Abstract
Data concerning the efficacy of SARS-CoV-2 vaccines in patients with non-oncological hematologic conditions are lacking. These include autoimmune cytopenias (autoimmune hemolytic anemia AIHA, immune thrombocytopenia ITP, and autoimmune neutropenia), and bone marrow failure syndromes (aplastic anemia, low risk myelodysplastic syndromes, and paroxysmal nocturnal hemoglobinuria). These conditions may relapse/reactivate after COVID-19 infection and vaccine. Moreover, they are mainly handled with immunosuppressive drugs that may hamper the response to vaccine. In this study, we prospectively evaluated the rate of seroconversion after mRNA SARS-CoV-2 vaccines in patients with autoimmune cytopenias or bone marrow failure syndrome after 2 ± 1 months from the last vaccine dose. Overall, 149 patients were tested and 135 (91%) seroconverted. The highest proportion of non-responders was observed in Evans syndrome (association of ITP and AIHA) and warm AIHA patients (p = 0.001), in those with lower levels of baseline serum IgG (p = 0.008), and in patients on active therapy with steroids (p = 0.03) who also had lower anti-Spike titers. The latter were inversely related with age, and a positively with lymphocyte counts. Additionally, patients who had received rituximab within 12 months from vaccination showed higher rates of non-response (p = 0.03) as compared to those treated before. Contrarily, cyclosporine alone, complement inhibitors, and bone marrow stimulating agents had no detrimental effect on seroconversion. These data suggest maintaining high vigilance and adherence to preventive/protective measures in this population since a proportion of cases may not respond or exhibit low anti-Spike titers.
Similar content being viewed by others
Introduction
There is increasing awareness about the reduced efficacy of SARS-CoV-2 vaccine in patients with hematologic neoplasms. The latter have been shown lower rate of seroconversion both after COVID-19 infection and after mRNA vaccines1,2,3. Most reports regard indolent non-Hodgkin lymphomas (NHL)4,5, and patients receiving B-cell depleting therapies, whilst data concerning non-oncological hematologic conditions are lacking6. The latter, encompass patients with autoimmune cytopenias (i.e. autoimmune hemolytic anemia AIHA, immune thrombocytopenia ITP, and autoimmune neutropenia AIN) and with bone marrow failure syndromes, including aplastic anemia (AA), low risk myelodysplastic syndromes (LR-MDS), and paroxysmal nocturnal hemoglobinuria (PNH). These conditions are rare and clinically heterogeneous, and several reports of disease exacerbations have been described both after COVID-19 infection and its vaccine7,8,9,10,11. More importantly, these diseases are mainly handled with immunosuppressive drugs, including steroids, the anti-CD20 monoclonal antibody rituximab, cytotoxic immunosuppressants such as cyclosporine A, anti-thymocyte globulin (in AA), and complement inhibitors that may hamper the response to vaccine. In this study, we prospectively evaluated the rate of seroconversion after mRNA SARS-CoV-2 vaccines in patients with autoimmune cytopenias or bone marrow failure syndrome undergoing vaccination at a single center in Milan, Italy. We focused on clinical and laboratory risk factors for lower seroconversion that may be used to optimize the timing of vaccination in this patient population.
Methods
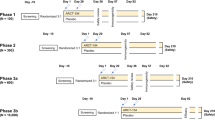
We prospectively evaluated patients with autoimmune cytopenias and bone marrow failure syndromes undergoing SARS-CoV-2 mRNA vaccination from March until October 2021 at two hematologic centers in Milan, Italy. Inclusion criteria were a diagnosis of AIHA, ITP, Evans syndrome (ES, the association of AIHA and ITP, and/or AIN), AA, LR-MDS, or PNH made according to current guidelines12,13,14,15 and the administration of a SARS-CoV-2 mRNA vaccine in the previous 3 months. The study was conducted in accordance with Helsinki Declaration and patients gave informed consent. Only patients ≥ 18 years of age have been enrolled. The study protocol was approved by the Ethical Committee of Istituto Nazionale per le Malattie Infettive Lazzaro Spallanzani, Rome, Italy with the code HECOVID.
Patients were sampled and tested for anti-Spike and anti-Nucleocapside IgG titer at 2 ± 1 months from the second vaccine dose or after the first vaccine dose if the subject had experienced COVID-19 infection. Seroconversion data were matched with clinical and laboratory variables to assess predictors of non-response.
For statistical analysis, we used Wilcoxon rank-sum and chi-squared test to compare categorical and quantitative variables, respectively. We calculated seroconversion proportions and 95% confidence intervals (CI) using the Agresti-Coull formula. Statistical analyses were performed with Stata 17 (StataCorp. 2021).
Ethics approval and consent to participate
Ethics approval and consent to participate were obtained for this study.
