Abstract
Despite the long-standing interest in the organization of ant communities across elevational gradients, few studies have incorporated the evolutionary information to understand the historical processes that underlay such patterns. Through the evaluation of phylogenetic α and β-diversity, we analyzed the structure of leaf-litter ant communities along the Cofre de Perote mountain in Mexico and evaluated whether deterministic- (i.e., habitat filtering, interspecific competition) or stochastic-driven processes (i.e., dispersal limitation) were driving the observed patterns. Lowland and some highland sites showed phylogenetic clustering, whereas intermediate elevations and the highest site presented phylogenetic overdispersion. We infer that strong environmental constraints found at the bottom and the top elevations are favoring closely-related species to prevail at those elevations. Conversely, less stressful climatic conditions at intermediate elevations suggest interspecific interactions are more important in these environments. Total phylogenetic dissimilarity was driven by the turnover component, indicating that the turnover of ant species along the mountain is actually shifts of lineages adapted to particular locations resembling their ancestral niche. The greater phylogenetic dissimilarity between communities was related to greater temperature differences probably due to narrow thermal tolerances inherent to several ant lineages that evolved in more stable conditions. Our results suggest that the interplay between environmental filtering, interspecific competition and habitat specialization plays an important role in the assembly of leaf-litter ant communities along elevational gradients.
Similar content being viewed by others
Introduction
Elucidating the mechanisms underlying the natural variation in species richness and composition across local communities has challenged ecologists for more than a century1. Since the development of community phylogenetics, a vast number of studies have used the phylogenetic approach to disentangle the relative importance of both deterministic and stochastic processes involved in the coexistence of species within a community2,3. The cornerstone of the phylogenetic approach is the assumption of phylogenetic niche conservatism, i.e., the tendency of species to conserve their niches over evolutionary history, with closely related species being ecologically more similar to each other than to distantly related species4. Based on this assumption, phylogenetically clustered communities (i.e., co-occurrence of closely related species) are typically thought to result from environmental filtering, where taxa are filtered by constraints imposed by the environment5. However, modern coexistence theory considers that phylogenetic clustering could also be driven by competitive exclusion where entire clades have higher competitive abilities leading to an exclusion of other lineages6. Conversely, phylogenetically overdispersed communities (i.e., co-occurrence of distantly related species) are thought to be structured by competition, which tends to select species with different ecological traits and thus low niche overlap7. Finally, a lack of phylogenetic structure suggests a predominance of neutral processes8 or the counteraction of both habitat filtering and interspecific competition9. Since these mechanisms can act simultaneously within communities10, the critical question is no longer which mechanism structures communities but rather which one plays a dominant role in community assembly along environmental gradients11.
Community ecologists have expanded the community phylogenetics framework from α-(richness) to β-diversity (composition) patterns (phylogenetic beta diversity, hereafter PBD) into ecological contexts12,13,14. PBD explicitly adds phylogenetic information to evaluate how evolutionary relationships of component lineages change between communities or biomes across space12. Furthermore, PBD links local processes (e.g., biotic interactions or environmental filtering) to more regional evolutionary processes (e.g., trait evolution, speciation and dispersal), hence providing further information about how current or historical environmental factors influence the variation in species compositions of communities across space12,15. Environmental filtering and dispersal limitation have been suggested to largely determine patterns of community composition16,17. Habitat filtering is expected to limit community members to habitats that resemble the ancestral niche where its lineage originated, i.e., habitat specialization18, resulting in closely-related species occupying similar portions of regional-scale climatic gradients. On the other hand, the dispersal limitation process predicts that community dissimilarity between sites will be correlated to geographical distances separating those sites, regardless of the environmental gradients16. Since environmental filtering and dispersal limitation are not mutually exclusive, a greater variation in community dissimilarity predicted by geography reflects a greater importance of dispersal limitation, whereas greater variation explained by environmental distances indicates that habitat filtering is the structuring force19.
Recent methodological advances have improved our understanding of the origin and maintenance of geographic patterns of PBD through its decomposition into two antithetic components that account for the replacement of lineages between sites (the turnover-resultant component) and differences in the phylogenetic diversity between nested assemblages (the nestedness-resultant component20,21). Although the turnover and nestedness components both contribute to total dissimilarity, their relative importance depends on the processes structuring communities. For instance, if environmental conditions vary across space, and species are adapted to particular conditions, the turnover component of PBD is more likely to shape community composition under environmental filtering. Conversely, nestedness tends to be a more common component due to several processes such as selective colonization, selective extinction, nestedness of habitats or passive sampling21. Analyzing the relationship between PBD (and its components) with climatic and geographic variables can provide a better understanding of community assembly along environmental gradients22.
Several studies have placed the phylogenetic α- and β-diversity into ecological and biogeographic contexts, such as the latitudinal pattern of phylogenetic diversity23, the phylogenetic modification of native communities under species invasions24 and the community structure along elevational gradients25,26,27. Particularly, ecological gradients occurring in mountains have successfully served to test community assembly processes considering species distributions are strongly affected by the environmental conditions found at particular elevations28,29. Particularly for thermophilic taxa (i.e., organisms with high-temperature affinity), such as ants, deterministic processes related to both temperature30,31,32 and precipitation26,33 have been shown to influence community membership at elevational gradients. In tropical mountains, as elevation increases, temperature tends to decrease whereas precipitation increases34 and, as a result, the abiotic conditions (cold and wet) at high-elevation sites become physiologically stressful for a majority of ant species in comparison with more favorable climatic conditions (warm and humid) found at low elevations. This spatial structure created by the interplay of temperature and precipitation has been used to explain the changing phylogenetic structure from overdispersed ant communities at low elevations towards clustered communities at higher elevations25,26,27. Despite these findings, the relative importance of deterministic and stochastic processes driving ant community organization under the phylogenetic perspective is still incipient, particularly when tropical regions are considered.
