Abstract
Citrus is among the most important fruit crops worldwide; however, numerous pests and diseases affect the orchards, increasing production costs. The psyllid Diaphorina citri, is a vector of the phloem-limited bacteria ‘Candidatus Liberibacter spp.’, the causal agent of Huanglongbing (HLB) disease. The lack of a cure for HLB requires management of the vector, mainly by intensive use of chemical insecticides, leading to the selection of resistant populations. Our study determined the effects of the entomopathogenic fungus Cordyceps fumosorosea on the probing behavior of D. citri at different time points after the fungus was applied by spraying. The electrical penetration graph technique was used to monitor the stylet activities of D. citri after application of the microbiological product. The effects were more pronounced between 30 and 96 h after the insects were sprayed, with significant disruption of the stylet activities related to the phloem and directly associated with the transmission of HLB. Our study indicated that the microbiological product Challenger®, with the active ingredient C. fumosorosea fungus, can significantly change the probing behavior of D. citri, may be helpful in more-sustainable management of the vector, and can be used to reduce the spread of HLB.
Similar content being viewed by others
Introduction
Citrus is one of the most important fruit crops in the world economically important for Brazil, which ranks among the main citrus producers after China, and is the largest producer of fresh oranges and orange juice1. However, citriculture is affected by many phytosanitary problems that cause significant losses and increases in production costs.
A serious pest in citriculture is the Asian citrus psyllid (ACP), Diaphorina citri Kuwayama (Hemiptera: Psyllidae), which causes little direct damage but is the main insect vector in citrus orchard, transmitting the phloem-limited α-proteobacteria 'Candidatus Liberibacter asiaticus' and 'Ca. L. americanus’, causal agents of the disease known as Huanglongbing (HLB) or citrus greening2,3. HLB is highly destructive and affects practically all existing commercial citrus cultivars, causing significant economic losses3,4. In the Americas, this disease was detected for the first time in 2004 in the State of São Paulo, Brazil5,6 and in 2005 in the State of Florida, USA7.
‘Candidatus Liberibacter spp.’ can be disseminated through grafting/vegetative propagation of infected plants and by the ACP, and the severity incidence of HLB is dependent on D.citri densities and movement from infected to non-infected citrus trees. These phytobacteria have a close relationship with their vector species and are transmitted in a persistent-propagative manner to new plants through insect feeding, being acquired when the insect ingests phloem sap of the infected citrus tree and inoculates the bacteria into healthy plants via its saliva8,9,10. The HLB-associated bacteria can be acquired by the vector after a few minutes of feeding on infected plants; the longer the feeding period, the greater the transmission efficiency11.
The Foundation of Citrus Growers of São Paulo State (FUNDECITRUS) estimated that the average incidence of symptomatic orange trees for HLB in 2019 was 19%, rising to 20.9% in 2020 in the citrus belt of São Paulo and southwestern Minas Gerais, Brazil12. Because no curative methods to HLB are available, the disease is managed by planting healthy citrus seedlings, using HLB-resistant rootstocks13, eliminating infected plants to reduce the source of inoculum, and controlling the insect vector D. citri with chemical insecticides. This last is the main management method used by citrus producers14,15.
Indiscriminate use of chemical insecticides to control D. citri, in addition to being economically unsustainable for producers, often generates imbalances in the agroecosystem, interfering with the management and biological control of other citrus pests. A wide variety of natural enemies could be used in the biological control of this psyllid species, thus reducing the need for massive applications of traditional insecticides. Among the main biological-control agents we can mention: parasitoid species such as Tamarixia radiata (Waterston, 1922) (Hymenoptera: Eulophidae) and Diaphorencytus aligarhensis (Shafee; Alan; Argawal, 1975) (Hymenoptera: Encyrtidae)16,17; predatory insects, mainly of the orders Coleoptera, Neuroptera and Diptera18,19,20; and entomopathogenic microorganisms21,22,23.
Among microorganisms, entomopathogenic fungi are the main microbiological agent of natural mortality of D. citri and spread horizontally among insects, being able to infect different pest species. Cordyceps fumosorosea (Wize) Kepler, Shrestha & Spatafora (Hypocreales: Cordycipitaceae) (basionym: Isaria fumosorosea), is a generalist species of fungus, commercially available and with high potential for D. citri control24,25.
Several studies have reported high insect mortality after C. fumosorosea application25,26,27,28,29. Others have evaluated the effects of traditional insecticides on vector probing behavior, with implications for the transmission of phytopathogens10,30,31,32. However, very few have investigated the effects of entomopathogenic fungi on the probing behavior of insect vectors before they are killed by the fungi33,34,35. These studies provide essential information for managing phytopathogens and their vectors since disruptions in stylet activities can affect the efficiency of transmission of pathogens to new host plants via vector feeding.
The probing behavior of sap-sucking insects can be monitored using the electrical penetration graph (EPG) technique36,37, which shows the stylet penetration into plant tissues in real-time on a computer monitor. The graph (waveforms) is later analyzed and correlated with biological activities, including the transmission of phytopathogens10,31,34.
In the present study, we hypothesized that by coming into direct contact with the insect (before its death), the entomopathogenic fungus C. fumosorosea would cause physiological and behavioral changes in the ACP D. citri due to the infection process, modulating the insect's stylet activities, affecting the ability to feed normally on phloem, and possibly reducing the chances of HLB transmission to new plants by bacteriliferous psyllids. We investigated if the fungus could modulate the stylet and probing activities of the ACP at different time points after spraying, allowing us to detect possible effects of the fungus on the insect´s probing behavior and the main period when the fungus affects the probing behavior before the insects die. The results would indicate if this biological-control agent would be efficient only in reducing psyllid populations or could also be an important means of reducing ACP feeding activities and/or HLB transmission in citrus orchards.
