Abstract
Coronavirus disease 19 (COVID-19) patients usually require long periods of mechanical ventilation and sedation, which added to steroid therapy, favours a predisposition to the development of delirium and subsequent mental health disorders, as well as physical and respiratory sequelae. The aim of this study was to determine the prevalence of post-intensive care syndrome (PICS) at 3 months after hospital discharge, in a cohort of mechanically ventilated patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). An ambispective, observational study was conducted in three hospitals with intensive care unit (ICU) follow-up clinics. We studied adults who survived a critical illness due to SARS-CoV-2 infection requiring invasive mechanical ventilation. A physical (muscle strength and pulmonary function), functional [12-Item Short Form Health Survey (SF-12), and Barthel score], psychological [hospital anxiety and depression (HADS) and posttraumatic stress disorder symptom severity scales], and cognitive [Montreal cognitive assessment (MoCA) test] assessment were performed. A total of 186 patients were evaluated at 88 days (IQR 68–121) after hospital discharge. Mean age was 59 ± 12 years old, 126 (68%) patients were men, and median length of mechanical ventilation was 14 days (IQR 8–31). About 3 out of 4 patients (n = 139, 75%) met PICS criteria. Symptoms of cognitive and psychiatric disorders were found in 59 (32%) and 58 (31%) patients, respectively. Ninety-one (49%) patients had muscle weakness. Pulmonary function tests in patients with no respiratory comorbidities showed a normal pattern in 93 (50%) patients, and a restrictive disorder in 62 (33%) patients. Also, 69 patients (37%) were on sick leave, while 32 (17%) had resumed work at the time of assessment. In conclusion, survivors of critical illness due to SARS-CoV-2 infection requiring mechanical ventilation have a high prevalence of PICS. Physical domain is the most frequently damaged, followed by cognitive and psychiatric disorders. ICU follow-up clinics enable the assistance of this vulnerable population.
Similar content being viewed by others
Introduction
The post-intensive care syndrome (PICS) represents a constellation of cognitive, psychiatric, physical, and pulmonary disorders frequently seen following admission into intensive care units (ICUs) in pre-pandemic studies1. At ICU discharge, nearly all survivors of critical illness experience impairments in one or more PICS domains. At 3 and 12 months, 64% and 56% of survivors experience one or more new post-intensive care problems, respectively, and co-occurrence is common2,3. Risk factors for developing PICS include longer periods of mechanical ventilation, delirium, treatment with steroids, vasoactive drugs and sedation, among others1.
Coronavirus disease 19 (COVID-19) has caused a worldwide surge in critical care demand. Up to 20% of hospitalized patients infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) require admission into the ICU, out of which more than 88% require endotracheal intubation and invasive mechanical ventilation (MV)4. Moreover, COVID-19 patients usually require longer periods of MV and sedation than non-COVID-19 critically ill patients, which added to steroid therapy, favour a predisposition to the development of delirium and subsequent mental health disorders, as well as physical and respiratory sequelae5.
Short and long-term sequelae of SARS-CoV-2 infection in patients admitted in ICU have been described6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22. However, PICS (in all 3 domains) in post-critical care mechanically ventilated COVID-19 patients has not been extensively studied10,11,17,19.
The aim of this study was to determine the prevalence of PICS in a cohort of mechanically ventilated SARS-CoV-2 patients, assessed after 3 months of hospital discharge, in the ICU follow-up consultation facilities of three major hospitals in Spain.
Methods
Study design
The study was designed in accordance with the Declaration of Helsinki23. We conducted an ambispective, observational study in three hospitals with ICU follow-up consultation facilities. We studied adult patients (≥ 18 years old) admitted to the ICU due to severe SARS-CoV-2 infection, requiring invasive MV and who were alive at the time of hospital discharge. Patients with previous severe psychiatric conditions, cognitive deficits, and any sort of functional dependency were excluded. Exclusion criteria also included patients from a different geographical area who were not willing to come for assessment to our centres and patients who refused to sign the informed consent form.