In this study, we measured the phylogenetic α- and β-diversity to infer whether deterministic and stochastic processes are driving the organization of leaf-litter ant communities occurring along a tropical elevational gradient in Mexico. Leaf-litter ants have numerous attributes that make them an ideal system to explore assembly mechanisms. For instance, they exhibit high levels of local co-occurrence; with up to 35 species co-existing in only one square meter35. Further, ant distributions are highly constrained by local and regional climate36,37. They display a wide variety of both individual38 and colony-level39 thermoregulatory strategies to cope with cold and hot conditions. Lastly, molecular phylogenetic analyses of the major ant lineages are providing a stable framework to understand the evolutionary relationships of the group40,41,42. Hinged on the assumption that ant species have retained their traits along their evolutionary history (i.e., phylogenetic niche conservatism23,43, we expect that (1) ant communities inhabiting in favorable and stable habitats (warm and humid habitats) found at low elevations will show phylogenetic overdispersion since negative interspecific interactions might be more intense in these environments. Conversely, cold and wet conditions at high elevations would lead to phylogenetically clustered communities considering that only closely related species of a subset of lineages possess the physiological traits that allow them to persist in the harsh conditions present at those elevations. In terms of PBD, it is expected that (2) lineages would have high habitat specialization to the conditions where they originated and thus drastic environmental changes along the elevational gradient would lead to high lineage replacement. This will result in a greater importance of turnover on total phylogenetic dissimilarity along the elevational gradient. Finally, we can expect that (3) pairwise dissimilarity values for PBD and their components would be mainly explained by environmental filtering (i.e., climatic distances), following their ancestral climatic affinities rather than dispersal limitation.
Results
α-Diversity
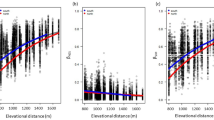
Overall, the tendency of the standardized effect sizes of phylogenetic diversity (SES.PD), mean-pairwise distance (SES.MPD) and mean nearest taxon distance (SES.MNTD) was negative values at low and some high elevational sites, whereas positive values at mid-elevations and the highest site. Considering the SES.PD, this means that three intermediate-elevational sites (1000, 1500 and 3000) contain higher evolutionary diversity in comparison with the rest of the sites (0, 600, 2000, 2500; Fig. 1a). This same trend was observed for the SES.MNTD (Fig. 1b), indicating phylogenetically diverse sites are related to more distantly species (overdispersion), whereas sites with lower phylogenetic diversity are inhabited by more closely-related species (clustering). For the SES.MPD, three mid-elevation sites (1000, 1500, 2000) and the highest site (3000) showed a tendency to overdispersion, whilst the rest of the sites (0, 600 and 2500) tended to a clustering pattern (Fig. 1c). All those trends were consistent across the 1000 phylogenetic trees and remained the same when the Maximum Clade Credibility (MCC tree) was used (Fig. 1; summary statistics for all metrics in Supplementary Table S1). Nevertheless, only the patterns at 2500 m for SES.PD and SES.MPD were significantly different from a null expectation at α = 0.05 (Supplementary Table S2).
Standardized effect sizes of (a) phylogenetic diversity, PD; (b) mean nearest taxon distance, MNTD; and (c) mean paiwise distance, MPD of the leaf-litter ant assemblages at seven elevations along the Cofre de Perote mountain. The inner horizontal line in each violin represents the mean of the 1000 SES values per elevation. Black point represents the SES values from the MCC tree. Area of no significance is delimited by the horizontal dashed lines at 1.96 and − 1.96.
When we assessed the contribution of climatic variables on explaining the alpha metrics patterns, the full model (Temp + Prec + Temp:Prec) explained a larger amount of variation in comparison with the additive or null model (Table 1; Supplementary Fig. S1). Moreover, the full model was considered the best statistically supported model in explaining SES.PD, SES.MPD and SES.MNTD patterns. However, in some instances, the additive model of temperature and precipitation resulted as equally probable models (Table 1, Supplementary Fig. S2), suggesting that the main effect of both variables is biologically important, yet its interaction effect refines model performance. Particularly for the full model, the general trend was positive interaction between temperature and precipitation (β > 0; Table 1).
