pneumonia by community health workers. Pathog. Glob. Health 106, 32–39 (2012).
Baragatti, M. et al. Social and environmental malaria risk factors in urban areas of Ouagadougou, Burkina Faso. Malar. J. 8, 13 (2009).
Musiime, A. K. et al. Impact of vector control interventions on malaria transmission intensity, outdoor vector biting rates and Anopheles mosquito species composition in Tororo, Uganda. Malar. J. 18, 445 (2019).
Wagman, J. et al. An observational analysis of the impact of indoor residual spraying with non-pyrethroid insecticides on the incidence of malaria in Ségou Region, Mali: 2012–2015. Malar. J. 17, 19 (2018).
DNDS-MALI, OIM, UNHCR. Matrice de suivi des Déplaces Internes. https://reliefweb.int/report/mali/mali-rapport-matrice-de-suivi-des-d-placements-dtm-juillet-2021 (2021).
Omumbo, J. A., Noor, A. M., Fall, I. S. & Snow, R. W. How well are malaria maps used to design and finance malaria control in Africa?. PLoS ONE 8, e53198 (2013).
Esayas, E. et al. Malaria epidemiology and stratification of incidence in the malaria elimination setting in Harari Region, Eastern Ethiopia. Infect. Dis. Poverty 9, 160 (2020).
Thawer, S. G. et al. Sub-national stratification of malaria risk in mainland Tanzania: A simplified assembly of survey and routine data. Malar. J. 19, 177 (2020).
Acknowledgements
We also thank the WHO offices Geneva and Bamako, the French ARTS grant from the French Research Institute for Development France (IRD) and by the JEAI (young team associated with the IRD) “Spatiotemporal Dynamics of Malaria Transmission in Changing Environments (DynaSTEC). The Non-Governmental Organization Prospective et Cooperation, which also provided technical support. We are very grateful to the National Malaria Control Program (NMCP) of Mali. We would also like to thank Abdoulaye Ongoiba at MRTC, IECR, USTTB, Mali for providing training on ArcGIS as well as Jennifer L. Kwan, PhD at NIAID, NIH, US for facilitating the acquisition of ArcGIS. Lastly, we would like to thank the Health and Human Services Grant Program at ESRI for providing the ArcGIS desktop software license and training to Mali Ministry of Health used for malaria data mapping.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
M.C. and I.S. designed the study; V.S., M.M., and L.S. conducted the field study coordinated by M.C.; M.C., J.G., and I.S. developed the statistical analysis plan with the participation of LS, MD, AKO, CSB, MDB, AMD, SD, and MBC; M.C. carried out the statistical analysis under the supervision of I.S., J.L., and J.G.; M.C. performed the cartographic analysis with the participation of AMD; I.S., J.G., D.T., I.C., B.S., and M.S. participated in the statistical analysis and validated the results; M.C., I.S., and J.G. drafted the manuscript; all authors read and approved to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cissoko, M., Magassa, M., Sanogo, V. et al. Stratification at the health district level for targeting malaria control interventions in Mali. Sci Rep 12, 8271 (2022). https://doi.org/10.1038/s41598-022-11974-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-11974-3
This article is cited by
-
Malaria risk stratification in Lao PDR guides program planning in an elimination setting
Scientific Reports (2024)
-
Malaria prevalence in Mauritania: a systematic review and meta-analysis
Malaria Journal (2023)
-
Geo-epidemiology of malaria incidence in the Vhembe District to guide targeted elimination strategies, South-Africa, 2015–2018: a local resurgence
Scientific Reports (2023)
-
Spatio-temporal modelling of routine health facility data for malaria risk micro-stratification in mainland Tanzania
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.