Data regarding demographic variables, treatment drugs (hypnotics, analgesics, muscle relaxants, corticoids and vasopressors), delirium, management procedures [length of MV, renal replacement therapy (RRT), extracorporeal membrane oxygenation (ECMO), extracorporeal CO2 removal (ECCO2R), tracheostomy] and complications [such as nosocomial infections, venous thromboembolism (VTE), pulmonary embolism (PE), and stroke] during the ICU stay were retrospectively registered from the patient´s electronic medical record. Only hyperactive delirium was registered in our analysis. Prolonged MV (PMV) was defined as the need for invasive MV for more than 14 days.
Follow-up assessment
In line with our usual clinical practice24, a follow-up appointment was arranged for all patients at three months after hospital discharge. This consultation included anamnesis and an assessment of potentially affected domains where PICS was evaluated: mental status, cognition, muscle strength, pulmonary function, dependence and functional status. All the variables related to this assessment were prospectively recorded.
Mental status was evaluated using the Hospital Anxiety and Depression Scale (HADS)25 and the Post-Traumatic Stress Disorder (PTSD) symptom severity scale26. The HADS scale combines two 7-item subscales evaluating symptoms of depression (HADS-D subscale) and anxiety (HADS-A subscale). We used a score of ≥ 8 in the anxiety or depression subscale to identify clinically relevant anxiety or depression. The PTSD symptom severity scale is a 0 to 3 scoring scale according to the frequency and intensity of symptoms, it has been validated in the Spanish population26 and has 17 items: five refer to re-experiencing symptoms (range 0 to 15 points), seven to avoidance symptoms (range 0 to 21 points), and five to symptoms of increased activation (range 0 to 15 points). A symptom is considered when it is scored with at least 2 points. In order to consider PTSD, the presence of one symptom is required in section A (re-experiencing), three symptoms in section B (avoidance), and two symptoms in section C (increased activation).
Cognition was assessed using the Montreal Cognitive Assessment (MoCA) test27. The MoCA test evaluates global cognitive function, including executive function, attention/working memory, episodic memory, and language. Total score ranges from zero to 30. Mild cognitive impairment was defined as a score of 18–25, moderate as a score of 10–17, and severe when the score is less than 102.
Muscle strength was assessed using a handgrip dynamometry, a basic method that is standardized by age groups and sex28. We used an electronic digital LCD device (Camry®, General ASDE SA, Spain, 93/42/CEE). Reference values are based on the study by Luna et al.29 in which they consider cut-off points of 85% from those obtained in a Spanish population of healthy volunteers (267 women and 229 men) aged between 17 and 97 years.
Pulmonary function was assessed by spirometry (Sibelmed® datospir touch. SIBEL. S.A.U. Barcelona, Spain). Protocol and interpretation were based on the 2005 American Thoracic Society (ATS) and European Respiratory Society (ERS) statements30, and Quanjer equations were used as reference values31.
Dependence was assessed using the Barthel score32. The Barthel score has 10 subheadings related to activities of daily living (ADL). Scoring ranges from zero to 100. A score of 100 is defined as being capable of ADL as well as complete self-care.
Quality of life was assessed using the 12 Item Short Form Healty Survey (SF-12)33, wich is a health-related quality of life questionnaire consisting of twelve items that measure eight health domains associated with physical and mental health. Physical health-related domains include general health, physical activities, usual role activities and body pain. Mental health-related scales include vitality, social activities, emotion influenced limitations in role activities and general mental health. The instrument was self-administered and two summary scores of the SF-12—physical and mental health—were calculated using the weighted means of the eight domains. A score under 50 indicates a poor health-related quality of life in relation to the reference population, whereas a score above 50 indicates good health-related quality of life.
Patients met PICS criteria if they had derangements in at least one of the domains assessed with the scales and tools used to detect long-term cognition, mental health, and physical function, as previously detailed, according to Needham et al.1 and adapted from Mikkelsen et al.2. A summary of outcome measures, including domains assessed, scale details, and interpretation, are shown in Table 1.
Statistical methods
Quantitative variables were expressed as mean ± standard deviation, or median and interquartile range (IQR) for variables not fitting a normal distribution according to Kolmogornov–Smirnov’s test. Chi-square test was used for analysing the association between qualitative variables. For qualitative variables in which the “n” was < 20 or any theoretical value was < 5, Fisher’s exact test was used. Differences were considered statistically significant if the p-value was < 0.05.
