Abstract
To establish whether obesity involves activation of endogenous ciliary neurotrophic factor (CNTF) signalling, we evaluated its plasma levels in patients with obesity and correlated its values with the major clinical and haematological indices of obesity, insulin resistance and systemic inflammation. This study involved 118 subjects: 39 healthy controls (19 men), 39 subjects with obesity (19 men) and 40 subjects with obesity and diabetes (20 men). Plasma CNTF and CNTF receptor α (CNTFRα) were measured using commercial ELISA kits. The results showed that plasma CNTF was significantly higher in males and females with obesity with and without diabetes than in healthy subjects. Women consistently exhibited higher levels of circulating CNTF. In both genders, CNTF levels correlated significantly and positively with obesity (BMI, WHR, leptin), diabetes (fasting insulin, HOMA index and HbA1c) and inflammation (IL-6 and hsCRP) indices. Circulating CNTFRα and the CNTF/CNTFRα molar ratio tended to be higher in the patient groups than in controls. In conclusion, endogenous CNTF signalling is activated in human obesity and may help counteract some adverse effects of obesity. Studies involving a higher number of selected patients may reveal circulating CNTF and/or CNTFRα as potential novel diagnostic and/or prognostic markers of obesity, diabetes and associated diseases.
Similar content being viewed by others
Introduction
Ciliary neurotrophic factor (CNTF) belongs to the interleukin (IL)-6 cytokine family along with IL-11, oncostatin M, leukaemia inhibitory factor (LIF), cardiotrophin (CT)-1 and CT-like cytokine factor 11,2. Rather than sequence homology, these cytokines share a four-α helical bundle structure containing discrete motifs responsible for binding high-affinity α receptors on target cells. Binding of CNTF to CNTF receptor α (CNTFRα) is followed by interaction with LIF receptor β (LIFRβ) on target cells and by activation of the widely distributed signal transducing protein gp1302,3. Originally identified for its role in supporting the survival of chick ganglion neurons during development in vitro4, CNTF has subsequently been shown to exhibit multiple and pleiotropic effects during nerve tissue development, including proliferation of neural progenitors, differentiation of sympathetic neurons, maturation of glial progenitor cells into astrocytes and oligodendrocytes and postnatal maintenance of sensory and motor neurons5,6.
Studies performed in experimental animals have found that CNTF is produced by a limited number of cell types in the body, including astrocytes and tanycytes in the brain7,8,9,10, Schwann cells in peripheral nerves7,11 and bone cells12. Interestingly, the cntf gene lacks a sequence coding for a peptide signal capable of directing the synthesis and secretion of the protein via the classic endoplasmic reticulum-Golgi pathway, and there is evidence that CNTF is a cytosolic protein11. These observations, together with the rapid upregulation of the cntf gene, seen in grey matter astrocytes after lesion or deafferentation8,13,14 and in sciatic nerve Schwann cells in response to lesion15,16, suggest a role for CNTF as a protective factor which becomes available after cell injury, death or other types of stress to repair target cells through paracrine and/or endocrine signalling. In this context, increased CNTF levels in cerebrospinal fluid and/or blood have been described in some human conditions characterized by variably extensive and/or evident tissue damage, including acute disseminated encephalomyelitis17, sporadic and familial amyotrophic lateral sclerosis (ALS)18,19, focal epilepsy20, autism21 and septic shock22.
The involvement of CNTF in human body metabolism has serendipitously been discovered in clinical trials evaluating its efficacy in ALS patients; although administration of human recombinant CNTF did not ameliorate their motor symptoms, it unexpectedly induced weight loss23,24. The weight-reducing effect of Axokine—a recombinant form of human CNTF characterized by greater efficacy and specificity—was later confirmed in a population of leptin-resistant patients with obesity, although the clinical trials were discontinued after a significant number of patients developed autoantibodies25. Studies of experimental animals have found that intravenous/intraperitoneal CNTF/Axokine acts on hypothalamic centres to reduce food intake, it induces adipose tissue lipolysis, stimulates brown fat-dependent non-shivering thermogenesis, promotes insulin sensitivity and reduces liver steatosis26,27. The metabolic effects of exogenous CNTF rely on the widespread distribution of CNTFRα, which, in striking contrast to its ligand, is widely expressed in metabolically important organs and cell types such as skeletal muscle fibres, white and brown adipocytes, hepatocytes, pancreatic islet cells and intestinal cells26,27,28, [freely available from the GTEx Portal].
Obesity is a chronic disorder associated with complex, interrelated and persistent metabolic dysfunctions, endocrine abnormalities and inflammatory conditions that over time affect and damage specific tissues and organs and impair some cellular functions, eventually leading to obesity-associated diseases such as insulin resistance, type 2 diabetes (T2D), non-alcoholic steatohepatitis, atherosclerosis, cardiovascular disease and even some cancers29,30,31.