Material and methods
Biological material: D. citri colony and plants
A colony of healthy D. citri was maintained in screened cages with an aluminum frame (35 × 35 × 53 cm) and acrylic door, containing orange jasmine plants, Murraya paniculata (L.) Jack (Rutaceae) in a climate-controlled room (25 ± 2 °C; photoperiod of 14L:10D; relative humidity 60–70%), under fluorescent lights at Escola Superior de Agricultura “Luiz de Queiroz” (ESALQ-USP), Piracicaba, Brazil. Healthy ‘Rangpur lime’ seedlings were used for the feeding-behavior assays. Each insect and seedling were used only once in the EPG assays.
Biological insecticide application
Before the probing behavior assays, adults (1–7 days old) of D. citri from the maintenance colony were placed in a glass tube and immobilized for 2–4 min in crushed ice. Thirty psyllids (not sexed) were placed in the center of an open Petri dish lined with filter paper. The insects were sprayed with a solution of the microbiological insecticide Challenger® (Cordyceps fumosorosea, strain ESALQ 1296) (Koppert Biological Systems) at the dose recommended by the manufacturers (400 µl of the commercial product/100 ml of water). Approximately 1.5 ml of solution (water + commercial product) was sprayed on each group of psyllids (at 1 m between the insects and the spray bottle). To ensure fungus infection, after the EPG recording the insects were placed in a humid chamber to certify sporulation, and only insects with confirmed mortality caused by C. fumosorosea were used in the analyses.
As a control, the same protocol was used but the insects were sprayed only with water. The time points used were: 0, 15, 30, 48, 72, 96, and 120 h after insect spraying, conforming to the general biological phases of the fungus (adhesion, germination, penetration, vegetative growth)38,39,40. Each time point was composed of the treatment with C. fumosorosea (treated insects) and its control (untreated insects). The insects were sprayed in groups of 30 and then kept in cages containing Rangpur lime seedlings to fulfill the necessary period for the EPG experiment, except for time point 0-h, when the insects were used immediately after spraying. Treated and untreated psyllids were kept in separate cages and rooms (25 ± 2 °C; photoperiod of 14L:10D) to avoid contamination.
Stylet activities and probing behavior of Diaphorina citri
The probing behavior of D. citri was evaluated in real-time using the EPG technique to monitor stylet activities at different time points after C. fumosorosea application.
To prepare the insects for the EPG assay, healthy adult females of D. citri (1–10 days old) from a colony maintained on orange jasmine were enclosed in a glass tube placed in an ice bath for 2–4 min to facilitate manipulation and then immobilized in a vacuum chamber under a dissecting microscope. Then, a gold wire (3 cm long, 18 µm in diameter; EPG Systems, Wageningen, The Netherlands) was attached to the psyllid pronotum with a small droplet of water-based silver glue. The opposite end of the gold wire was glued to a thin copper wire (2 cm long), which was connected to the EPG probe. Another copper electrode (10 cm long, 2 mm wide) was inserted into the soil of the plant.
After a 1-h starvation period, each psyllid was placed individually on the abaxial surface of a young Rangpur lime leaf. The EPG waveforms were recorded for 8 h inside a Faraday cage (for electrical noise isolation) in a climate-controlled room (25 ± 1 °C) using a Direct Current eight-channel EPG device, model Giga-8d, with Stylet+ for Windows software (EPG Systems). Twenty-two replicates were analyzed per each treatment (treated and untreated insects) at each time point. Also, the mortality of the insects analyzed in the feeding behavior assays were evaluated after 24 h of the beginning of each EPG recording.
Statistical analysis
The EPG data were analyzed according to the waveforms described for D. citri by Bonani et al.41: non-probing (waveform np); intercellular apoplastic stylet pathway and salivary sheath secretion (waveform C); first phloem contact (waveform D); salivation into the phloem (waveform E1); passive phloem-sap ingestion (waveform E2); and active intake of xylem sap (waveform G).
The output of EPG recordings given by the EPG-Excel Data Workbook 5.0 of Sarria et al.42 for each insect was used to calculate the treatment mean for the sequential and non-sequential variables of each EPG. The selected EPG variables (mean ± SE) were calculated and compared between treatments as described by Backus et al.43: PPW: proportion of individuals that generated a particular waveform type; NWEI: number of waveform events per insect; WDI: total waveform duration (min) per insect; and sequential variables: ‘Time to 1st probe from the start of EPG’, ‘Number of probes to the 1st E1’, and ‘Time from start of EPG to 1st E’.
Before analysis, normality and homogeneity of variance were checked, and when necessary, the data were transformed with ln (x + 1) or √ (x + 1) to reduce heteroscedasticity and improve normal distribution. All parameters were analyzed with a parametric Student’s t-test (data with Gaussian distribution) or a nonparametric Mann–Whitney U test (data with non-Gaussian distribution). A chi-squared test was used to analyze the proportion of individuals that generated a particular waveform type (PPW). All data were analyzed using IBM Statistics SPSS 22.0 software44.
The insects treated with C. fumosorosea vs. untreated insects (control) were compared at the same time point. A combined analysis was not performed because the experiments at each time point were performed separately; in addition, due to the post-spray period, the individuals did not have the same age at the different time points.
The EPG records considered valid for statistical analysis were those in which: (a) the insects were on the plant and glued to the wire after 8 h of recording; (b) the insects performed some feeding activity in at least 5 of the 8 h evaluated; (c) insects that, after recording, were placed in a humid chamber and sporulated (confirming infection by C. fumosorosea or were negative for the untreated insects, ensuring that the changes in behavior were due to infection by the fungus.
Ethical approval
The authors have permission to use all biological materials used in this study. Also, all methods were carried out in accordance with relevant guidelines and regulations.