Relationship between the quantitative variables used as PICS criteria and mental or physical SF-12 component was assessed by Pearson correlation analysis. For assessing the independent association of each variable with PICS, a multivariate logistic regression analysis including those variables with a p < 0.05 in bivariate analysis was developed. The method used for regression was forward elimination.
Data were processed by the Statistical Package for the Social Sciences Software (version 26.0) for Windows (SPSS Inc, Chicago, IL).
Ethics approval
The study was approved by the Ethical Committee of Clinical Research of University Hospital La Paz, IdiPAZ, Madrid, Spain (reference number: #PI-4325) and was adopted for all participating centres, as required by Spanish legislation. All patients provided written informed consent for its inclusion in the study.
Results
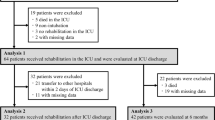
From February 27th, 2020 to May 10th, 2021, a total of 1093 patients with acute respiratory failure due to SARS-CoV-2 were admitted to the ICUs of participating hospitals: 820 patients (75%) required invasive MV and 86 (10%) were transferred to other centres for lack of ICU beds. Out of the remaining 734 patients, 332 (45%) survived to hospital discharge. On May 10th, those patients (n = 88) who met inclusion criteria but did not complete the period from hospital discharge, and had not been assessed in follow-up clinic had to be excluded. Other 58 patients were excluded due to other reasons (had exclusion criteria, were from other health areas, or due to language barriers). Finally, 186 patients were enrolled in the present study (Fig. 1). Patients were assessed in the ICU follow-up consultation at 88 days (IQR 68–121) after hospital discharge [a median of 110 days (IQR 84–167) after ICU discharge]. Demographic and clinical features are shown in Table 2.
Thirty-three patients (18%) were transferred to a rehabilitation centre after being discharged from the hospital, while 41 patients (22%) needed domiciliary oxygen, and 52 patients (28%) needed home care.
At the time of assessment at the ICU follow-up clinic, 139 patients (75%) met PICS criteria: 86 (46%), 40 (21%) and 13 (7%) patients had derangements of one, two or three PICS domains, respectively.
Regarding physical assessment, the most common symptoms were dyspnea (n = 106, 57%), muscle weakness (n = 91, 49%), and joint pain (n = 83, 45%) (Table 3). After excluding 7 patients with a history of chronic respiratory disease, 93 patients (50%) had a normal pulmonary function pattern, in 16 patients (10%) spirometry tests were not evaluable due to lack of patient’s collaboration, 62 patients (33%) showed a restrictive disorder, 5 patients (3%) had a mixed (obstructive-restrictive) disorder, and 3 patients (2%) showed an obstructive spirometry pattern. Values of spirometry are shown in Table 3.
Cognitive and psychiatric disorders were found in 59 (32%), and 58 (31%) patients respectively. In 47 patients (25%), cognitive disorders were mild, whereas 12 patients (6%) had moderate cognitive disorders. In our study, no patients showed severe cognitive disorders. Symptoms of psychiatric disorders are shown in Table 4.
Summary of MoCA test, Barthel score, and SF-12 score are shown in Table 3. The physical component of the SF-12 showed a correlation with the degree of dyspnea (mMRC) (r = − 0.32, p < 0.001) and Barthel scale (r = 0.49 p < 0.001), whereas the SF-12 mental component was strongly related to HADS-A (r = − 0.68, p < 0.001) and HADS-D scales (r = − 0.67, p < 0.001).
PICS was associated with PMV (p = 0.01), use of benzodiazepines (p = 0.002), and nosocomial infection (p = 0.04) (Table 5). Associations between these variables and domains of PICS are shown in Table 6. Nosocomial infection, use of benzodiazepines, and PMV were included in the stepwise multiple regression logistic analysis which only retained as independent variable the PMV (adjusted odds ratio: 2.271, 95%CI 1.140–4.524, p = 0.020).
Eighty-six patients (46%) were transferred to other clinical specialists, after assessing their needs. Thirty-one patients (17%) were transferred to the Department of Mental Health, 19 patients (10%) were transferred to Physical Medicine and Rehabilitation Department, and 7 patients (4%) were transferred to both departments. Thirty-four patients (18%) were already treated by those departments and did not required further transfer. Twenty-nine patients (16%) were transferred to other specialists (including respiratory medicine, internal medicine, and neurology, among others).