β-Diversity
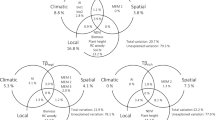
We found that total phylogenetic dissimilarity derived from multiple-site calculations exhibited considerable high values (PBDmulti.sor = 0.73 ± 0.006). Decomposition demonstrated that the turnover component (PBDmulti.sim = 0.52 ± 0.02) had a greater contribution to total dissimilarity compared with the relatively low values of the nestedness-resultant component (PBDmulti.nes = 0.21 ± 0.01). These patterns remained when the MCC tree was used (Supplementary Fig. S3). When assessing the patterns under the adjacent approach, we observed that the total dissimilarity (PBDadj.sor) increased with elevation (e.g., β = 0.08, p-value: 0.04 when values from the MCC tree were regressed with elevation). More specifically, lower values (< 0.5) were observed at lowland sites (< 2000), whereas higher values (> 0.5) at the highlands (> 2000; Fig. 2a). In most cases, the βratio calculation displayed values higher than 0.5 indicating that total dissimilarity is determined dominantly by turnover. A deviation of this general trend was observed at 1500–2000 sites where the nestedness (PBDadj.nes) dominated over the turnover component (Fig. 2b; summary statistics in Supplementary Table S3). Pairwise patterns of PBD showed that an increase of elevational distance results in greater total dissimilarity (PBDpair.sor) and is produced by a mixture of turnover (PBDpair.sim) and nestedness (PBDpair.nes) values operating between different elevational ranges (Supplementary Fig. S4).
Patterns in the (a) phylogenetic total dissimilarity and (b) the relative contribution of the turnover over the total dissimilarity (i.e., βratio = PBDadj.sim/PBDadj.sor) between adjacent sites along the tropical mountain of Cofre de Perote, Mexico. βratio > 0.5 indicates that beta diversity is determined dominantly by species turnover, whereas βratio < 0.5 indicates the predominance of the nestedness component. The inner horizontal line in each violin represents the mean of the 1000 PBD values per site comparison. Black point represents the SES values from the MCC tree.
According to GDM analysis, the full model explained a considerably high variation of PBD pairwise patterns. Particularly, 79.2% of deviance was explained for total dissimilarity (PBDpair.sor), followed by 49.9% for nestedness (PBDpair.nes) and in minor instance the turnover (PBDpair.sim) with 26.4%. Statistical significance was observed for all total dissimilarity models, however, turnover and nestedness were poorly supported with three and seventy-two (out of 1000) significant models respectively (Table 2). These results are consistent when the MCC tree was used in the analysis (Supplementary Table S4). In all PBD components, the variance explained by the temperature was much larger than explained by geographic distance or precipitation, indicating that thermal affinity has played a much greater role than affinity to precipitation and dispersal limitation in shaping the phylogenetic composition of ant assemblages across our study area (Table 2).
Discussion
In this study, we analyzed the phylogenetic α- and β- diversity to unravel the assembly of leaf-litter ant communities along a tropical mountain in Mexico. Overall, lowland and some highland sites show low phylogenetic diversity and are composed of closely-related species (phylogenetic clustering), whereas communities at intermediate elevations and the highest site present higher phylogenetic diversity and are inhabited of distantly-related species (phylogenetic overdispersion). All these patterns were highly supported not only across all the α-phylogenetic metrics (PD, MPD, MNTD) but also across 1000 hypothetical trees and the maximum clade credibility tree, thus patterns remained regardless of phylogenetic uncertainty. We found a positive relationship between temperature interacting with precipitation, suggesting that communities showing high phylogenetic diversity and tending to overdispersion are higher in environments with warm temperatures along with wet and seasonal precipitation regimens. Conversely, communities showing a low phylogenetic diversity and clustering tendency are associated to cold-dry sites with less-seasonal precipitations. Both the additive and full effects of precipitation with temperature were equally supported, yet the full model explained higher variation of phylogenetic α-diversity patterns for all metrics. Phylogenetic total dissimilarity was mainly driven by turnover, thus highland lineages are not subsets of lowland assemblages but instead, they are communities with different lineage compositions. Finally, the temperature differences between sites emerged as the most important driver of total dissimilarity, turnover and nestedness components. In sum, our findings provide additional evidence that evolutionary processes mediated by climate-related (deterministic) mechanisms are strongly involved in the assembly of ant communities along elevational gradients.
Contrary to our expectations, ant communities at the lowland and highland sites (except for the 3000 site) showed a tendency to phylogenetic clustering. Such a pattern has been typically associated with the gradual loss of ant lineages due to abiotic constraints (i.e., environmental filtering) where only a subset of species possesses the necessary adaptations to persist under stressful conditions25,26,27. In our study area, the drastic reduction of temperature coupled with relatively higher precipitation with increasing elevation may act as a strong filter on total diversity and the members of the regional species pool, such that the resultant high-elevation community would be phylogenetically clustered with taxa possessing wet and cold-resistant traits for dealing with this stress. Here, we found that > 50% of species inhabiting phylogenetically clustered communities at high elevations belong mainly to two cold-specialist genera Stenamma and Temnothorax (Supplementary Table S5). Strategies such as the hibernation of the ant colony during the winter season in Stenamma species44 or the production of glycerol and other antifreeze substances in Temnothorax45 may account as likely explanations for the ant persistence in this high, cold environments. Conversely, phylogenetic clustering at lowlands has never been reported for elevational ant studies. The reasons for this deviation require further study, but we propose two likely explanations. First, in comparison with the upper part of the mountain, the lowlands of the Cofre de Perote are characterized by high temperatures and marked precipitation seasonality. This means that all (or almost all) precipitation is concentrated in a relatively short time (3-months), leaving a prolonged drought season for the rest of the year. This drought period interacting with high temperatures may drastically prone ant species to desiccation46, and as a result, increase the importance of environmental filtering at those communities47,48. The second explanation includes the competition displacement, in which native ants are locally excluded by tramp and alien ant species. The competition displacement may lead to phylogenetic clustering since it is expected that only closely-related taxa of the introduced species can subsist under these biological invasions24. In fact, an observable characteristic of our lowland communities is the high dominance of three tramp and alien species: Hypoponera opaciceps (Mayr, 1887), Solenopsis geminata (Fabricius, 1804) and Wasmannia auropunctata (Roger, 1863; Supplementary Table S5). The well-known effects of these ant species in disrupting and displacing native ants49,50,51 suggest that patterns of phylogenetic clustering observed at lowland communities may not be driven by contemporary climate alone, but is also a result of the invasion of these ant species.