Consent for publication
All authors approved present submission.
Results
We enrolled 149 patients (male/female ratio 1.12, median age 73 years, range 17–93) with the following diagnoses: 21 warm type AIHA (wAIHA), 16 cold type AIHA (cAIHA), 25 ITP, 11 ES, 11 AA, 19 PNH, and 46 LR-MDS. Overall, 108 patients were on active treatment, 31 were receiving steroids, 19 cyclosporine A, 20 complement inhibitors (including 3 cAIHA subjects on C1s inhibitor sutimlimab and 17 C5 inhibitors in PNH), 10 cyclosporine combined with steroids, and 28 a bone marrow stimulating agent including the thrombopoietin receptor agonist (TPO-RA) eltrombopag (in ITP) and recombinant erythropoietin (in LR-MDS). The remaining patients were out of therapy: 21 autoimmune cytopenias in remission, and 20 LR-MDS on clinical follow up. Patients received either BNT162b2 (Pfizer-BioNTech, 45%) or mRNA-1273 (Moderna, 55%) vaccine, and 135 (91%) mounted an IgG anti-Spike titer > 0.8 U/mL (the cut-off of our laboratory). As shown in Table 1 and Fig. 1, the highest proportion of non responders was observed in ES and wAIHA patients (36% and 29%, respectively) as compared to < 15% for other diseases (p = 0.001). Non responders displayed lower levels of baseline serum IgG (p = 0.008), whilst neutrophil and lymphocyte counts had no effect. Treatment status had a significant impact (p = 0.03), with a lower rate of seroconversion in patients on active therapy with steroids alone (77%). The detrimental effect of steroids on seroconversion was not clearly related with the dose, as we observed the same rate of non response in patients receiving > or < 20 mg/day of prednisone (25 and 20%, respectively). A similar lower frequency of seroconversion was observed in patients receiving cyclosporine associated with steroids (80%), whilst cyclosporine single agent and complement inhibitors had no effect. No patients were receiving rituximab during vaccination, however 38 had received the drug with a median time from rituximab to vaccination of 24 (1–156) months. Patients who had received rituximab had a higher prevalence of non-response 20% versus 9% compared to rituximab naïve cases (p = 0.04) and those who seroconverted had a longer time from rituximab to vaccination (p = 0.06). Once categorized, patients who had received rituximab within 12 months from vaccination showed higher rates of non response (38% versus 7%, p = 0.03) as compared to those treated before (Fig. 1). Finally, BM stimulating agents had no impact on seroconversion.
Regarding anti-Spike IgG titers (median 283, 1–40,000 U/mL), a great heterogeneity was noted among responding patients. In particular, an inverse correlation was observed with age (r = − 0.28, p = 0.009), and a positive one with lymphocyte counts (r = 0.47, p = 0.0003) (Fig. 2). Moreover, IgG titers distributed differently across disease types and therapies (Fig. 3): patients with AIHA, ES, and AA had IgG anti-Spike titers below the median of the entire cohort (93, 1.1–12,500 U/mL versus 356, 1–40,000 U/mL, p = 0.04), and those receiving steroids alone or combined to CyA displayed significant lower anti-Spike IgG titers (median 73, 1.1–12,500 U/mL for steroids, 113, 3–12,500 U/mL for cyclosporine plus steroids) as compared to the others (356, 3–40,000 U/mL, p = 0.007), irrespective of steroid dose.
As expected, previous COVID19 infection was associated with a high frequency of anti-Spike seroconversion (100% of cases) and higher median anti-Spike titers (6346 U/mL, 76–40,000 versus 283 U/mL, 1.1–9836, p < 0.001). Anti-Nucleocapside titers were positive in all these cases.
Discussion
Here we show for the first time that SARS-CoV-2 double-vaccinated patients with autoimmune cytopenias and bone marrow failure syndromes display a frequency of seroconversion (> 90%), nearly comparable to that of the general healthy population16. This is in line with recent reports dealing with patients with autoimmune rheumatic diseases that showed seroconversion rates exceeding 80% after 2 doses of anti-SARS-CoV-2 vaccine17,18,19.