Regarding the social aspects of life, 32 patients (17%) had resumed work at the time of assessment, 69 patients (37%) were on sick leave, 57 patients (31%) were retired prior to hospital admission, 7 patients (13%) remained unemployed, and 15 patients (8%) were housekeepers (as prior to hospital admission). Eighty patients (43%) had resumed driving, and 48 patients (26%) had normalized their sexual activities.
Discussion
The main finding of this study is that about three out of four survivors of severe COVID-19 met PICS criteria: 46%, 21%, and 7% of them had derangements of one, two, or three PICS domains, respectively. Physical domain was the most frequently damaged.
Prior to our work, post-ICU COVID-19 related sequelae has been assessed in other reports.
Some of them with larger samples, but with a smaller number of patients requiring invasive mechanical ventilation6,7,21. Only two studies17,19 have assessed the three PICS domains focused on ventilated patients: the first one17 assessed a cohort of 47 patients 6 months after hospital discharge, and the second one19 analyzed a cohort of 178 patients at 3 and 12 months after hospital discharge.
We found a prevalence of PICS of 75% with a 28% co-occurrence of symptoms, with dyspnea being the most frequent symptom (57%). The highest prevalence of PICS in COVID-19 patients reported to date has been 91% in 45 patients (out of which 90% had been on MV) and 58% had at least two main domains affected16. Of note, follow-up consultations in that study16 were via telematics at 1 month after hospital discharge. Gamberini et al.19 found that most of the patients reported persistent symptoms 1 year after ICU discharge, with dyspnea (58%) being the most frequent symptom. Heesakers et al.21, also 1 year after ICU treatment, reported physical symptoms in 74% (weakness 38.9%), mental symptoms in 26% and cognitive symptoms in 16% of the patients, of whom 81% underwent mechanical ventilation. Overall, 31% of the survivors reported symptoms in at least 2 domains, and 10% experienced symptoms in all 3 domains.
Main conditions associated with PICS included large duration of MV, treatment with benzodiazepines, and nosocomial infection, although PMV was the independent variable associated to PICS. PMV and deep sedation have been classically associated with PICS1. In the COVID-19 era, a large number of patients required PMV and deep sedation (with the coexistence of two or more hypnotics) and relaxation to facilitate MV in the prone position. There have been substantial concerns about respiratory sequelae due to COVID-19. At 3 months after hospital discharge, we observed that pulmonary function was normal in over 50% of patients in our study. Also, 43% did not manifest dyspnea, and in those who did, the mean modified Medical Research Council (mMRC) dyspnea scale34 was 1.7 ± 0.9 which corresponds to a low symptom intensity (mainly breathlessness only on strenuous exercise). Other authors35 have published similar spirometry results. Morin et al.7 included functional and morphological assessment, reporting that severe pulmonary sequelae were infrequent, although all had experienced a severe or very severe form of COVID-19 associated pneumonia. In contrast, both, prevalence of restrictive spirometry results in our patients, was slightly higher compared to COVID-19 pneumonia patients who did not require ICU admission36. Mean SF-12 physical and mental summary scores in our cohort was lower when compared to previous reports at three months of follow-up20. These findings can be explained by a longer ICU stay and PMV. However, in line with the results of these authors20 we also found a correlation between degree of dyspnea and physical component of SF-12.
The published prevalence for cognitive and psychiatric disorders in post-critical COVID-19 patients covers a wide range, probably due to methodological heterogeneity among studies. Psychiatric disorders have been described in up to 49%16 while cognitive disturbances reach 57%35 at 1 month and 6 weeks of follow-up respectively. At 1 year following ICU treatment, mental symptoms were reported by 38%19 and cognitive symptoms by 16%21.
Unlike other pre-pandemic37,38 and pandemic8,16,19studies, our work has been carried out using face-to-face consultation. The scales we have used are recommended for these entities2 except for PTSD assessment. Due to easy-to-use characteristics, PTSD was evaluated using the symptom severity scale26, a structured interview developed in Spain that takes into account severity and intensity of symptoms. The main drawback of this scale is that it uses DSM-IV criteria as a reference.