We found intermediate elevations tending to phylogenetically overdispersed communities. Typically, phylogenetic overdispersion is interpreted as evidence of interspecific competition since a long history of competitive interactions should cause evolutionary divergence in species niches52. Interspecific competition among ant species is intense and often involved in the configuration of ant communities53, yet evidence suggests that the importance of competition may be higher in favorable, stable environments where abiotically stressful factors are absent27,54. Intermediate elevations at Cofre de Perote reflect these conditions considering that at these elevations temperature is not too low to freeze available water nor too high to evaporate it (Supplementary Fig. S4). Besides, productivity, an ecological proxy of the amount of niches and resource heterogeneity in an ecosystem55, is expected to peak at this point since productivity is limited by drought at lowlands and cold temperatures at highlands. Thus, if competition is the driving mechanism at these intermediate elevations, we should observe communities containing a series of species with different evolutionary histories56. Indeed, clades are well represented in these communities with seventeen tribes (out of eighteen) containing a mixture of species from both tropical and temperate origins distributed at low and high elevations respectively.
Whilst competitive interaction is congruent as a structuring force in phylogenetically overdispersed communities at more favorable habitats found at intermediate elevations, it is unlikely that this hypothesis stands for the isolated overdispersed community at the highest stressful elevation (i.e., 3000 m). Particularly, we observed that the ant community at this elevation was composed of eight species, each one belonging to different genera dispersed across the phylogeny. Some studies have posited that geographic isolation for historical climatic variations has played a key role in the distribution of species at high-elevation habitats57,58,59. Thus, the presence of species with contrasting evolutionary histories may suggest that communities at 3000 m could be acting as refugia, maintaining relict lineages that migrated from distant regions with temperate climate or were more widespread in the past but became geographically isolated as a consequence of habitat contractions in the last glaciations58.
The complementary use of the multiple-site, adjacent, and pairwise approaches, coupled with the decomposition of total dissimilarity into the turnover and nestedness components significantly contributed to unveil the underlying mechanisms influencing dissimilarity variation. For instance, all approaches showed a high dominance of the turnover component for total dissimilarity. This result indicates that the turnover of species is actually a turnover of entire lineages or clades12. Besides, it suggests that ant lineages are established at specific elevations (habitat specialization) corresponding to the climatic conditions where they originated18. A deeper examination of the distribution of large clades along the Cofre de Perote may support this assumption. On one hand, several ant genera within the tribes Attini (e.g., Octostruma, Pheidole and Strumigenys) and Solenopsidini (e.g., Solenopsis, Monomorium, Megalomyrmex) belonging to the subfamily Myrmicinae are highly restricted to the warm conditions found at lowlands probably resembling their neotropical origin42,60. On the other hand, middle elevations are highly dominated by Adelomyrmex species. This genus is considered as pantropically distributed but its dominance in cloud forests, such as those predominating in our sampled intermediate elevations, is well documented61,62. Finally, lineages with more temperate origins such as Stenamma63 and Temnothorax64 show a tendency to specialize at more high elevations and rarely spread to lowlands (Supplementary Table S5). Taken together, our results bring evidence that habitat specialization is not the only key driver of compositional dissimilarity of species (i.e.22,25,32), but also an important process scaling up to entire lineages (i.e., phylogenetic niche conservatism), in such a way that evolutionary history strongly constraints the elevational distribution of ant species25.
Despite the adjacent turnover component having a preponderant impact on total dissimilarity, we observed a breakpoint between 1500 and 2000 where the phylogenetic nestedness prevailed over turnover. The possible reason for this dominant nestedness in PBD is that selective extinction, operating through environmental filtering, is playing an important role in shaping the patterns of phylogenetic dissimilarity at this particular elevation21. Because environmental filtering favors certain traits over others, a high number of ant lineages are lost as a result of the temperature decrease and humidity increase occurring from 1500 to 2000 site65. Indeed, we noticed that the distribution of certain ant species was restricted to low and intermediate elevations (Supplementary Table S5). Particularly, several well-known neotropical species belonging to Brachymyrmex, Europhalothrix and Strumigenys genera completely disappear at 2000 site (or higher elevation sites; Supplementary Table S5). These ant genera are mainly restricted to more tropical environments found in lowland areas, with one or few species within the same clade capable to cross to high elevations62. Therefore, we speculate that this point of the mountain can serve to broadly separate two well-distinguished ant fauna: low-montane fauna, distributed from sea level to below the 2000 m of elevation, and high-montane fauna habiting at 2000 elevation and greater.