From a quantitative perspective, seroconversion was predicted by diseases type, with lower rates of response in subjects with autoantibody mediated diseases (i.e. ES and wAIHA) and maximal response in those with bone marrow failure syndromes (i.e. AA, PNH, and LR-MDS) where autoimmunity is reckoned to be more cellular-mediated. This tendency is also mirrored by the favorable association of seroconversion with higher polyclonal IgG levels pre-vaccine. The latter are a good surrogate of the fitness of the humoral system and are therefore a good predictor of seroconversion possibly useful in clinical practice. On the other hand, therapy had also an effect on seroconversion with patients receiving steroids at the time of vaccine showing lower response rates. Interestingly, the detrimental effect of steroids on seroconversion and anti-Spike titers seemed independent from the dose. This is somewhat unexpected since doses < 20 mg/day of prednisone are usually considered safe and have been hypothesized to have negligible effect on seroconversion by recent guidelines17,18,19. Additionally, impairment of humoral response by B-cell depleting treatment with rituximab within the last 12 months was associated with a worse response, whilst T-cell targeting agent cyclosporine had no effect on seroconversion. Finally, complement inhibitors used in PNH and CAD had also no effect on seroconversion, and this is important given the long-term nature of such therapies. On the whole, our experience is similar to that of rheumatic diseases, where treatment with steroids, rituximab, and mycophenolate mofetil significantly reduced the rate of seroconversion18,19. Interestingly, Boekel et al.17 observed that repeated exposure to SARS-CoV-2 via infection or vaccination might abrogate the impairment of immune response in autoimmune patients treated with immunosuppressants. Other strategies to improve seroconversion may be the choice of an alternative treatment if available (i.e. TPO-RA versus rituximab in ITP), vaccinating before B-cell depleting drugs, and allowing enough time for immune reconstitution in patients treated with B-cell depleting agents, as already suggested for lymphoproliferative disorders20,21. In this regard, Tanguay et al. showed that rates of seroconversion raised to 88% if lymphoma patients had received B-cell depletion > 2 years prior to vaccine (versus 5% in those treated within 1 year before vaccine)20.
“Qualitative” response to SARS-CoV-2 vaccine seems more heterogeneous, with some patients exhibiting anti-Spike titers > 10,000 U/mL and other close to the lower cut off of positivity. Lower titers were associated with older age, with autoantibody mediated diseases (ES and wAIHA), with lower lymphocyte counts pre-vaccine, and with ongoing steroid treatment. Although there is great uncertainness regarding the clinical significance of anti-Spike titers to be considered “protective”, it may be hypothesized that lower titers may predict a weaker immune response to SARS-CoV-2 infection. Ferri et al., by studying 478 unselected patients with autoimmune systemic diseases observed significantly lower anti-Spike neutralizing antibodies levels compared to controls (286 (53–1203) versus 825 (451–1542) U/mL) and suggested that these subjects may be named as “suboptimal responders” that should be prioritized for a booster-dose of vaccine, whilst those not responding at all may be administered a different type of vaccine18.
Our study carries several limitations mainly regarding the limited number of subjects and the heterogeneity of the diseases included. The latter do however point at the need of tailored approaches in the choice of treatment type and timing to/from vaccine in each condition, rather than a “one size fits all”. Additionally, we did not test for T-cell response to SARS-CoV-2 vaccine, that has been reckoned to lead immune competence in patients undergoing B-cell depletion. In a recent study including patients with autoimmune rheumatic and glomerular diseases, 82.6% of double vaccinated subjects developed a T-cell response that was diminished in those receiving tacrolimus19. Thus, it may be hypothesized that our patients treated with CyA, although seroconverted, might have decreased T-cell response to SARS-CoV-2 vaccine, suggesting not to lower preventive measures even in this setting.
In conclusion, patients with autoimmune cytopenias and bone marrow failure syndromes showed high frequency of seroconversion after SARS-CoV2 vaccine. However, high vigilance and adherence to preventive/protective measures (use of personal protective equipment and social distancing) are pivotal since a proportion of cases may not respond or exhibit low anti-Spike titers. These cases are mainly those with impaired humoral immunity as shown by reduced polyclonal IgG or lymphocytes pre-vaccine, and those on active steroid therapy or who received rituximab within the last 12 months.
Data availability
All data are available within the manuscript and further may be available upon reasonable request to the corresponding author.
References
Greenberger, L. M. et al. Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell 39(8), 1031–1033. https://doi.org/10.1016/j.ccell.2021.07.012 (2021) (Epub 2021 Jul 22).
Maneikis, K. et al. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: A national prospective cohort study. Lancet Haematol. 8(8), e583–e592. https://doi.org/10.1016/S2352-3026(21)00169-1 (2021) (Epub 2021 Jul 2).
Malard, F. et al. Weak immunogenicity of SARS-CoV-2 vaccine in patients with hematologic malignancies. Blood Cancer J. 11(8), 142. https://doi.org/10.1038/s41408-021-00534-z (2021).
Perry, C. et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with B-cell non-Hodgkin lymphoma. Blood Adv. 5(16), 3053–3061. https://doi.org/10.1182/bloodadvances.2021005094 (2021).
Ehmsen, S. et al. Antibody and T cell immune responses following mRNA COVID-19 vaccination in patients with cancer. Cancer Cell 39(8), 1034–1036. https://doi.org/10.1016/j.ccell.2021.07.016 (2021) (Epub 2021 Jul 27).