Results
0 h after spraying
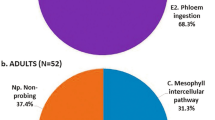
In the experiment with ACP at the time point 0-h after spraying, that is, sprayed and immediately connected to the EPG system to record the activities, no differences were observed in non-sequential variables, for number (NWEI) and total duration (WDI) of non-phloem (wave C, np, G) (Fig. 1) and phloem activities (D, E1 and E2) (P > 0.05) (Fig. 2; Supplementary material Table S1) and in the proportion of insects (PPW) that performed salivation into the phloem (E1) (x2 = 1.504; P = 0.36) and ingested phloem sieve (E2) (x2 = 1.467; P = 0.36) (Fig. 4).
Number of waveform event per insect (NWEI) and Total waveform duration per insect (WDI) of non-probing (A,B) and waveform C (intercellular stylet pathway) (C,D) of Asian-citrus-psyllids Diaphorina citri on ‘Rangpur lime’ seedlings at different time points after microbiological insecticide pulverization (Cordyceps fumosorosea, strain ESALQ 1296). The columns and bars represent the mean and the standard error for each variable and time point. Bars with an asterisk (*) indicate a statistically significant difference (P < 0.05) according to t-Student test or Mann–Whitney U test between untreated vs. treated psyllids in the same time point (ns non-significant).
Number of waveform event per insect (NWEI) and Total waveform duration per insect (WDI) of E1 (phloem salivation) (A,B) and E2 (phloem ingestion) (C,D) of Asian-citrus-psyllids Diaphorina citri on ‘Rangpur lime’ seedlings at different time points after microbiological insecticide pulverization (Cordyceps fumosorosea, strain ESALQ 1296). The columns and bars represent the mean and the standard error for each variable and time point. Bars with an asterisk (*) indicate a statistically significant difference (P < 0.05) according to Mann–Whitney U test between untreated vs. treated psyllids in the same time point. (ns non-significant).
The only difference observed for the sequential variables was in the 'Number of probes for the 1st E1': ACP treated with C. fumosorosea performed fewer probes before the first phloem salivation (E1) than untreated insects (U = 152,000; df = 1; P = 0.03) (Fig. 3).
EPG sequential variables of probing behavior of Asian-citrus-psyllids Diaphorina citri on ‘Rangpur lime’ seedlings at different time points after microbiological insecticide pulverization (Cordyceps fumosorosea, strain ESALQ 1296). The columns and bars represent the mean and the standard error for each variable and time point. Bars with an asterisk (*) indicate a statistically significant difference (P < 0.05) according to t-Student test or Mann–Whitney U test between untreated vs. treated psyllids in the same time point (ns non-significant).
15 h after spraying
The effects of C. fumosorosea on the stylet activities of the ACP were more apparent when EPG recordings were started 15 h after insect spraying (Supplementary Table S2) However, the differences were limited to non-phloem parameters such as the number (NWEI) of non-probing (t = 3.859; df = 1; P < 0.01) and intercellular stylet pathway (waveform C) (t = 3.849; df = 1; P < 0.01) activities. Treated ACP performed these activities less often than untreated insects, without affecting the duration (WDI) of the activities (Fig. 1).
Treated ACP spent more time (23.8 ± 6.9 min) to perform the ‘1st probe from start of EPG’ than untreated insects (8.4 ± 5.0 min) (t = − 3.328; df = 1; P < 0.01), and as well as at time point 0-h, treated ACP performed fewer probes to the 1st E1 than untreated insects (U = 159.500; df = 1; P < 0.03) (Fig. 3).
No differences were observed between treatments in the PPW of E1 (x2 = 1.467; P = 0.36) and E2 (x2 = 1.467; P = 0.36) (Fig. 4).
Proportion (PPW) of Asian-citrus-psyllids Diaphorina citri that generated salivation into phloem sieve elements (waveform E1) (A) and passive phloem-sap ingestion (waveform E2) (B) on ‘Rangpur lime’ seedlings at different time points after microbiological insecticide pulverization (Cordyceps fumosorosea, strain ESALQ 1296). Bars with an asterisk (*) indicate a statistically significant difference (P < 0.05) according to chi-square (x2) test with Bonferroni correction for specific pairwise comparisons.
30 h after spraying
The effects of the treatment with C. fumosorosea started to become more apparent and reached phloem parameters when EPG recordings were started 30 h after the insect was sprayed (Supplementary Table S3).
The proportion (PPW) of treated ACP that performed phloem-feeding activities was significantly lower compared to untreated insects, for both E1 (x2 = 9.167; P < 0.01) and E2 (x2 = 7.379; P = 0.01) (Fig. 4). Treated ACP that were able to reach the phloem vessels salivated (waveform E1) (NWEI: U = 157,000; df = 1; P = 0.03) and ingested (waveform E2) (NWEI: U = 162.500; df = 1; P = 0.04) phloem sap less often than untreated insects (Fig. 2). However, only the ingestion time was affected, and treated ACP ingested phloem sap for less than one hour, in contrast to untreated insects, which ingested phloem content for 2.5 h on average (WDI: U = 126.000; df = 1; P < 0.01) (Fig. 2). Thus, the fungus shortened the phloem phase (E = E1 + E2) (treated ACP: 48.8 ± 18.46 min; untreated ACP: 156.52 ± 27.93 min) (U = 113,500; df = 1; P = 0.02).
Unlike the earlier periods, 30 h after spraying, treated ACP took longer, from the beginning of the EPG recording, to perform the first activity in the phloem than the untreated ACP (U = 146,500; df = 1; P = 0.02) (Fig. 3).