We show here that phylogenetic total dissimilarity was best explained by temperature differences among pairwise sites regardless of the precipitation or geographical differences among them. More specifically, higher total dissimilarity is expected between two sites differing in their temperatures in comparison with two sites sharing similar temperatures. This result agrees with those found by Liu et al.25 who documented that PBD in ant composition dissimilarity of the Hengduan mountain was mainly driven by climatic distance (in which a set of temperature-related variables were included). This high importance of temperature distance shaping PBD may be explained by the climate variability hypothesis proposed by Janzen66. The climate variability hypothesis proposes that species exposed to variable climates (such that occurring at higher elevations) evolve broad thermal tolerances, allowing those species to traverse climatic gradients found across elevations, resulting in a wider geographic distribution than thermal specialists from stable climates (generally found at lower elevations). Therefore, we should expect lineages that originated in more tropical regions (e.g., neotropical) to be characterized by narrower thermal tolerances resulting in more restricted distribution in comparison with lineages originated in more variable climates (e.g., nearctic). The fact that eighty ant species (54%) were found in only one elevation (see Fig. S1 in32), many of them restricted to the lowlands and belonging to genera with a neotropical origin (e.g., Brachymyrmex, Camponotus, Pheidole, Strumigenys and Octostruma; Supplementary Table S5) supports this assumption. This consistent effect of thermal adaptations constraining not only species distributions32,67 but those of entire lineages support that climatic niches are conserved over the evolutionary history of the ant clade25,43. Taken together, these results highlight the role of species sorting processes68, where the phylogenetic composition is mainly driven by deterministic processes (i.e., habitat filtering) in response to local environmental conditions rather than stochastic processes (i.e., dispersal limitation). The simultaneous examination of the phylogenetic α- and β-diversity (and its components of turnover and nestedness) enhances our understanding of the relative importance of deterministic and stochastic processes in structuring patterns of ant diversity.
Here, we showed that environmental filtering, interspecific competition and habitat specialization jointly structure the leaf-litter ant communities along the Cofre de Perote. These results highlight the importance of deterministic (niche-based) processes over stochastic processes. Further, our results provide insights about phylogenetic niche conservatism since some ant lineages have retained the necessary traits to colonize harsher environments (the colder habitats at the summit). Additionally, the large evolutionary history accumulated in the lineages inhabiting each elevational site along with the remarkable rates of phylogenetic turnover contributing to total phylogenetic dissimilarity confirms the importance of mountains not only as centers of species diversity but crucial reservoirs of unique evolutionary history69,70. In concordance with other studies2,27, our results highlight the importance of well-focused conservation strategies in mountain systems considering that the increase of anthropogenic influence and global warming threatens the diversity patterns across the tropical mountains. For instance, the expected increase of temperature coupled with the high importance of thermal tolerances in ant species may disrupt lowland communities by forcing species to move up the mountain, leading to losses of distinct evolutionary histories found at the summit due to no possible migration upwards71. Altogether, this work builds on the theory that not only contemporary but historical factors also influence the structure of leaf-litter ant assemblages along environmental gradients and this can be detected by integrating α- and β-phylogenetic diversities.
Methods
Study area and sant sampling
This study was conducted along the eastern slope of the Cofre de Perote mountain, in Veracruz, Mexico. This region is located at the junction of the Trans-Mexican volcanic belt and the Sierra Madre Oriental. We selected eight study sites spanning an elevational gradient from 0 to 3500 m (Fig. 3). All sites were systematically separated with an elevational difference of 500 m on average between each other. We placed our study sites at the following elevations above sea level: 30–50 m, 610–670 m, 900–1010 m, 1470–1650 m, 2020–2230 m, 2470–2600 m, 3070–3160 m and 3480–3540 m, however, for simplicity, we will refer to each site as discrete units (i.e., 0, 600, 1000, 1500, 2100, 2500, 3100, 3500 m). Geographical distance among adjacent sites varied greatly, ranging from 1.6 km between the closest sites (2000–2500), 44.6 km separating the furthest (0–500) and an average of 14.26 km.
Altitudinal map showing the location of the eight sampling points (white dots and numbers) along the Eastern slope of the Cofre de Perote, Mexico. Elevation data were obtained from INEGI (https://www.inegi.org.mx/app/geo2/elevacionesmex/) and contour lines from CONABIO (https://www.gob.mx/conabio, CONABIO 1998). Map is projected in World Geodetic System of 1984 (WGS84) and was created using ArcGis desktop 10.3, http://www.esri.com/software/arcgis/arcgis-for-desktop.
At each sampling point (i.e., elevational site), we collected 40 independent 1 m2 forest floor samples separated at least 20 m from each other (i.e., 320 m2 across the entire mountain). Ant communities at each elevational site were obtained by pooling all the species from these 40 samples (see32 for a complete description of sampling design). In each 1-m2 quadrat, we collected the leaf litter inside and sifted it through a coarse mesh screen of 1-cm grid size to remove the largest fragments and concentrate the fine litter. The concentrated fine litter from each sample was suspended in independent mini-Winkler sacks for 3 days in the laboratory. Falling arthropods were collected into a container with 95% ethanol. Ant workers were removed from each container and identified at the species level or assigned to a morphospecies number. Since no ant species were collected at 3500 m, all analyses were conducted excluding this site (see32 and Supplementary Table S5).
Ethics approvals
All applicable international, national and institutional guidelines for the collection of ant specimens and leaf-litter material were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted. The material was collected following the permits issued by SEMARNAT (license number: FAUT-0312).