The prevalence of delirium in ICU patients is estimated between 32 and 87%, although these figures vary considerably depending on whether the studied population had received MV39. All patients in our study underwent MV, and due to excessive workload during the pandemic, delirium and PICS prevention measures were inapplicable40,41. Prevalence of hyperactive delirium was found to be lower than previous published experiences, in COVID-1942, but somehow higher than the figure published in a large study of COVID-19 population41. No association between benzodiazepines and worse long-term cognitive scores was found, as described in previous studies43. Despite a 64% prevalence of hyperactive delirium, no influence on subsequent cognitive assessment was established, unlike other studies specifically designed for this purpose with a larger sample size43,44. Of note, since our study was not specifically designed for the prevalence of delirium, only hyperactive delirium was registered due to the diagnostic challenge posed by hypoactive delirium. Unlike prepandemic studies, hyperactive delirium is much more frequent than hypoactive delirium in COVID-19 patients42. Nevertheless, the hypothesis about a possible neuro-invasive potential of SARS-CoV-245 and its influence on mental or psychiatric disorders remains open, since to date, its pathophysiology is poorly understood39.
Data on socio-occupational issues is absent in most studies. In our study, almost a third of previously active patients had resumed work within 88 days after hospital discharge, 43% had resumed driving, and about 25% had normalized their sexual life. Of note, two-thirds of our patients had a Barthel score of 100 points. These results differ drastically from those published by Rousseau et al.46 in 32 COVID-19 patients (where 30 patients required MV) who reported that only 6% of patients fully recovered and had normal MoCA, IES-R and Barthel scores three months after hospital discharge.
Our study has several strengths. First, our group has experience in post-ICU follow-up consultation since 201647 and post-ICU follow-up is part of our usual clinical practice. Second, participating ICUs followed the same protocol, reducing heterogeneity among centres. Third, this is the largest study involving mechanically ventilated COVID-19 patients. Fourth, all patients in our study were assessed in a face-to-face consultation room with an intensivist and the support of a “post-ICU-team” (physiatrists, psychiatrists, psychologists and physiotherapists). No patient was assessed by phone or any other telematics mean. However, we acknowledge some limitations of our study. First, our study is an uncontrolled study design and, therefore, comparison with non-COVID-19 patients could not be established. Second, since our design is ambispective, some variables were retrospectively recorded. Third, 49% of studied patients were survivors of the first wave, the most devastating and challenging wave in which patient individualization and PICS prevention was inapplicable, along with changes in treatment protocol and patient upmake over time. Fourth; although according to our methodology patients with psychiatric impairment, severe cognitive impairment and patients with severe neuromuscular or neurological diseases were excluded from the PICS evaluation, baseline like HADS scale, MOCA test or muscle strength are unknown. Many of complaints related to these domains are often underdiagnosed. All of this probably may have overestimated its incidence in this patient population. Fifth, although our study is the largest cohort of mechanically ventilated COVID-19 patients, sample size is still limited to draw definitive conclusions. Participation of more centres would have been desirable to enlarge sample size. However, the number of centres with ICU follow-up clinics is still limited and some of them were forced to close due to the pandemic. Sixth, pulmonary function was assessed using forced spirometry. This test has obvious limitations, especially if it is not accompanied by other diagnostic procedures. Seventh, the possibility of performing lung morphological analysis at 3 months exceeded the objectives of the present study, which focused mainly on evaluating classically described PICS in a population of critical COVID-19 patients.
Conclusions
This is the largest study addressing PICS in SARS-CoV-2 mechanically ventilated patients, assessed in follow-up ICU clinics. About three out of four survivors of severe COVID-19 meet PICS criteria. Physical domain is the most frequently damaged, followed by cognitive and psychiatric disorders. In line with the findings by other authors48, ICU follow-up clinics allow the assistance of this vulnerable population, as well as making advances in the understanding of PICS and COVID-19 sequelae.