48 h after spraying
Forty-eight hours after ACP spraying, both non-phloem and phloem-feeding activities were significantly affected by C. fumosorosea infection.
Treated ACP performed non-probing periods (waveform np) more often (NWEI: t = − 3.984; df = 1; P < 0.01) and the total duration of these periods was significantly longer (WDI: t = − 3.404; df = 1; P < 0.01) than in untreated ACP. Also, intercellular stylet pathway activities (waveform C) were performed more frequently by treated ACP than by untreated ones (NWEI: t = − 3.527; df = 1; P < 0.01), although without affecting the duration of this parameter (WDI: U = 163.000; df = 1; P = 0.06) (Fig. 1).
The proportion (PPW) of treated ACP that performed the phloem-feeding phase was significantly lower than in untreated ACP, for phloem salivation (waveform E1) (x2 = 9.402; P < 0.01) and phloem ingestion (waveform E2) (x2 = 7.503; P = 0.01) (Fig. 4). Treated ACP performed fewer phloem-salivation (waveform E1) (NWEI: U = 130.000; df = 1; P = 0.01) and phloem-ingestion (waveform E2) (NWEI: U = 130.500; df = 1; P < 0.01) activities than untreated psyllids (Fig. 2). The same tendency was observed for the total duration of the phloem salivation period (WDI: U = 122.000; df = 1; P < 0.01) and phloem ingestion period (WDI: U = 103.000; df = 1; P < 0.01), which were significantly shorter in treated than untreated psyllids (Fig. 2).
Differences were observed in all sequential parameters evaluated. The 'Time to 1st probe from starts of EPG' was shorter in treated ACP (19.19 ± 6.33 min) than untreated psyllids (40.29 ± 12.19 min) (t = 2.152; df = 1; P = 0.04). The 'Number of probes to the 1st E1' was high for treated than untreated ACP (t = − 4.397; df = 1; P < 0.01). The same tendency was observed for 'Time from start of EPG to 1st E' (U = 91.000; df = 1; P < 0.01) (Fig. 3; Supplementary Table S4).
72 h after spraying
As in the 48-h time point, the probing behavior of ACP sprayed 72 h before the beginning of EPG recording was affected, both in non-phloem (Fig. 1) and phloem activities (Fig. 2; Supplementary Table S5).
Treated ACP performed non-probing periods (waveform np) more often (NWEI: t = − 5.364; df = 1; P < 0.01); the total duration of these periods was three times longer (WDI: U = 73.000; df = 1; P < 0.01) (Figure) than in untreated psyllids. The number of stylet pathways (waveform C) was also higher for treated than for untreated ACP (NWEI: t = − 3.711; df = 1; P < 0.01), however without influencing the duration of this parameter (WDI: U = 73.000; df = 1; P = 0.09) (Fig. 1).
The proportion (PPW) of treated ACP that performed phloem activities was significantly lower compared to untreated ACP, for E1 (x2 = 19,250; P < 0.01) and E2 (x2 = 11.023; P < 0.01) (Fig. 4). Also, treated ACP performed fewer E1 (NWEI: U = 101.500; df = 1; P = 0.01) and E2 (NWEI: U = 100.500; df = 1; P < 0.01), with shorter durations ((WDI-E1: U = 91.500; df = 1; P < 0.01); (WDI-E2: U = 84.000; df = 1; P < 0.01) than untreated psyllids (Fig. 2). Furthermore, treated ACP performed three times more probes before the first E1 than untreated psyllids (t = − 5.481; df = 1; P < 0.01), as well as taking twice as long from the start of the EPG to the first contact with the phloem vessels than untreated insects did (U = 61.500; df = 1; P < 0.01) (Fig. 3).
96 h after spraying
Four days (96 h) after the insects were treated, only the non-probing duration was affected regarding non-phloem parameters: treated ACP remained three times longer with their stylets outside the plant tissue than did untreated psyllids (WDI: U = 59.000; df = 1; P < 0.01) (Fig. 1; Supplementary Table S6).
The proportion (PPW) of treated ACP that performed phloem salivation (E1 waveform) was significantly lower than in untreated insects (x2 = 5.867; P = 0.01) (Fig. 4). Also, treated insects performed fewer E1 (NWEI: U = 134,000; df = 1; P < 0.01) and E2 (NWEI: U = 159,500; df = 1; P = 0.03), and the duration of these activities was also significantly shorter ((WDI-E1: U = 133.000; df = 1; P < 0.01); (WDI-E2: U = 162.000; df = 1; P = 0.03) (Fig. 2).
The sequential parameters differed only in the 'Time from start of EPG to 1st E', which was longer in the treated ACP (U = 142.00; df = 1; P = 0.01) (Fig. 3).
120 h after spraying
Few effects on probing behavior were observed 5 days (120 h) after insects were treated. The non-probing time of treated ACP was longer than in untreated insects (NWEI: t = 1.832; df = 1; P = 0.04) (Fig. 1). Treated ACP also took three times longer to perform the 1st probe from the start of EPG (treated ACP 47.43 ± 12.47 min; untreated ACP 14.22 ± 4.64 min) (t = − 3.766; df = 1; P < 0.01) (Supplementary Table S7).
No differences were observed in parameters associated with phloem (Fig. 2) or in the PPW (Fig. 4) (P > 0.05).
Twenty-four hours after the beginning of the recordings, it was evaluated the number of living and dead insects connected to the EPG system in the control and in the treatment with C. fumosorosea at all time-points, in order to verify possible effects of product application. In the control treatment, the survival of the insects ranged from 90 to 100%, whereas in the treatment where the insects were sprayed with C. fumosorosea ranged from 63 to 95.4% (Table 1).