Phylogenetic tree constructions
Ideally, one would use a complete, species-level phylogeny of all ant species present in your study area to calculate phylogenetic diversity, yet our current understanding of ant relationships is still limited. As an alternative, we built a genus-level phylogeny based on the tree by Moreau and Bell72, but using the phylogenetic relationships and divergence times within Myrmicinae from Ward42. This phylogeny was then pruned to keep only a single species per genus to generate a genus-level phylogeny. To maximize taxonomic coverage, we replaced genera that were missing from those studies with closely-related lineages that were not present in our dataset using other phylogenetic studies73,74,75. We then used the list of species (Supplementary Table S5) in our dataset to simulate a species-level phylogeny in which the relationships within genera were obtained from a Yule (pure-birth) process using the genus.to.species.tree function in the “phytools” package76. A total of 1000 simulated trees were obtained to account for phylogenetic uncertainty (see77 and78 for similar approach). Additionally, we constructed a Maximum Clade Credibility tree (hereafter MCC tree) which was used to summarize the uncertainty of the 1000 simulated trees. The MCC tree was constructed from the sample of the 1000 trees with the maxCladeCred function incorporated in the “ape” package79. Both the 1000 hypothetical trees and the MCC tree were used in downstream analyses (Supplementary Fig. S6).
Alpha and beta phylogenetic diversity
Phylogenetic alpha diversity patterns of leaf-litter ant assemblages at each site were estimated using three metrics: Faith’s PD80, mean pairwise distance (MPD) and mean neighbor taxon distance (MNTD). PD measures the total phylogenetic branch length that joins the basal node to the tips of all the species in the sample80 and is used as a measure of absolute phylogenetic diversity81. MPD and MNTD are two phylogenetic divergence metrics7,81, with the former being an estimate of the average phylogenetic relatedness between all possible pairs of taxa in a local community, whereas the latter is an estimate of the mean phylogenetic relatedness between each taxon in a local community and its nearest relatives.
To investigate which processes may be influencing the patterns of phylogenetic alpha diversity, we calculated the standardized effect sizes (SES) for each α-diversity metric (i.e., SES.PD, SES.MPD, SES.MNTD). SES values were calculated by taking the difference between the observed value of PD, MPD and MNTD and their corresponding mean random values derived from null communities, then dividing these differences by the standard deviation across randomizations82. The null communities were generated by randomizing the community data matrix using the “independentswap” algorithm with 1000 iterations. Positive and negative SES.PD values indicate species in a community accumulated more or less evolutionary history than expected by null communities, respectively83. Whereas for MPD and MNTD, positive SES values suggest phylogenetic overdispersion, whereas clustering is inferred by negative values7. Statistical significance is inferred if SES values are greater than 1.96 or less than -1.96. All these analyses were conducted using the ses.pd, ses.mpd and ses.mntd functions incorporated in the “picante” package84 of the R software85.
We investigated the patterns of β-diversity through three complementary approaches: (i) the multiple-site approach (PBDmulti), used to summarize in one value the overall dissimilarity in the mountain, (ii) the adjacent approach (PBDadj), used to investigate the unidirectional β-diversity variation focusing only on adjacent sites towards the summit, and (iii) the pairwise approach (PBDpair), used to investigate how β-diversity patterns were related to the environmental and geographical distance between all pairwise sites. For all approaches, we calculated the total dissimilarity through the PhyloSor distance (multiple-site: PBDmulti.sor, adjacent: PBDadj.sor; pairwise: PBDpair.sor) and further decomposed it into the turnover (multiple-site: PBDmulti.sim, adjacent: PBDadj.sim; pairwise: PBDpair.sim) and the nestedness (multiple-site: PBDmulti.nes, adjacent: PBDadj.nes; pairwise: PBDpair.nes) components. Under the phylogenetic framework, total dissimilarity captures the proportion of shared and exclusive branch lengths among assemblages, turnover measures ‘true’ lineage turnover and nestedness considers the differences in Faith’s PD between assemblages21. Multiple-site calculations were obtained using the multi.phylo function, whereas pairwise and adjacent values with the phylo.beta.pair function incorporated in the “betapart” package86.
To assess the relative contribution of the spatial turnover component to the total dissimilarity between adjacent sites, we calculated the ratio of turnover over total dissimilarity (hereafter βratio) following Dobrovolski et al.87: PBDadj.sim/ PBDadj.sor. Thus, βratio > 0.5 indicates that total dissimilarity is determined dominantly by the turnover, and βratio < 0.5 indicates nestedness is the dominant component87,88. We did not conduct such analyses for the pairwise approach since the raw-unconverted data is necessary for GDM analyses (see “Statistical analyses” section).
Climatic predictors
Considering that ants do not respond to elevation directly but rather a suite of covarying abiotic factors89,90, we focused in evaluate whether local climate account for the observed phylogenetic diversity patterns. For this purpose we used the 19 climatic variables from the bioclimatic raster available for Mexico at 3 arc-second resolution (~ 90 m91) coincident with the twenty georeferenced points at each elevational site. Considering that some sampling points are close enough to prompt to pseudo-replication, only one sampling site was permitted in each predictor raster pixel. This was achieved by excluding duplicated geolocations by using the gridSample function in “dismo” package92 which retains a single point from each raster pixel. Thus, all values were calculated as the mean of all the independent rasters within each elevational site. We first divided those 19 variables into temperature- and precipitation-related subsets. Then, we used separate principal components analyses (PCA) to generate a synthetic uncorrelated climatic variable that represents the original variables contained in each climatic subset. Before PCA analyses all variables were standardized to remove the unit and were centered (mean = 0, SD = 1). Since the first principal component accounted for a high variation contained in each subset of temperature (PC1Temperature:85.3%) and precipitation (PC1Precipitation:67.5%; Supplementary Table S6), we conducted the consecutive analyses using only these vectors (hereafter referred simply as “temperature” and “precipitation” respectively). We noted that elevation highly correlated with temperature (ρ = 0.99, p-value < 0.0001) but no with precipitation (ρ = − 0.03, p-value = 0.94).