In obesity, some types of tissue/cell damage may involve activation of endogenous CNTF signalling which, by virtue of its catabolic action, has the potential to counteract some adverse effects of obesity and of the associated metabolic syndrome. In this observational study, we measured circulating CNTF and CNTFRα in a cohort of patients with obesity with or without diabetes and in age- and gender-matched healthy subjects. Collectively, our findings indicate that circulating CNTF levels are upregulated in such patients and that they correlate with several clinical and/or haematological indices of obesity, inflammation and insulin resistance.
Materials and methods
Patients
Blood samples were obtained from 118 patients (median age, 60 years; interquartile range, IQR, 56–65) admitted to the Italian National Research Centre on Aging (INRCA), Ancona, Italy32. All subjects provided written informed consent and the original study protocol was approved by the Institutional Review Board of IRCCS INRCA hospital (approval no. 34/CdB/03). The study was carried out in accordance with the Declaration of Helsinki. Participants came from the provinces of Ancona, Ascoli Piceno, Macerata, Fermo and Pesaro-Urbino (central Italy) and provided information such as vital signs, anthropometric measures, medical history and behavioural data including diet and physical activity. All subjects consumed a Mediterranean diet. They were categorized into 3 age- and gender-matched groups (Table 1): control subjects (C; n = 39, 19 men), patients with obesity (O; n = 39, 19 men) and patients with obesity and T2D (OD; n = 40, 20 men). Control subjects came from a larger population enrolled in a T2D prevention programme and were considered healthy because at the time of blood collection they had normal haematological parameters and were free of clinically evident major diseases. Obesity was defined as a body mass index (BMI) ≥ 30 kg/m2 according to the World Health Organization classification. T2D was diagnosed according to American Diabetes Association criteria33. The presence or absence of T2D complications was established as described previously34. Of the 40 patients with obesity and diabetes, 17 had at least one T2D complication and 11 patients had 2 or more; specifically, 8 patients had diabetic neuropathy, 8 had diabetic nephropathy, 6 had diabetic retinopathy, 9 had atherosclerotic vascular disease and 6 had a history of major adverse cardiovascular events. At the time of enrolment, 36 patients with obesity and diabetes were receiving the following medications, alone or in combination: 23, metformin; 15, sulphonylureas; 2, glinides; 5, insulin.
Laboratory assays
Peripheral venous blood was drawn from all participants in the morning after overnight fasting. EDTA plasma was obtained within 4 h by centrifugation at 2.000 rpm for 20 min at 4 °C and frozen and stored at − 80 °C until use. The biochemical parameters were assessed by standard procedures. Estimated glomerular filtration rate (eGFR) was calculated according to the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation based on serum creatinine, age, gender and ethnicity35. The homeostasis model assessment (HOMA) index was calculated as fasting insulin (μU/mL) × fasting glucose (mg/dL)/40536. The plasma concentrations of CNTF, CNTFRα, leptin, adiponectin, plasminogen activator inhibitor-1 (PAI-1) antigen and IL-6 were assessed using the following ELISA kits according to the manufacturer’s instructions: Human Ciliary Neurotrophic Factor ELISA kit (# CSB-E04527h, Cusabio, Houston, TX, USA); Human CNTFRα ELISA kit (# LS-F49895, LSBio, Seattle, WA, USA); Human Leptin ELISA kit (# EZHL-80SK, Millipore, MO, USA); Human Adiponectin ELISA kit (# AG-45A-0001YEK-KI01, Adipogen Life Sciences, Switzerland); Human PAI-1 ELISA Kit (# RAB0429, Sigma- Aldrich, Missouri, USA); Human IL-6 Quantikine HS ELISA kit (# HS600C, RRID:AB_2893335, R&D System, Minneapolis, MN, USA). All measurements were performed in duplicate. Missing data were as follows: fasting glucose, total cholesterol, HDL-cholesterol, triglycerides, azotaemia, eGFR, creatinine, uric acid, alkaline phosphatase, aspartate aminotransferase and alanine aminotransferase, 1; adiponectin and leptin, 3; IL-6, 6; and fibrinogen, 24.