Discussion
The present study has shown firsthand the effects of C. fumosorosea on the probing behavior of D. citri using the EPG technique. The effects were more pronounced between 30 and 96 h after the insects were sprayed, with significant disruption of the stylet activities mainly in the phloem-vessels, directly associated with the transmission of HLB.
The use of entomopathogenic microorganisms such as fungi and bacteria has been suggested as an alternative to conventional control of agricultural pests, which is based on intensive applications of chemical insecticides, leading to several undesired secondary effects, including the selection of resistant populations to the available chemicals. In recent years there has been a significant increase in studies demonstrating that entomopathogens, especially fungi, play important roles in the ecosystem, as endophytes in plants34, growth promoters45,46, and phytopathogen antagonists47,48.
Much of the research has reported that members of the fungal order Hypocreales such as Beauveria bassiana (Hypocreales: Cordycipitaceae) (Bals. -Criv) Vuill. and C. fumosorosea act as important biological-control agents for different species of hemipterans, such as psyllids24,25,27, aphids34,49, and whiteflies26,50. The effects of these agents on insect behavior are still poorly understood, especially regarding insect vectors of plant diseases.
In general, entomopathogenic microorganisms require time to effectively infect their hosts and kill them, even though the capacity of entomopathogenic fungi to produce many types of secondary metabolites/mycotoxins during the initial phase of colonization of the insect body is well known51,52,53. The insects may continue to feed, causing damage due to probing behavior and transmission of phytopathogens54. Because of the longer time needed to kill the target insect, many producers opt for traditional insecticides, as they are apparently more “aggressive and efficient” (in the eyes of the producer) in terms of how quickly they kill the pest.
Most available insecticides (chemical and microbiological) can cause high mortality in the insect vector population10,21,27,31,55, however, many insecticides cannot affect the probing behavior soon enough to prevent stylet activities associated with transmission of phytopathogens to new host plants. Therefore, for successful control of insect vectors, it is necessary to understand the insect´s characteristics and mechanisms of action of insecticides on its mortality, but also the modes of transmission of plant pathogens and stylet activities associated with transmission10,31,32,34,56.
In general, research on insect vectors and entomopathogenic fungi is mainly focused on evaluating their effects on mortality and indirect quantification of feeding through honeydew excretion21, or on reducing the symptoms of phytopathogens on the plants54. From this information, it is not possible to determine if there really was a change in the stylet activities of the insects in these plants.
The effects of an entomopathogenic fungus on the stylet activities of an insect vector using the EPG technique were evaluated by Gonzáles-Mas et al.34. They evaluated the probing behavior of Aphis gossypii Glover (Hemiptera: Aphididae) and the transmission of the persistent-circulative Cucurbit aphid-borne yellow virus persistent virus (CABYV) (Polerovirus) and the non-persistent Cucumber mosaic virus (CMV) (Cucumovirus) in melon plants colonized endophytically by B. bassiana. They showed that endophytic B. bassiana can be used in integrated pest management programs to reduce virus transmission by A. gossypii, since transmission of both viruses was lower when receptor plants had been treated with a suspension of B. bassiana conidia.
Chen et al.33 observed changes in the probing behavior of the green peach aphid Myzus persicae (Sulzer) (Hemiptera: Aphididae) when infected with the entomopathogenic fungus Pandora neoaphidis (Remaud. & Hennebert) Humber (Entomophthoromycotina: Entomophthorales). The authors found that the number and total duration of brief intracellular probes (pd) were greater for infected than uninfected aphids, which suggests that P. neoaphidis infection may increase the acquisition of a non-persistent virus during such intracellular probes. On the other hand, if infected aphids spent less time ingesting phloem sieve (WDI E2), this can reduce the spread of persistent viruses, which are transmitted during phloem phase.
For transmission of HLB-associated bacteria, the longer the acquisition access period (AAP)11 and inoculation access period (IAP), the greater the transmission efficiency, although the insects can acquire the bacteria within shorter periods57. However, between pathogen acquisition from an infected plant and inoculation into a healthy plant, there is a period required for the insect to be able of inoculating the pathogen, known as the latency period58. The mean latency period for 'Candidatus Liberibacter asiaticus' in adults of D. citri was estimated by Canale et al.59 as 17.8 days and the persistence of transmission by D. citri is approximately 5 weeks, which means that the insects can inoculate the bacterium into new plants for almost their entire life. Thus, there is a considerable period for entomopathogens to act before HLB transmission occurs.
Avery et al.21 observed that D. citri infected with C. fumosorosea produced significantly fewer honeydew droplets per day than the control psyllids, suggesting that they ate less when infected with the entomopathogenic fungus.
We observed that the stylet activities were considerably altered as soon as 30 h and the changes persisted until just over 96 h after spraying. At times shorter than 30 h and longer than 96 h, although the insects were "infected” with the fungus (as shown by the sporulation), the fungus did not disrupt the psyllids’ probing behavior regarding the phloem-feeding activities.
We hypothesize that before 30 h, the fungus did not have time to produce enough mycotoxins to disrupt the probing behaviors, and after 96 h of infection, although infected with C. fumosorosea, we assume that the psyllids that survive these days, may have been more resistant or had a defense system that prevented changes in their stylet activities, since, to infect the insect, the entomopathogenic fungi needs to display several steps, as adhesion of the spores, enzyme production, germination, hemocoel penetration, and replication. Therefore, the fungus must time to overstep the insect´s defense mechanisms to complete the infection process60,61,62.
Lei et al.62 evaluating the mode of action of C. fumosorosea on the diamondback moth larvae, Plutella xylostella (L.) (Lepdoptera: Plutellidae), observed that the first signs of germination started 4 h following inoculation and penetrate the epidermis and began to enter the hemocoel 24 h after. Thirty-six hours post-inoculation, the hyphal bodies colonized the insect body cavity, and 48 h post-inoculation the hyphae reproduced massively. This study shows that the entomopathogen needs time to effectively infect the host and alter its behavior.