The examination of variable loading in each principal component revealed that almost all variables included in the analysis (75% of the total) highly contributed (i.e., large weights) to each first component (Supplementary Table S6). Therefore, any interpretation using PC1Temperature and PC1Precipitation should largely reflect the broad variation in terms of temperature and precipitation occurring along the Cofre de Perote mountain.
Statistical analyses
To evaluate which climatic variables (i.e., temperature and precipitation) better explained the phylogenetic alpha diversity, we implemented multiple regression models where the 1000 SES values of each alpha metric (SES.PD, SES.MPD, SES.MNTD) were regressed with the main effects and the interaction terms of the first principal component of each climatic subset (temperature and precipitation). Simultaneously, we constructed a null model with the same response variables modeled against the intercept. Normality assumption was checked in the residuals of all the adjusted models using the Shapiro test at α = 0.05. To avoid spurious interpretation, a second run of regression models was conducted including only models which met the normality assumption (models syntaxis and number of trees included in final analyses are condensed in Supplementary Table S7).
The null and multiple regression models were evaluated and the model with the lowest Bayesian Information Criterion (BIC) was chosen as the best model93. We selected BIC over the Akaike information criterion (AIC) since BIC is based on the assumption that a true model exists among the set of candidate models94. We considered this scenario true since temperature and precipitation (and their interaction) have been documented as the most important predictors of ant diversity (e.g.,30,33,62). We considered a model equally probable to the best fit model if the difference in BIC (ΔBIC) between the focal model and the model with the lowest BIC were < 2. Further, we extracted the coefficients of determination (R2) and the slope coefficient (β) to evaluate the proportion of variance explained by each model and the relationship between each phylogenetic alpha metric with the climatic predictors respectively. Regression models and normality tests were conducted through the lm and shapiro.test functions respectively, whereas model performance was conducted using the bictab function incorporated in the “AICmodavg” package95.
To assess whether environmental filtering (climate distances) or dispersal limitation (geographical distances) better explained pairwise PBD patterns, we used Generalized Dissimilarity Modelling (GDM96). GDM uses a nonlinear matrix regression technique for analyzing spatial patterns in compositional dissimilarity, providing fitted I-splines to describe the relationships between a dissimilarity matrix (response) and both climatic and geographical predictors, coupled with the partial deviance explained by each predictor97. Moreover, GDM standardizes variables so they can be directly compared with one another and is highly robust to multicollinearity among predictors96. To conduct GDM, we first converted the observed pairwise dissimilarity matrices (PBDpair.sor, PBDpair.sim, PBDpair.nes) into a GDM site-pair table using the formatsitepair function setting the type 3 in the “bioFormat” argument. The gdm function was used to fit the model which included the climatic variables (temperature and precipitation) and the geographical coordinates corresponding to the centroid of the total sampling points located at each site. Finally, the function gdm.varImp was used to extract the total deviance explained by each model, the significance of the full model and the importance of each predictor. Predictor importance is quantified as the percent change in deviance explained by the full model and the deviance explained by a model fit with that variable permuted98. We used 1000 permutations to estimate predictor importance and full model significance. Since this complete procedure was ran across the 1000 matrices of each PDB component, we thus calculated the ratio between the number of significant values (p < 0.05) out of the 1000 phylogenetic trees. GDM analyses were conducted using the functions incorporated in the “gdm” package98.
All packages employed for statistical analyses and the “ggplot2” package99 used for graph building are incorporated in the R project software (v. 4.1.2)85.
Data availability
The taxonomic matrix along with the 1000 simulated trees and the Maximum Clade Credibility tree constructed in each elevational site are available on the Zenodo digital repository (https://doi.org/10.5281/zenodo.5646220).
References
Ricklefs, R. E. A comprehensive framework for global patterns in biodiversity. Ecol. Lett. 7, 1–15 (2004).
Dolson, S. J. et al. Diversity and phylogenetic community structure across elevation during climate change in a family of hyperdiverse neotropical beetles (Staphylinidae). Ecography 44, 740–752 (2021).
Montaño-Centellas, F. A., McCain, C. & Loiselle, B. A. Using functional and phylogenetic diversity to infer avian community assembly along elevational gradients. Glob. Ecol. Biogeogr. 29, 232–245 (2020).
Wiens, J. J. et al. Niche conservatism as an emerging principle in ecology and conservation biology. Ecol. Lett. 13, 1310–1324 (2010).
Cavender-Bares, J., Kozak, K. H., Fine, P. V. A. & Kembel, S. W. The merging of community ecology and phylogenetic biology. Ecol. Lett. 12, 693–715 (2009).
Mayfield, M. M. & Levine, J. M. Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecol. Lett. 13, 1085–1093 (2010).