Data availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ADL:
-
Activities of daily living
- ATS:
-
American Thoracic Society
- BMI:
-
Body mass index
- COPD:
-
Chronic obstructive pulmonary diseases
- COVID-19:
-
Coronavirus disease-2019
- ECCO2R:
-
Extracorporeal CO2 removal
- ECMO:
-
Extracorporeal membrane oxygenation
- ERS:
-
European Respiratory Society
- FEV1 :
-
Forced expiratory volume at 1 s
- FVC:
-
Forced vital capacity
- HADS:
-
Hospital Anxiety and Depression Scale
- ICU:
-
Intensive care unit
- IQR:
-
Interquartile range
- mMRC:
-
Medical Research Council Dyspnea Scale
- MoCA:
-
Montreal cognitive assessment
- MV:
-
Mechanical ventilation
- PE:
-
Pulmonary embolism
- PICS:
-
Post-intensive care syndrome
- PMV:
-
Prolonged mechanical ventilation
- PTSD:
-
Post-traumatic stress disorder
- RRT:
-
Renal replacement therapy
- SF-12:
-
12-Item Short Form Health Survey
- VTE:
-
Venous thromboembolism
References
Needham, D. M. et al. Improving long-term outcomes after discharge from intensive care unit: Report from a stakeholders’ conference. Crit. Care Med. 40(2), 502–529. https://doi.org/10.1097/CCM.0b013e318232da75 (2012).
Mikkelsen, M. E. et al. Society of critical care medicine’s international consensus conference on prediction and identification of long-term impairments after critical illness. Crit. Care Med. 48(11), 1670–1679. https://doi.org/10.1097/CCM.0000000000004586 (2020).
Marra, A. et al. Co-occurrence of post-intensive care syndrome problems among 406 survivors of critical illness. Crit. Care Med. 46(9), 1393–1401. https://doi.org/10.1097/CCM.0000000000003218 (2018).
Ziehr, D. R. et al. Pathophysiology of mechanically ventilated patients with COVID-19: A cohort study. Am. J. Respir. Crit. Care Med. 201(12), 1560–1564. https://doi.org/10.1164/rccm.202004-1163LE (2020).
Cadd, M. & Nunn, M. An A-E assessment of post-ICU COVID-19 recovery. J. Intens. Care 9(1), 29. https://doi.org/10.1186/s40560-021-00544-w (2021).
Huang, C. et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 397(10270), 220–232. https://doi.org/10.1016/S0140-6736(20)32656-8 (2021).
Morin, L. et al. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA 325(15), 1525–1534. https://doi.org/10.1001/jama.2021.3331 (2021).
Todt, B. C. et al. Clinical outcomes and quality of life of COVID-19 survivors: A follow-up of 3 months post hospital discharge. Respir. Med. 184, 106453. https://doi.org/10.1016/j.rmed.2021.106453 (2021).
Tarsitani, L. et al. Post-traumatic stress disorder among COVID-19 survivors at 3-month follow-up after hospital discharge. J. Gen. Intern. Med. 36(6), 1702–1707. https://doi.org/10.1007/s11606-021-06731-7 (2021).
van Gassel, R. J. J. et al. High prevalence of pulmonary sequelae at 3 months after hospital discharge in mechanically ventilated survivors of COVID-19. Am. J. Respir. Crit. Care Med. 203(3), 371–374. https://doi.org/10.1164/rccm.202010-3823LE (2021).
Van Gassel, R. et al. Functional outcomes and their association with physical performance in mechanically ventilated coronavirus disease 2019 survivors at 3 months following hospital discharge: A cohort study. Crit. Care Med. 49(10), 1726–1738. https://doi.org/10.1097/CCM.0000000000005089(2021) (2019).
Lerum, T. V. et al. Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. Eur. Respir. J. 57(4), 2003448. https://doi.org/10.1183/13993003.03448-2020 (2021).
Mongodi, S., Salve, G., Tavazzi, G., Politi, P. & Mojoli, F. COVID-19 post-ICU team. High prevalence of acute stress disorder and persisting symptoms in ICU survivors after COVID-19. Intens. Care. Med. 47, 616–618. https://doi.org/10.1007/s00134-021-06349-7 (2021).
Prevel, R. et al. Psychological evaluation and support in COVID-19 critically ill patients: A feasibility study. Crit. Care 25(1), 218. https://doi.org/10.1186/s13054-021-03642-1 (2021).
van den Borst, B. et al. Comprehensive health assessment three months after recovery from Acute Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 73(5), e1089–e1098. https://doi.org/10.1093/cid/ciaa1750(2021) (2019).