The results from the use of the EPG technique indicated that the commercial product containing the fungus C. fumosorosea as the active ingredient disrupts the probing and stylet activities of D. citri. This product has the potential to reduce the psyllid´s capacity for HLB transmission and can be used as part of integrated pest and citrus disease management, being compatible with other control strategies. More detailed studies are needed to better understand these relationships among fungus colonization, secondary metabolites, and probing behavior.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary material files.
References
United States Department of Agriculture (USDA) Foreign Agricultural Service. Citrus: World Markets and Trade. Preprint at https://apps.fas.usda.gov/psdonline/app/index.html#/app/home (2021).
Capoor, S. P., Rao, D. G. & Viswanath, S. M. Diaphorina citri: A vector of the greening disease of citrus in India. Indian J. Agric. Sci. 37, 572–576 (1967).
Bové, J. M. Huanglongbing: A destructive, newly emerging, century-old disease of citrus. J. Plant Pathol. 88, 7–37 (2006).
Gottwald, T. R. Current epidemiological understanding of citrus Huanglongbing. Annu. Rev. Phytopathol. 48, 119–139 (2010).
Coletta-Filho, H. D. et al. First report of the causal agent of Huanglongbing (“Candidatus Liberibacter asiaticus”) in Brazil. Plant Dis. 88, 1382 (2004).
Teixeira, D. C. et al. Citrus huanglongbing in São Paulo State, Brazil: PCR detection of the ‘Candidatus’ Liberibacter species associated with the disease. Mol. Cell. Probes 19, 173–179 (2005).
Halbert, S. The discovery of huanglongbing in Florida. In Proceedings of the Second International Citrus Canker and Huanglongbing Research Workshop; Florida Citrus Mutual: Orlando, FL, USA. Preprint at https://swfrec.ifas.ufl.edu (2005).
Ammar, E. D., Shatters, R. G. & Hall, D. G. Localization of Candidatus Liberibacter asiaticus, associated with citrus huanglongbing disease, in its psyllid vector using fluorescence in situ hybridization. J. Phytopathol. 159, 726–734 (2011).
Ammar, E. D., Ramos, J. E., Hall, D. G., Dawson, W. O. & Shatters, R. G. Jr. Acquisition, replication and inoculation of Candidatus Liberibacter asiaticus following various acquisition periods on huanglongbing-infected citrus by nymphs and adults of the Asian citrus psyllid. PLoS One 11, e0159594 (2016).
Carmo-Sousa, M., Garcia, R. B., Wulff, N. A., Fereres, A. & Miranda, M. P. Drench application of systemic insecticides disrupts probing behavior of Diaphorina citri (Hemiptera: Liviidae) and inoculation of Candidatus Liberibacter asiaticus. Insects 11, 314 (2020).
Pelz-Stelinski, K. S., Brlansky, R. H., Ebert, T. A. & Rogers, M. E. Transmission parameters for Candidatus Liberibacter asiaticus by Asian citrus psyllid (Hemiptera: Psyllidae). J. Econ. Entomol. 103, 1531–1541 (2010).
FUNDECITRUS. Levantamento da incidência das doenças dos citros: Greening , CVC e cancro cítrico no cinturão citrícola de São Paulo e Triângulo/Sudoeste mineiro. Araraquara, SP. www.fundecitrus.com.br (2020).
Urbaneja-Bernat, P., Carrillo, D. & Jaques, J. A. Behavior of Diaphorina citri: An investigation of the potential risk to the most commonly used citrus rootstock in Europe. Entomol. Gen. 40, 79–86 (2020).
Belasque, J. Jr. et al. Lessons from huanglongbing management in São Paulo state, Brazil. J. Plant Pathol. 92, 285–302 (2010).
Bassanezi, R. B. et al. Epidemiologia do huanglongbing e suas implicações para o manejo da doença. Citrus Res. Technol. 31, 11–23 (2010).
Grafton-Cardwell, E. E., Stelinski, L. L. & Stansly, P. A. Biology and managenent of Asian citrus psyllid, vector of the huanglongbing pathognes. Annu. Rev. Entomol. 58, 413–432 (2013).
Kondo, T. et al. A checklist of natural enemies of Diaphorina citri Kuwayama (Hemiptera: Liviidae) in the department of Valle del Cauca, Colombia and the world. Insecta Mundi 457, 1–14 (2015).
Michaud, J. P. & Olsen, L. E. Suitability of Asian citrus psyllid, Diaphorina citri, as prey for ladybeetles. Biocontrol 49, 417–431 (2004).
Gravena, S. Manual prático de manejo ecológico de pragas dos citros. (ed. Gravena). (2005).
Yamamoto, P. T. Parra, J. R. P. Manejo integrado de pragas dos citros in Citros (eds. Mattos Junior, D., Negri, J. D., Pio, R. M. & Pompeu Junior, J.) 729–768 (Centro Apta Citros, 2005).
Avery, P. B. et al. Effects of the fungus Isaria fumosorosea (Hypocreales: Cordycipitaceae) on reduced feeding and mortality of the Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Biocontrol Sci. Technol. 21, 1065–1078 (2011).
Hall, D. G. et al. Observations on the entomopathogenic fungus Hirsutella citriformis attacking adult Diaphorina citri (Hemiptera: Psyllidae) in a managed citrus grove. Biocontrol 57, 663–675 (2012).
Pinto, A. P. F., Filho, A. B., de Almeida, J. E. M. & Wenzel, I. M. Beauveria bassiana pathogenicity to Diaphorina citri and compatibility of the fungus with phytosanitary products Pesqui. Agropecu. Bras. 47, 1673–1680 (2012).