Statistical analysis
The power of the study was assessed for plasma CNTF and CNTFRα levels using two-way analysis of variance, a significance level of 5% and the partial omega-squared effect size, which provides a less biased estimate in studies involving a small sample size37. The sample size of the study ensured a power greater than 99% to test an effect size of 0.39 for the three-level factor (healthy controls, patients with obesity and patients with obesity and diabetes) and an effect size of 0.27 for the two-level factor (males and females); it had a power of 91% to test an effect size of 0.09 for the interaction between the two factors. The clinical characteristics and plasma parameters of participants were analysed according to their health conditions. Since the quantitative variables did not follow a normal distribution, a non-parametric approach was employed. The quantitative variables were summarized using the median and IQR. Comparisons among conditions were evaluated with the Kruskal–Wallis test. Spearman coefficients and 95% confidence intervals (95% CI) stratified by gender were calculated to estimate the correlation between CNTF and (i) indices of obesity (BMI and waist-to-hip ratio [WHR]); (ii) parameters of glucidic metabolism (fasting glucose, fasting insulin, glycosylated haemoglobin [HbA1c] and HOMA index); (iii) lipid profile (total cholesterol, HDL-cholesterol, LDL-cholesterol, apolipoprotein A1, apolipoprotein B and triglycerides); (iv) kidney function (azotaemia, eGFR, creatinine and uric acid); (v) liver function (alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase); (vi) inflammatory indices (high-sensitivity C-reactive protein [hsCRP] and IL-6); (vii) prothrombotic factors (fibrinogen and PAI-1); (vi) adipokines (leptin and adiponectin); and (viii) plasma CNTFRα levels. The correlation coefficients were considered significant if the 95% CI did not include the zero value. The multiple quantile regression model was applied to evaluate the association of CNTF and CNTFRα, the dependent variables, while health condition and gender were considered as independent variables, by estimating the 50th percentile. The point and 95% CI of regression coefficients for each independent variable and the interaction term were estimated; in the regression model of CNTF, the interaction term between fasting insulin and gender was also included in the analysis. The regression coefficients were considered significant when the 95% CI did not include the zero value. All analyses were performed using R software 4.0.5.
Results
Plasma CNTF is increased in men and women with obesity with or without diabetes
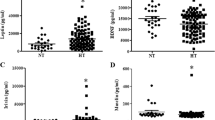
As reported in Table 1, the clinical data of participants showed that there were no significant differences in age or gender distribution among the groups. Notably, patients with obesity and patients with obesity and diabetes showed significantly higher BMI, WHR, fasting insulin, hsCRP and leptin and significantly lower HDL-cholesterol and adiponectin levels than Control subjects. Fasting glucose, the HOMA index, triglycerides and IL-6 rose progressively from participants in the Control to Obesity to Obesity and Diabetes cohorts. In the Obesity and Diabetes group, HbA1c and fibrinogen were higher than in the Obesity and Control groups, whereas creatinine was higher and eGFR lower than in Control group. CNTF was detected in the plasma of all subjects. Notably, the two patient groups had significantly higher plasma CNTF than Control subjects; however, although patients with obesity and diabetes exhibited a higher median plasma CNTF level than those with obesity alone, the difference was not statistically significant (Fig. 1A).
Obesity is a sexually dimorphic disease and even its complications have a different incidence and prevalence in men and women29,30,31. Accordingly, plasma CNTF levels were stratified both by clinical condition and gender. Among women, BMI, WHR, fasting insulin, hsCRP, IL-6 and leptin values were higher and adiponectin was lower in the two patient groups than in healthy participants. The HOMA index progressively increased in the three groups. Patients with obesity and diabetes had higher fasting glucose, HbA1c and triglycerides than subjects with obesity or controls, while having significantly lower HDL-cholesterol and eGFR than Control participants (Supplementary Table S1). Among men, the two patient groups had significantly higher BMI, WHR, fasting insulin and leptin and significantly lower HDL-cholesterol levels than Control subjects. In patients with obesity and diabetes, fasting glucose, the HOMA index, HbA1c and IL-6 were significantly higher than in participants in the obesity group and control group. In addition, they showed significantly higher triglycerides, hsCRP and fibrinogen and significantly lower eGFR values than Control subjects. Adiponectin was significantly lower in patients with obesity than in the other two groups (Supplementary Table S2). All female and male patients had significantly higher median values of circulating CNTF than healthy subjects, with marked differences between the genders (Fig. 1B); in particular, unlike women with obesity with or without diabetes, whose CNTF levels were comparable, men with obesity and diabetes showed a higher median CNTF plasma concentration than men with obesity, even though the difference was not statistically significant. In all three groups, circulating CNTF was always higher in women than in men; in particular, the median value was about 2.5-fold higher in Control women, more than 3-fold higher in women with obesity, and about 1.5-fold higher in women with obesity and diabetes. The multiple quantile regression analysis confirmed the independent effect of obesity, alone or associated with diabetes, and gender on plasma CNTF levels; in addition, fasting insulin was positively associated with plasma CNTF, with higher levels in females (Supplementary Table S3).
Collectively, these data show that obesity, with or without diabetes, is associated with increased circulating CNTF levels in both genders. However, whereas in women CNTF secretion was higher in the Obesity group, in men it was higher in group with obesity and diabetes. This suggests that different, gender-related mechanisms underpin its production and secretion in patients with obesity and metabolic syndrome.
Circulating CNTF correlates with obesity, insulin resistance and inflammation indices
To establish whether circulating CNTF correlates with the major clinical and/or haematological parameters of obesity or metabolic syndrome, we calculated the Spearman correlation coefficient and 95% CI between plasma CNTF concentrations and the parameters listed in Table 1 in each gender, pooling the data of all male and female participants. The values of the Spearman correlation coefficient were graded as follows: 0.20–0.40, weak; 0.41–0.60, moderate; 0.61–0.80 strong; 0.81–1, very strong correlation.