Martillo, M. A. P. et al. Postintensive care syndrome in survivors of critical illness related to coronavirus disease 2019: Cohort study from a New York City critical care recovery clinic. Crit. Care Med. 49(9), 1427–1438. https://doi.org/10.1097/CCM.0000000000005014(2021) (2019).
Carenzo, L. et al. Short-term health-related quality of life, physical function and psychological consequences of severe COVID-19. Ann. Intens. Care 11(1), 91. https://doi.org/10.1186/s13613-021-00881-x (2021).
Janiri, D. et al. Posttraumatic stress disorder in patients after severe COVID-19 infection. JAMA Psychiat. 78(5), 567–569. https://doi.org/10.1001/jamapsychiatry.2021.0109 (2021).
Gamberini, L. et al. Health-related quality of life profiles, trajectories, persistent symptoms and pulmonary function one year after ICU discharge in invasively ventilated COVID-19 patients, a prospective follow-up study. Respir. Care 189, 106665. https://doi.org/10.1016/j.rmed.2021.106665 (2021).
Gonzalez, J. et al. Pulmonary function and radiologic features in survivors of critical COVID-19. A 3-month prospective cohort. Chest 160(1), 187–198. https://doi.org/10.1016/j.chest.2021.02.062 (2021).
Heesakkers, H. et al. Clinical outcomes among patients with 1-year survival following intensive care unit treatment for COVID-19. JAMA 327(6), 559–565. https://doi.org/10.1001/jama.2022.0040 (2022).
Groff, D., Sun, A. & Ssentongo, A. E. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection. A systematic review. JAMA 4(10), e2128568. https://doi.org/10.1001/jamanetworkopen.2021.28568 (2021).
World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 310(20), 2191–2194. https://doi.org/10.1001/jama.2013.281053 (2013).
Extremera, P., Añón, J. M. & García de Lorenzo, A. Are outpatient clinics justified in Intensive Care Medicine? Med. Intens. 42(2), 110–113. https://doi.org/10.1016/j.medin.2017.07.010 (2018).
Bjelland, I., Dahl, A. A., Haug, T. T. & Neckelmann, D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J. Psychosom. Res. 52(2), 69–77. https://doi.org/10.1016/s0022-3999(01)00296-3 (2002).
Echeburúa, E., Corral, P., Amor, P. J., Zubizarreta, I. & Sarasua, B. Escala de Gravedad de Síntomas del Trastorno de Estrés Postraumático: Propiedades psicométricas. Anál. y Modif. de Conducta 23(90), 503–526 (1997).
Nasreddine, Z. S. et al. The Montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53(4), 695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x (2005).
Spies, C. D. et al. Instruments to measure outcomes of post-intensive care syndrome in outpatient care settings. Results of an expert consensus and feasibility field test. J. Intens. Care Soc. 22(2), 159–174. https://doi.org/10.1177/1751143720923597 (2021).
Luna-Heredia, E., Martín-Peña, G. & Ruiz-Galiana, J. Valores normales y límites de la normalidad de la fuerza de la mano determinados con dinamometría. Nutr. Hosp. 19, 1 (2004).
Miller, M. R. et al. ATS/ERS Task Force. Standardisation of spirometry. Eur. Respir. J. 26(2), 319–338. https://doi.org/10.1183/09031936.05.00034805 (2005).
Pellegrino, R. et al. Interpretative strategies for lung function tests. Eur. Respir. J. 26(5), 948–968. https://doi.org/10.1183/09031936.05.00035205 (2005).
Mahoney, F. I. & Barthel, D. W. Functional evaluation: The Barthel Index. Md. State. Med. J. 14, 61–65 (1965).
Ware, J., Kosinski, M. & Keller, S. D. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med. Care 34(3), 220–233. https://doi.org/10.1097/00005650-199603000-00003 (1996).
Mahler, D. A. & Wells, C. K. Evaluation of clinical methods for rating dyspnea. Chest 93(3), 580–586. https://doi.org/10.1378/chest.93.3.580 (1998).
Ramani, C. et al. Post-ICU COVID-19 outcomes. A case series. Chest 159(1), 215–218. https://doi.org/10.1016/j.chest.2020.08.2056 (2021).