Conceschi, M. R., D’Alessandro, C. P., Moral, R. A., Demétrio, C. G. B. & Delalibera, I. Transmission potential of the entomopathogenic fungi Isaria fumosorosea and Beauveria bassiana from sporulated cadavers of Diaphorina citri and Toxoptera citricida to uninfected D. citri adults. Biocontrol 61, 567–577 (2016).
Ausique, J. J. S., D’Alessandro, C. P., Conceschi, M. R., Mascarin, G. M. & Delalibera, I. Efficacy of entomopathogenic fungi against adult Diaphorina citri from laboratory to field applications. J. Pest Sci. 90, 947–960 (2017).
Wraight, S. P. et al. Evaluation of the entompathogenic fungi Beauveria bassiana and Paecilomyces fumosoroseus for microbial control of the silverleaf whitefly, Bemisia argentifolii. Biol. Control 17, 203–217 (2000).
Avery, P. B. et al. Diaphorina citri (Hemiptera: Psyllidae) infection and dissemination of the entomopathogenic fungus Isaria fumosorosea (Hypocreales: Cordycipitaceae) under laboratory conditions. Fla. Entomol. 92, 608–618 (2009).
Hunter, W. B., Avery, P. B., Pick, D. & Powell, C. A. Broad spectrum potential of Isaria fumosorosea against insect pests of citrus. Fla. Entomol 94, 1051–1054 (2011).
Stauderman, K., Avery, P., Aristizábal, L. & Arthurs, S. Evaluation of Isaria fumosorosea (Hypocreales: Cordycipitaceae) for control of the Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Biocontrol Sci. Technol. 22, 747–761 (2012).
Miranda, M. P., Yamamoto, P. T., Garcia, R. B., Lopes, J. P. A. & Lopes, J. R. S. Thiamethoxam and imidacloprid drench applications on sweet orange nursery trees disrupt the feeding and settling behavior of Diaphorina citri (Hemiptera: Liviidae). Pest Manag. Sci. 72, 1785–1793 (2016).
Maluta, N. K. P., Lopes, J. S. P., Fiallo-Olivé, E., Navas-Castillo, J. & Lourenção, A. L. Foliar spraying of tomato plants with systemic insecticides: effects on feeding behavior, mortality, and oviposition of Bemisia tabaci (Hemiptera: Aleyrodidae) and inoculation efficiency of tomato chlorosis virus. Insects 11, 559 (2020).
Maluta, N. K. P., Lopes, J. S. P., Fiallo-Olivé, E., Navas-Castillo, J. & Lourenção, A. L. Foliar application of systemic insecticides disrupts feeding behavior of the whitefly Bemisia tabaci MEAM1 and the transmission of tomato chlorosis virus in potato plants. J. Pest Sci. 94, 1265–1276 (2021).
Chen, C., Ye, S., Hu, H., Xue, C. & Yu, X. Use of electrical penetration graphs (EPG) and quantitative PCR to evaluate the relationship between feeding behaviour and Pandora neoaphidis infection levels in green peach aphid, Myzus persicae. J. Insect Physiol. 104, 9–14 (2018).
González-Mas, N., Quesada-Moraga, E., Plaza, M., Fereres, A. & Moreno, A. Changes in feeding behaviour are not related to the reduction in the transmission rate of plant viruses by Aphis gossypii (Homoptera: Aphididae) to melon plants colonized by Beauveria bassiana (Ascomycota: Hypocreales). Biol. Control 130(95), 103 (2019).
Fingu-Mabola, J. C., Bawin, T. & Francis, F. Direct and indirect effect via endophytism of entomopathogenic fungi on the fitness of Myzus persicae and its ability to spread PLRV on tobacco. Insects 12, 89 (2021).
Tjallingii, W. F. Electronic recording of penetration behavior by aphids. Entomol. Exp. Appl. 24, 721–730 (1978).
Tjallingii, W. F. Electrical recording of stylet penetration activities. In Aphids: Their Biology. Natural Enemies and Control (eds. Minks, A. K. & Harrewijn, P. J.) 95–108, (Elsevier, 1988).
Boomsma, J. J., Jensen, A. B., Meyling, N. V. & Eilenberg, J. Evolutionary interaction networks of insect pathogenic fungi. Annu. Rev. Entomol. 59, 467–485 (2014).
Lacey , L. A. Microbial Control of Insect and Mite Pests: From Theory to Practice. (ed. Lacey, L. A) (Academic Press, 2017).
Alves, S. B. Fungos entomopatogênicos. In Controle Microbiano de Insetos. (ed. Alves, S. B.) 289–381 (FEALQ, 1998).
Bonani, J. P. et al. Caracterization of electrical penetration graphs of the Asian citrus psyllid, Diaphorina citri, in sweet orange seedlings. Entomol. Exp. Appl. 134, 35–49 (2010).
Sarria, E., Cid, M., Garzo, E. & Fereres, A. Excel Workbook for automatic parameter calculation of EPG data. Comput. Electron. Agric. 67, 35–42 (2009).
Backus, E. A., Cline, A. R., Ellerseick, M. R. & Serrano, M. S. Lygus hesperus (Hemiptera: Miridae) feeding on cotton: New methods and parameters for analysis of non-sequential electrical penetration graph data. Ann. Entomol. Soc. Am. 100, 296–310 (2007).
IBM Corp. IBM SPSS Statistics for Windows, Version 22.0. (IBM Corp., 2013).
Sasan, R. K. & Bidochka, M. J. The insect-pathogenic fungus Metarhizium robertsii (Clavicipitaceae) is also an endophyte that stimulates plant root development. Am. J. Bot. 99, 101–107 (2012).