In women (Fig. 2), plasma CNTF showed a moderate correlation with WHR; notably, there was a strong correlation with BMI, the insulin resistance indices fasting insulin and HOMA index and the inflammatory markers hsCRP and IL-6, as well as a very strong correlation with leptin. There was a weak positive correlation with fasting glucose and HbA1c and a weak negative correlation with adiponectin, whose circulating levels are inversely related with body weight, visceral fat accumulation and metabolic syndrome38. A weak correlation was also found with the prothrombotic markers PAI-1 and fibrinogen. Finally, plasma CNTF exhibited a moderate correlation with triglycerides and some markers of renal function, including azotaemia and uric acid, and a weak inverse correlation with HDL-cholesterol and eGRF (Supplementary Fig. S1).
In men (Fig. 3), circulating CNTF showed a weak correlation with WHR; notably, similar to women, there was a strong or very strong correlation with BMI and leptin, respectively. Importantly, there was also a positive correlation with fasting glucose, fasting insulin, HOMA index, HbA1c and the inflammatory markers hsCRP and IL-6. At variance with women, plasma CNTF showed a barely appreciable inverse correlation with adiponectin and a moderate correlation with a single prothrombotic factor, fibrinogen. Finally, there was a weak positive association with triglycerides and creatinine and a weak negative correlation with eGFR and HDL-cholesterol (Supplementary Fig. S2).
Plasma CNTFRα and the CNTF/CNTFRα molar ratio tend to increase in patients with obesity
Soluble CNTFRα is detectable in serum and cerebrospinal fluid39. Importantly, murine and human cell lines not usually responsive to CNTF become responsive when treated with a combination of CNTF and the soluble form of CNTFRα39. These data suggest that, as originally shown for IL-640, systemic CNTF signalling may be sustained, and amplified, by trans-signalling. As a result, the coexistence in the blood of CNTF and its receptor would lead to the formation of a circulating heterodimer that induces responses in discrete target cells originally devoid of the specific cytokine receptor but endowed with LIFRβ and gp130. To determine whether this was the case for CNTF signalling in patients with obesity, we measured plasma CNTFRα and found a progressive increase in median CNTFRα values from the Control group to the Obesity group and the Obesity and Diabetes group, although the difference achieved significance only between patients with obesity and diabetes compared with the Control subjects (Fig. 4A). Gender stratification disclosed lower median CNTFRα concentrations in healthy men and higher CNTFRα levels in women with obesity and diabetes; no further gender-related patterns were evident (Fig. 4B). Interestingly, the presence in each patient group of a substantial number of samples with high/very high CNTFRα levels suggests that in selected patient categories, which were probably underrepresented in our cohort, CNTFRα may be markedly upregulated. Finally, no significant correlation was found between CNTF and CNTFRα plasma levels either in women or in men (data not shown). The results of multiple quantile regression analysis, showing an independent effect of obesity associated with diabetes and gender on plasma CNTF levels, are reported in Supplementary Table S4.
Since the molecular ratio of CNTF-CNTFRα complexes is 1:141, we calculated the CNTF/CNTFRα molar ratio and used it as an index of free circulating CNTF. The ratio was always positive and was higher in patients with obesity with or without diabetes than in healthy subjects, even though the difference was significant only between the Obesity and Control groups (Fig. 4C). Gender stratification confirmed that patients, especially women with obesity, tended to show a higher ratio, but the difference was never significant (Fig. 4D). Collectively, these results demonstrate that human blood contains a higher number of molecules of CNTF than of CNTFRα and that free CNTF tends to increase in patients with obesity.
Discussion
This cross-sectional study demonstrates for the first time that circulating CNTF values are higher in patients with obesity and in patients with obesity and diabetes than in healthy subjects, and that its plasma concentrations are significantly associated with obesity, diabetes and inflammation indices. Collectively, these findings suggest that in patients with obesity CNTF signalling is activated in a still unknown tissue/organ and that the peptide is released into the circulation, potentially transducing signals to distant organs and affecting body metabolism.
Even in obese patients, where circulating CNTF was highest, its median levels were 2 ng/ml or less. In vitro studies have found variable, often higher, EC50 values of human CNTF ranging from 90 ± 45 pg/ml42 to 60 ng/ml43, depending on the cell type studied and the biological effects measured. Thus, the increase in circulating CNTF documented in our study could also reflect passive leakage of the peptide into the circulation following CNTF signalling activation in a still uncharacterized site of the body. However, evidence of activation of the specific Janus kinase-signalling transducer and activator of transcription 3 pathway in human adipocytes at a concentration as low as 2 ng/ml44 and other possible variables, including linkage with plasma protein and trans-signalling, lend support to the notion that the CNTF oversecreted in patients with morbid obesity may act on discrete cell types at specific sites and play adaptive metabolic roles.