Griffiths, E. A. & Segal, B. H. Immune responses to COVID-19 vaccines in patients with cancer: Promising results and a note of caution. Cancer Cell 39(8), 1045–1047. https://doi.org/10.1016/j.ccell.2021.07.001 (2021) (Epub 2021 Jul 3).
Barcellini, W., Giannotta, J. A. & Fattizzo, B. Are patients with autoimmune cytopenias at higher risk of COVID-19 pneumonia? The experience of a reference center in northern Italy and review of the literature. Front. Immunol. 11, 609198. https://doi.org/10.3389/fimmu.2020.609198 (2021).
Fattizzo, B., Giannotta, J. A., Cecchi, N. & Barcellini, W. SARS-CoV-2 vaccination induces breakthrough hemolysis in paroxysmal nocturnal hemoglobinuria on complement inhibitor. Am. J. Hematol. 96(9), E344–E346. https://doi.org/10.1002/ajh.26262 (2021) (Epub 2021 Jun 18).
Fattizzo, B., Pasquale, R., Bellani, V., Barcellini, W. & Kulasekararaj, A. G. Complement mediated hemolytic anemias in the COVID-19 era: Case series and review of the literature. Front. Immunol. 12, 791429. https://doi.org/10.3389/fimmu.2021.791429 (2021).
Barcellini, W. et al. COVID-19 in patients with paroxysmal nocturnal haemoglobinuria: An Italian multicentre survey. Br. J. Haematol. 194(5), 854–856. https://doi.org/10.1111/bjh.17558 (2021) (Epub 2021 May 25).
Fattizzo, B., Giannotta, J. A., Cecchi, N. & Barcellini, W. SARS-CoV-2 vaccination in patients with autoimmune cytopenias: The experience of a reference center. Am. J. Hematol. 96(11), E413–E416. https://doi.org/10.1002/ajh.26345 (2021) (Epub 2021 Sep 16).
Jäger, U. et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: Recommendations from the First International Consensus Meeting. Blood Rev. 41, 100648. https://doi.org/10.1016/j.blre.2019.100648 (2020) (Epub 2019 Dec 5).
Neunert, C. et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 3(23), 3829–3866. https://doi.org/10.1182/bloodadvances.2019000966 (2019) (Erratum in: Blood Adv. 2020;4(2):252).
Fenaux, P. et al. Myelodysplastic syndromes: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 32(2), 142–156. https://doi.org/10.1016/j.annonc.2020.11.002 (2021) (Epub 2020 Nov 19).
Killick, S. B. et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br. J. Haematol. 172(2), 187–207. https://doi.org/10.1111/bjh.13853 (2016) (Epub 2015 Nov 16, Erratum in: Br J Haematol. 2016 Nov;175(3):546).
Lombardi, A. et al. SARS-CoV-2 anti-spike antibody titres after vaccination with BNT162b2 in naïve and previously infected individuals. J. Infect. Public Health. 14(8), 1120–1122. https://doi.org/10.1016/j.jiph.2021.07.005 (2021) (Epub 2021 Jul 17).
Boekel, L. et al. Antibody development after COVID-19 vaccination in patients with autoimmune diseases in the Netherlands: A substudy of data from two prospective cohort studies. Lancet Rheumatol. 3(11), e778–e788 (2021).
Ferri, C. et al. Impaired immunogenicity to COVID-19 vaccines in autoimmune systemic diseases. High prevalence of non-response in different patients’ subgroups. J. Autoimmun. 125, 102744 (2021).
Prendecki, M. et al. Humoral and T-cell responses to SARS-CoV-2 vaccination in patients receiving immunosuppression. Ann. Rheum. Dis. 80(10), 1322–1329 (2021).
Tanguay, M. et al. B-cell cytopenia and time to last B-cell-depleting therapy predict response to SARS-COV-2 vaccines in patients with lymphoproliferative disorders. Vaccine. 40(9), 1203–1207 (2022).
Fattizzo, B. et al. Seroconversion to mRNA SARS-CoV-2 vaccines in hematologic patients. Front. Immunol. (2022) (in Press)
Author information
Authors and Affiliations
Contributions
B.F., M.B., J.A.G., S.C., and W.B. followed patients, collected data, and wrote the manuscript. D.C. performed statistical analysis. All Authors revised the manuscript for important intellectual content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fattizzo, B., Bortolotti, M., Giannotta, J.A. et al. Seroconversion to mRNA SARS-CoV-2 vaccines in patients with autoimmune cytopenias and bone marrow failures. Sci Rep 12, 7743 (2022). https://doi.org/10.1038/s41598-022-11857-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-11857-7
This article is cited by
-
Chronic cold agglutinin disease after a third COVID-19 mRNA vaccination
International Journal of Hematology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.