Jaber, L. R. & Enkerli, J. Effect of seed treatment duration on growth and colonization of Vicia faba by endophytic Beauveria bassiana and Metarhizium brunneum. Biol Control 103, 187–195 (2016).
Jaber, L. R. & Salem, N. M. Endophytic colonization of squash by the fungal entomopathogen Beauveria bassiana (Ascomycota: Hypocreales) for managing Zucchini yellow mosaic virus in cucurbits. Biocontrol Sci. Technol. 24, 1096–1109 (2014).
Jaber, L. R. Grapevine leaf tissue colonization by the fungal entomopathogen Beauveria bassiana s.l. and its effect against downy mildew. Biocontrol 60, 103–112 (2015).
Jandricic, S. E., Filotas, M., Sanderson, J. P. & Wraight, S. P. Pathogenicity of conidiabased preparations of entomopathogenic fungi against the greenhouse pest aphids Myzus persicae, Aphis gossypii, and Aulacorthum solani (Hemiptera: Aphididae). J. Invertebr. Pathol. 118, 34–46 (2014).
Wu, J., Yang, B., Xu, J., Cuthbertson, A. G. S. & Ali, S. Characterization and toxicity of crude toxins produced by Cordyceps fumosorosea against Bemisia tabaci (Gennadius) and Aphis craccivora (Koch). Toxins 13, 220 (2021).
Xu, Y. et al. Biosynthesis of the cyclooligomer depsipeptide beauvericin, a virulence factor of the entomopathogenic fungus Beauveria bassiana. Chem. Biol. 15, 898e907 (2008).
Schrank, A. & Vainstein, M. H. Metarhizium anisopliae enzymes and toxins. Toxicon 56, 1267e1274 (2010).
Qasim, M. et al. Characterization of mycotoxins from entomopathogenic fungi (Cordyceps fumosorosea) and their toxic effects to the development of asian citrus psyllid reared on healthy and diseased citrus plants. Toxicon 188, 39–47 (2020).
Lacey, L. A. et al. Entomopathogenic fungi (Hypocreales) for control of potato psyllid, Bactericera cockerelli (Šulc) (Hemiptera: Triozidae) in an area endemic for zebra chip disease of potato. Biol. Control 56, 271–278 (2011).
Lacey, L. A., de la Rosa, F. & Horton, D. R. Insecticidal activity of entomopathogenic fungi (Hypocreales) for potato psyllid, Bactericera cockerelli (Hemiptera: Triozidae): Development of bioassay techniques, effect of fungal species and stage of the psyllid. Biocontrol Sci. Technol. 19, 957–970 (2009).
Garzo, E., Moreno, A., Plaza, M. & Fereres, A. Feeding behavior and virus-transmission ability of insect vectors exposed to systemic insecticides. Plants 9, 895 (2020).
Capoor, S., Rao, D. & Viswanath, S. Greening disease of citrus in the Deccan Trap Country and its relationship with the vector, Diaphorina citri Kuwayama. In Proc. 6th Conf. Int. Organization of Citrus Virologists, 43–49. Preprint at https://escholarship.org/uc/item/6rm6x1tw (1974).
Purcell, A. H. Insect vector relationships with procaryotic plant pathogens. Annu. Rev. Phytopathol. 20, 397–417 (1982).
Canale, M. C., Tomaseto, A. F., Haddad, M. L., Coletta-Filho, E. D. & Lopes, J. R. S. Latency and persistence of ‘Candidatus Liberibacter asiaticus’ in its psyllid vector, Diaphorina citri (Hemiptera: Liviidae). Phytopathol. 107, 264–272 (2017).
Téllez-Jurado, A., Cruz, R. M. G., Flores, M. Y., Asaff, T. A. & Arana- Cuenca, A. Mecanismos de acción y respuesta en la relación de hongos entomopatógenos e insectos. Rev. Mex. Micol. 30, 73–80 (2009).
Mora, M. A. E., Castilho, A. M. C. & Fraga, M. E. Classification and infection mechanism of entomopathogenic fungi. Arq. Inst. Biol. 84, 1–10 (2017).
Lei, Y. et al. Unraveling the mode of action of Cordyceps fumosorosea: Potential biocontrol agent against Plutella xylostella (Lepidoptera: Plutellidae). Insects 12, 179 (2021).
Acknowledgements
We thank Janet Reid, at JWR Associates: Biological Consulting and Editing Services, for revising the English and for the valuable comments. We also thank Koppert Biological Systems for providing the product Challenger® used in the assays. Thanks to FAPESP [Grant number 2018/02317-5] as part of the SPARCBIO (São Paulo Advanced Research Center for Biological Control) located at ESALQ/USP with support of FAPESP, Koppert, and University of São Paulo (USP). Finally, we thank the anonymous reviewers for many useful comments and suggestions, which helped to improve the manuscript.
Funding
FAPESP [Grant number 2018/02317-5] as part of the SPARCBIO (São Paulo Advanced Research Center for Biological Control).
Author information
Authors and Affiliations
Contributions
N.M., T.R.C. and J.R.S.L. conceived and designed the research. N.M. conducted all experiments, processed, and analyzed the data. All authors contributed to the writing and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maluta, N., Castro, T. & Lopes, J.R.S. Entomopathogenic fungus disrupts the phloem-probing behavior of Diaphorina citri and may be an important biological control tool in citrus. Sci Rep 12, 7959 (2022). https://doi.org/10.1038/s41598-022-11789-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-11789-2
This article is cited by
-
Tamarixia radiata global distribution to current and future climate using the climate change experiment (CLIMEX) model
Scientific Reports (2023)
-
DC-electrical penetration graph waveforms for Dalbulus maidis (Hemiptera: Cicadellidae) and the effects of entomopathogenic fungi on its probing behavior
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.