To date, animal studies have failed to provide strong evidence of a role for endogenous CNTF in energy balance control; importantly, neither obesity nor hyperphagia have been described in CNTF-knockout mice45, whereas studies of humans with genetic CNTF deficiency do not allow firm conclusions to be drawn46,47. However, possible metabolic functions of endogenous CNTF may be revealed under distinctive metabolic challenges that may give rise to tissue and cell damage. Our group has studied CNTF and CNTFRα expression and the distribution of CNTF-producing and CNTF-responsive cells in the hypothalamus of mice rendered obese by a high-fat diet or fed a calorie restriction diet. We found that obesity is associated with increased CNTF hypothalamic signalling, whereas calorie restriction induced its reduction48. These data support the hypothesis that, at least at the level of the hypothalamus, obesity can activate endogenous CNTF signalling, which may be involved in energy balance regulation. Notably, aside from the debated role of IL-6 in obesity and insulin resistance, other members of the IL-6 family of cytokines, including CT-149, are oversecreted in obesity and affect the energy balance.
In animal models, it has consistently been demonstrated that CNTF administration not only reduces food intake by acting on hypothalamic50,51,52 and brainstem53 feeding centres, but that it also improves obesity-associated hyperglycaemia, hyperinsulinaemia and hyperlipidaemia via metabolic effects exerted through actions on peripheral organs26,27. In skeletal muscle, exogenous CNTF increases fatty acid oxidation and reduces insulin resistance54; in the liver of db/db obese mice55 and of obese rats fed a high-fat diet56, it reduces hepatic steatosis and enhances insulin responsiveness; in mice with alloxan-induced57 and streptozotocin-induced58 diabetes it protects pancreatic islet cells from cytokine-induced apoptosis, it increases β cell mass and reduces insulin clearance. CNTF also exerts important effects on adipose tissue; in particular, reduces lipogenesis and promotes mitochondrial biogenesis, fatty acid oxidation and insulin sensitivity in white adipocytes44,59 and enhances β3-adrenergic induction of mitochondrial uncoupling protein 1 in brown adipocytes60, possibly promoting thermogenesis-dependent energy expenditure. Although some of these effects may be amplified by the supraphysiological doses of exogenous CNTF used in the experiments, these findings collectively indicate that CNTF exerts central and peripheral anti-obesogenic effects and that in subjects with obesity and diabetes its overproduction could counteract some of the adverse aspects of morbid obesity and metabolic syndrome by reducing energy intake, promoting energy consumption and improving insulin resistance.
The source(s) of endogenous circulating CNTF are unknown. The few cell types where it is known to be constitutively expressed are clearly probable candidates. In patients with obesity, glial cell-derived CNTF may diffuse to the general circulation through the leaking blood–brain barrier61, whereas at the periphery metabolic, inflammatory and/or mechanical stress could instigate CNTF secretion by Schwann and/or bone cells. Extensive screening of tissue and cell types from experimental animals challenged with specific metabolic stimuli and selective knockout mouse models are clearly required to identify the cellular source(s) of circulating CNTF.
Interestingly, our data document marked differences between men and women, since the latter show higher constitutive and inducible levels of circulating CNTF. Whereas this finding suggests that sex hormones may instigate differential CNTF production and secretion, the higher amount of this metabolically beneficial cytokine may be involved in the lower and later incidence of some obesity-related complications in female patients29,30,31.
The association between CNTF levels and obesity with or without diabetes was confirmed even when the multiple regression analysis was adjusted for fasting insulin and the interaction between fasting insulin and gender. The positive correlation between fasting insulin levels and body fat mass62 suggests the interesting hypothesis that circulating CNTF levels may correlate with adiposity, and that the gender differences observed in our study reflect differences in body fat content between men and women. The accurate measurement of body fat mass by appropriate techniques may enable to establish whether plasma CNTF levels correlate with adiposity and, conceivably, with greater fat accumulation at specific sites (visceral and intra-organ), where it plays a prominent role in the pathophysiology of morbid obesity.
Although human CNTF displays high affinity for CNTFRα, it also binds both membrane-bound and soluble human IL-6 receptor α63. Consequently, the high CNTF levels measured in the patient groups may also promote IL-6 signalling, or trans-signalling, compounding the metabolic effects of circulating CNTF. The pleiotropic and redundant functions of IL-6 cytokine family members are also mediated by trans-signalling, as clearly described for IL-640 and IL-1164. Trans-signalling occurs when cytokine receptors, secreted or cleaved from the cell membrane, form a circulating complex with the cytokine and extend the signal to a higher number of target cells. CNTFRα lacks a conventional transmembrane domain and is anchored to the cell membrane by a glycosyl-phosphatidylinositol linkage39. Thus, specific phospholipases may break down this linkage and elicit the regulated release of the receptor, enabling trans-signalling. In some conditions the circulating heterodimeric complex CNTF-CNTFRα may therefore act on target cells expressing the widely distributed LIFRβ and gp130 proteins, eliciting and/or amplifying responsiveness to circulating CNTF. Even though the higher levels of circulating CNTFRα found in patients with obesity with or without diabetes, agree with the activation of systemic CNTF signalling in obesity, they do not allow conclusions to be drawn with regard to a specific role of CNTF trans-signalling in the pathophysiology of obesity. Finally, the activation of endogenous CNTF signalling in obesity is supported by the higher CNTF/CNTFRα molar ratio found in these subjects, where a larger number of free circulating CNTF molecules are available to signal to CNTFRα-expressing target cells.