Núñez-Fernández, M. et al. Alterations in respiratory function test three months after hospitalisation for COVID-19 pneumonia: Value of determining nitric oxide diffusion. J. Clin. Med. 10(10), 2119. https://doi.org/10.3390/jcm10102119 (2021).
Huang, M. et al. Psychiatric symptoms in acute respiratory distress syndrome survivors: A 1-year national multicenter study. Crit. Care Med. 44(5), 954–965. https://doi.org/10.1097/CCM.0000000000001621 (2016).
Hatch, R. et al. Anxiety, depression and post traumatic stress disorder after critical illness: A UK-wide prospective cohort study. Crit. Care 22(1), 310. https://doi.org/10.1186/s13054-018-2223-6 (2018).
Wilcox, M. E., Girard, T. D. & Hough, C. L. Delirium and long term cognition in critically ill patients. BMJ 373, n1007. https://doi.org/10.1136/bmj.n1007 (2021).
Devlin, J. W. et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit. Care Med. 46(9), e825–e873. https://doi.org/10.1097/CCM.0000000000003299 (2018).
Pun, B. T. et al. Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): A multicenter cohort study. Lancet Respir. Med. 9(3), 239–250. https://doi.org/10.1016/S2213-2600(20)30552-X (2021).
Helms, J. et al. Delirium and encephalopathy in severe COVID-19: A cohort analysis of ICU patients. Crit. Care 24(1), 491. https://doi.org/10.1186/s13054-020-03200-1 (2020).
Pandharipande, P. P. et al. Long-term cognitive impairment after critical illness. New. Engl. J. Med. 369(14), 1306–1316. https://doi.org/10.1056/NEJMoa1301372 (2013).
Girard, T. D. et al. Clinical phenotypes of delirium during critical illness and severity of subsequent long-term cognitive impairment: A prospective cohort study. Lancet. Respir. Med. 6(3), 213–222. https://doi.org/10.1016/S2213-2600(18)30062-6 (2018).
Stollings, J. L. et al. Delirium in critical illness: Clinical manifestations, outcomes and management. Intensive. Care. Med. 47(10), 1089–1103. https://doi.org/10.1007/s00134-021-06503-1 (2021).
Rousseau, A. F. et al. Post-intensive care syndrome after a critical COVID-19: Cohort study from a Belgian follow-up clinic. Ann. Intens. Care 11(1), 118. https://doi.org/10.1186/s13613-021-00910-9 (2021).
Añón, J. M. et al. Postintensive care syndrome and follow-up clinics: Results after a two-year pilot. Minerva. Anestesiol. 86(11), 1246–1248. https://doi.org/10.23736/S0375-9393.20.14754-0 (2020).
Prescott, H. C. Outcomes for patients following hospitalization for COVID-19. JAMA 325(15), 1511–1512. https://doi.org/10.1001/jama.2021.3430 (2021).
Funding
This study did not receive any funding or financial support. JMA and JV are funded by Grants from the Instituto de Salud Carlos III, Spain (CB06/06/1088, PI19/00141).
Author information
Authors and Affiliations
Contributions
K.N.N., A.G.d.L. and J.M.A. contributed to the initial study concept. K.N.N., L.L.P., C.G.E., I.R.B., E.C., M.S.A., D.D.D., B.H., A.G.M., M.A.R., M.Q., M.R.U., A.S., M.V.B., F.G.R., M.L.T., J.M.A. enrolled patients into the study and participated in the data collection and data analysis. J.M.A., J.V., C.G.E., F.G.R. and L.L.P. participated in the data interpretation. K.N.N., J.V. and J.M.A. contributed to the first and subsequent drafts of the manuscript. K.N.N., L.L.P., C.G.E., E.C., F.G.R. and J.M.A., had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nanwani-Nanwani, K., López-Pérez, L., Giménez-Esparza, C. et al. Prevalence of post-intensive care syndrome in mechanically ventilated patients with COVID-19. Sci Rep 12, 7977 (2022). https://doi.org/10.1038/s41598-022-11929-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-11929-8
This article is cited by
-
Rehabilitative interventions in patients with persistent post COVID-19 symptoms—a review of recent advances and future perspectives
European Archives of Psychiatry and Clinical Neuroscience (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.