In conclusion, human obesity is associated with high plasma CNTF levels, which may have a role in counteracting obesity-induced tissue damage, inflammation and dysmetabolism. Further insights into the metabolic role of endogenous CNTF in homeostatic and pathological conditions and of its sources, target cells and signalling pathways are expected to provide a better understanding of the pathophysiology of metabolic syndrome and improve treatment. Notably, studies involving a higher number of selected patients may disclose circulating CNTF as a potential novel diagnostic and/or prognostic marker of obesity, diabetes and associated diseases.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Kang, S., Narazaki, M., Metwally, H. & Kishimoto, T. Historical overview of the interleukin-6 family cytokine. J. Exp. Med. 217, e20190347 (2020).
Metcalfe, R. D., Putoczki, T. L. & Griffin, M. D. W. Structural understanding of interleukin 6 family cytokine signaling and targeted therapies: Focus on interleukin 11. Front. Immunol. 11, 1424 (2020).
Heinrich, P. C. et al. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 374, 1–20 (2003).
Adler, R., Landa, K. B., Manthorpe, M. & Varon, S. Cholinergic neuronotrophic factors: Intraocular distribution of trophic activity for ciliary neurons. Science 204, 1434–1436 (1979).
Sleeman, M. W., Anderson, K. D., Lambert, P. D., Yancopoulos, G. D. & Wiegand, S. J. The ciliary neurotrophic factor and its receptor, CNTFRα. Pharm. Acta Helv. 74, 265–272 (2000).
Deverman, B. E. & Patterson, P. H. Cytokines and CNS development. Neuron 64, 61–78 (2009).
Stockli, K. A. et al. Molecular cloning, expression and regional distribution of rat ciliary neurotrophic factor. Nature 42, 920–923 (1989).
Guthrie, K. M., Woods, A. G., Nguyen, T. & Gall, C. M. Astroglial ciliary neurotrophic factor mRNA expression is increased in fields of axonal sprouting in deafferented hippocampus. J. Comp. Neurol. 386, 137–148 (1997).
Dallner, C., Woods, A. G., Deller, T., Kirsch, M. & Hofmann, H.-D. CNTF and CNTF receptor alpha are constitutively expressed by astrocytes in the mouse brain. Glia 37, 374–378 (2002).
Severi, I., Carradori, M. R., Amici, A., Cinti, S. & Giordano, A. Constitutive expression of ciliary neurotrophic factor in mouse hypothalamus. J. Anat. 220, 622–631 (2012).
Stöckli, K. A. et al. Regional distribution, developmental changes, and cellular localization of CNTF-mRNA and protein in the rat brain. J. Cell Biol. 115, 447–459 (1991).
McGregor, N. E. et al. Ciliary neurotrophic factor inhibits bone formation and plays a sex-specific role in bone growth and remodeling. Calcif. Tissue Int. 86, 261–270 (2010).
Lee, M. Y., Deller, T., Kirsch, M., Frotscher, M. & Hofmann, H. D. Differential regulation of ciliary neurotrophic factor (CNTF) and CNTF receptor alpha expression in astrocytes and neurons of the fascia dentata after entorhinal cortex lesion. J. Neurosci. 134, 1137–1146 (1997).
Watt, J. A., Bone, S., Pressler, M., Cranston, H. J. & Paden, C. M. Ciliary neurotrophic factor is expressed in the magnocellular neurosecretory system of the rat in vivo: Evidence for injury- and activity-induced upregulation. Exp. Neurol. 197, 206–214 (2006).
Sendtner, M., Stöckli, K. A. & Thoenen, H. Synthesis and localization of ciliary neurotrophic factor in the sciatic nerve of the adult rat after lesion and during regeneration. J. Cell Biol. 118, 139–148 (1992).
Friedman, B. Regulation of ciliary neurotrophic factor expression in myelin-related Schwann cells in vivo. Neuron 9, 295–305 (1992).
Ichiyama, T. et al. Elevated cerebrospinal fluid level of ciliary neurotrophic factor in acute disseminated encephalomyelitis. J. Neurol. Sci. 177, 146–149 (2000).
Iłzecka, J. Increased serum CNTF level in patients with amyotrophic lateral sclerosis. Eur. Cytokine Netw. 14, 192–194 (2003).
Laaksovirta, H., Soinila, S., Hukkanen, V., Röyttä, M. & Soilu-Hänninen, M. Serum level of CNTF is elevated in patients with amyotrophic lateral sclerosis and correlates with site of disease onset. Eur. J. Neurol. 15, 355–359 (2008).
Shpak, A. et al. Increased ciliary neurotrophic factor in blood serum and lacrimal fluid as a potential biomarker of focal epilepsy. Neurol. Sci. 43, 493–498 (2022).
Brondino, N. et al. Increased CNTF levels in adults with autism spectrum disorders. World J. Biol. Psychiatry 20, 742–746 (2019).
Guillet, C., Fourcin, M., Chevalier, S., Pouplard, A. & Gascan, H. ELISA detection of circulating levels of LIF, OSM, and CNTF in septic shock. Ann. N. Y. Acad. Sci. 762, 407–409 (1995).
ACTS. A double-blind placebo-controlled clinical trial of subcutaneous recombinant human ciliary neurotrophic factor (rHCNTF) in amyotrophic lateral sclerosis. ALS CNTF Treatment Study Group. Neurology 46, 1244–1249 (1996).
Miller, R. G. et al. A placebo-controlled trial of recombinant human ciliary neurotrophic (rhCNTF) factor in amyotrophic lateral sclerosis. rhCNTF ALS Study Group. Ann. Neurol. 39, 256–260 (1996).
Ettinger, M. P. et al. Recombinant variant of ciliary neurotrophic factor for weight loss in obese adults: A randomized, dose-ranging study. JAMA 289, 1826–1832 (2003).
Pasquin, S., Sharma, M. & Gauchat, J. F. Cytokines of the LIF/CNTF family and metabolism. Cytokine 82, 122–124 (2016).
Cron, L., Allen, T. & Febbraio, M. A. The role of gp130 receptor cytokines in the regulation of metabolic homeostasis. J. Exp. Biol. 219, 259–265 (2016).
Beltran, W. A. et al. Cloning, mapping, and retinal expression of the canine ciliary neurotrophic factor receptor alpha (CNTFRalpha). Investig. Ophthalmol. Vis. Sci. 44, 3642–3649 (2003).
Giordano, A., Frontini, A. & Cinti, S. Convertible visceral fat as a therapeutic target to curb obesity. Nat. Rev. Drug Discov. 15, 405–424 (2016).
Heymsfield, S. B. & Wadden, T. A. Mechanisms, pathophysiology, and management of obesity. N. Engl. J. Med. 376, 254–266 (2017).
Saltiel, A. R. & Olefsky, J. M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 127, 1–4 (2017).
Testa, R. et al. Leukocyte telomere length is associated with complications of Type 2 diabetes mellitus. Diabet. Med. 28, 1388–1394 (2011).
American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care 43, S14–S31 (2020).
Mensà, E. et al. Circulating miR-146a in healthy aging and type 2 diabetes: Age- and gender-specific trajectories. Mech. Ageing Dev. 180, 1–10 (2019).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612 (2009).
Matthews, D. R. et al. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 (1985).
Yiğit, S. & Mendes, M. Which effect size measure is appropriate for one-way and two-way ANOVA models? A Monte Carlo simulation study. REVSTAT Stat. J. 16, 295–313 (2018).
Kishida, K., Funahashi, T. & Shimomura, I. Adiponectin as a routine clinical biomarker. Best Pract. Res. Clin. Endocrinol. Metab. 28, 119–130 (2014).
Davis, S. et al. Released form of CNTF receptor alpha component as a soluble mediator of CNTF responses. Science 259, 1736–1739 (1993).
Mackiewicz, A., Schooltink, H., Heinrich, P. C. & Rose-John, S. Complex of soluble human IL-6-receptor/IL-6 up-regulates expression of acute-phase proteins. J. Immunol. 149, 2021–2027 (1992).
Panayotatos, N., Everdeen, D., Liten, A., Somogyi, R. & Acheson, A. Recombinant human CNTF receptor alpha: production, binding stoichiometry, and characterization of its activity as a diffusible factor. Biochemistry 33, 5813–5818 (1994).
Krüttgen, A. et al. Human ciliary neurotrophic factor: A structure-function analysis. Biochem. J. 309, 215–220 (1995).
Johnson, R. M. et al. Recombinant human ciliary neurotrophic factor stimulates the metabolic activity of SH-SY5Y cells as measured by a cytosensor microphysiometer. Brain Res. 646, 327–331 (1994).
Perugini, J. et al. Biological effects of ciliary neurotrophic factor on hMADS adipocytes. Front. Endocrinol. 10, 768 (2019).
Masu, Y. et al. Disruption of the CNTF gene results in motor neuron degeneration. Nature 365, 27–32 (1993).
Takahashi, R. et al. A null mutation in the human CNTF gene is not causally related to neurological diseases. Nat. Genet. 7, 79–84 (1994).
O’Dell, S. D. et al. Null mutation in human ciliary neurotrophic factor gene confers higher body mass index in males. Eur. J. Hum. Genet. 10, 749–752 (2002).
Severi, I. et al. Opposite effects of a high-fat diet and calorie restriction on ciliary neurotrophic factor signaling in the mouse hypothalamus. Front. Neurosci. 7, 263 (2013).
Moreno-Aliaga, M. J. et al. Cardiotrophin-1 is a key regulator of glucose and lipid metabolism. Cell Metab. 14, 242–253 (2011).
Lambert, P. D. et al. Ciliary neurotrophic factor activates leptin-like pathways and reduces body fat, without cachexia or rebound weight gain, even in leptin-resistant obesity. Proc. Natl. Acad. Sci. USA 98, 4652–4657 (2001).
Anderson, K. D. et al. Activation of the hypothalamic arcuate nucleus predicts the anorectic actions of ciliary neurotrophic factor and leptin in intact and gold thioglucose-lesioned mice. J. Neuroendocrinol. 15, 649–660 (2003).
Venema, W. et al. Ciliary neurotrophic factor acts on distinctive hypothalamic arcuate neurons and promotes leptin entry into and action on the mouse hypothalamus. Front. Cell. Neurosci. 14, 140 (2020).
Senzacqua, M., Severi, I., Perugini, J., Cinti, S. & Giordano, A. Action of administered ciliary neurotrophic factor on the mouse dorsal vagal complex. Front. Neurosci. 10, 289 (2016).
Watt, M. J. et al. CNTF reverses obesity-induced insulin resistance by activating skeletal muscle AMPK. Nat. Med. 12, 541–548 (2006).
Sleeman, M. W. et al. Ciliary neurotrophic factor improves diabetic parameters and hepatic steatosis and increases basal metabolic rate in db/db mice. Proc. Natl. Acad. Sci. USA 100, 14297–14302 (2003).
Cui, M. X. et al. Alleviative effect of ciliary neurotrophic factor analogue on high fat-induced hepatic steatosis is partially independent of the central regulation. Clin. Exp. Pharmacol. Physiol. 44, 395–402 (2017).
Rezende, L. F., Santos, G. J., Santos-Silva, J. C., Carneiro, E. M. & Boschero, A. C. Ciliary neurotrophic factor (CNTF) protects non-obese Swiss mice against type 2 diabetes by increasing beta cell mass and reducing insulin clearance. Diabetologia 55, 1495–1504 (2012).
Rezende, L. F., Santos, G. J., Carneiro, E. M. & Boschero, A. C. Ciliary neurotrophic factor protects mice against streptozotocin-induced type 1 diabetes through SOCS3: The role of STAT1/STAT3 ratio in β-cell death. J. Biol. Chem. 287, 41628–41639 (2012).
Crowe, S., Turpin, S. M., Ke, F., Kemp, B. E. & Watt, M. J. Metabolic remodeling in adipocytes promotes ciliary neurotrophic factor-mediated fat loss in obesity. Endocrinology 149, 2546–2556 (2008).
Ott, V., Fasshauer, M., Dalski, A., Klein, H. H. & Klein, J. Direct effects of ciliary neurotrophic factor on brown adipocytes: Evidence for a role in peripheral regulation of energy homeostasis. J. Endocrinol. 173, R1–R8 (2002).
Van Dyken, P. & Lacoste, B. Impact of metabolic syndrome on neuroinflammation and the blood–brain barrier. Front. Neurosci. 12, 930 (2018).
Denti, L. et al. Effects of aging on dehydroepiandrosterone sulfate in relation to fasting insulin levels and body composition assessed by bioimpedance analysis. Metabolism 46, 826–832 (1997).
Schuster, B. et al. Signaling of human ciliary neurotrophic factor (CNTF) revisited. The interleukin-6 receptor can serve as an alpha-receptor for CTNF. J. Biol. Chem. 278, 9528–9535 (2003).
Lokau, J. et al. Proteolytic cleavage governs interleukin-11 trans-signaling. Cell Rep. 14, 1761–1773 (2016).
Acknowledgements
This work was supported by grants from Fondazione Cariverona (COD. SIME 2018.0777 Bando Progetti Scientifici di Eccellenza 2018 to AG).The authors are grateful to Dr Silvia Modena for her assistance with language and style. We would like to dedicate this work to the memory of Francesco Osculati, Professor of Human Anatomy. His untiring efforts on behalf of young researchers will forever stand as an eminent example of a passion for biological research and dedication to university teaching.
Author information
Authors and Affiliations
Contributions
J.P.: performance of experiments, data analysis and interpretation, manuscript writing; E.Di.M., A.G. and J.S.: performance of experiments, data analysis and interpretation; A.R.B. and E.T.: providing human plasma samples; I.S., S.C. and F.O.: critical revision of the manuscript; C.W.leR.: conception and design, critical revision of the manuscript; R.G.: conception and design, statistical analysis, critical revision of the manuscript; A.G.: conception and design, financial support, data analysis and interpretation, manuscript writing, final approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Perugini, J., Di Mercurio, E., Giuliani, A. et al. Ciliary neurotrophic factor is increased in the plasma of patients with obesity and its levels correlate with diabetes and inflammation indices. Sci Rep 12, 8331 (2022). https://doi.org/10.1038/s41598-022-11942-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-11942-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.