Webb, C. O., Ackerly, D. D., McPeek, M. A. & Donoghue, M. J. Phylogenies and community ecology. Annu. Rev. Ecol. Syst. 33, 475–505 (2002).
Hubbell, S. P. The Unified Neutral Theory of Biodiversity and Biogeography (MPB-32) Vol. 32 (Princeton University Press, 2001).
Kraft, N. J. B., Cornwell, W. K., Webb, C. O. & Ackerly, D. D. Trait evolution, community assembly, and the phylogenetic structure of ecological communities. Am. Nat. 170, 271–283 (2007).
Cadotte, M. W. & Tucker, C. M. Should environmental filtering be abandoned?. Trends Ecol. Evol. 32, 429–437 (2017).
Mouchet, M. A. et al. Functional diversity measures: An overview of their redundancy and their ability to discriminate community assembly rules. Funct. Ecol. 24, 867–876 (2010).
Graham, C. H. & Fine, P. V. A. Phylogenetic beta diversity: Linking ecological and evolutionary processes across space in time. Ecol. Lett. 11, 1265–1277 (2008).
Qian, H., Jin, Y., Leprieur, F., Wang, X. & Deng, T. Geographic patterns and environmental correlates of taxonomic and phylogenetic beta diversity for large-scale angiosperm assemblages in China. Ecography 43, 1706–1716 (2020).
Swenson, N. G. et al. Phylogenetic and functional alpha and beta diversity in temperate and tropical tree communities. Ecology 93, 112–125 (2012).
Qian, H., Hao, Z. & Zhang, J. Phylogenetic structure and phylogenetic diversity of angiosperm assemblages in forests along an elevational gradient in Changbaishan, China. J. Plant Ecol. 7, 154–165 (2014).
Chase, J. M. & Myers, J. A. Disentangling the importance of ecological niches from stochastic processes across scales. Philos. Trans. R. Soc. B Biol. Sci. 366, 2351–2363 (2011).
Leibold, M. A., Economo, E. P. & Peres-Neto, P. Metacommunity phylogenetics: Separating the roles of environmental filters and historical biogeography. Ecol. Lett. 13, 1290–1299 (2010).
Ricklefs, R. E. Evolutionary diversification and the origin of the diversity-environment relationship. Ecology 87, 3–13 (2006).
Zhang, J. L. et al. Phylogenetic beta diversity in tropical forests: Implications for the roles of geographical and environmental distance. J. Syst. Evol. 51, 71–85 (2013).
Baselga, A. The relationship between species replacement, dissimilarity derived from nestedness, and nestedness. Glob. Ecol. Biogeogr. 21, 1223–1232 (2012).
Leprieur, F. et al. Quantifying phylogenetic beta diversity: Distinguishing between ‘true’ turnover of lineages and phylogenetic diversity gradients. PLoS ONE https://doi.org/10.1371/journal.pone.0042760 (2012).
Bishop, T. R., Robertson, M. P., van Rensburg, B. J. & Parr, C. L. Contrasting species and functional beta diversity in montane ant assemblages. J. Biogeogr. 42, 1776–1786 (2015).
Economo, E. P., Narula, N., Friedman, N. R., Weiser, M. D. & Guénard, B. Macroecology and macroevolution of the latitudinal diversity gradient in ants. Nat. Commun. 9, 1–8 (2018).
Lessard, J. P., Fordyce, J. A., Gotelli, N. J. & Sanders, N. J. Invasive ants alter the phylogenetic structure of ant communities. Ecology 90, 2664–2669 (2009).
Liu, C., Dudley, K. L., Xu, Z. H. & Economo, E. P. Mountain metacommunities: climate and spatial connectivity shape ant diversity in a complex landscape. Ecography 41, 101–112 (2018).
Smith, M. A., Hallwachs, W. & Janzen, D. H. Diversity and phylogenetic community structure of ants along a Costa Rican elevational gradient. Ecography 37, 720–731 (2014).
Machac, A., Janda, M., Dunn, R. R. & Sanders, N. J. Elevational gradients in phylogenetic structure of ant communities reveal the interplay of biotic and abiotic constraints on diversity. Ecography 34, 364–371 (2011).
Guo, Q. et al. Global variation in elevational diversity patterns. Sci. Rep. 3, 1 (2013).
Kluge, J., Kessler, M. & Dunn, R. R. What drives elevational patterns of diversity? A test of geometric constraints, climate and species pool effects for pteridophytes on an elevational gradient in Costa Rica. Glob. Ecol. Biogeogr. 15, 358–371 (2006).
Sanders, N. J., Lessard, J. P., Fitzpatrick, M. C. & Dunn, R. R. Temperature, but not productivity or geometry, predicts elevational diversity gradients in ants across spatial grains. Glob. Ecol. Biogeogr. 16, 640–649 (2007).
Malsch, A. K. F. et al. An analysis of declining ant species richness with increasing elevation at Mount Kinabalu, Sabah, Borneo. Asian Myrmecol. 2, 33–49 (2008).
Pérez-Toledo, G. R., Valenzuela-González, J. E., Moreno, C. E., Villalobos, F. & Silva, R. R. Patterns and drivers of leaf-litter ant diversity along a tropical elevational gradient in Mexico. J. Biogeogr. 48, 2515 (2021).
Szewczyk, T. M. & McCain, C. M. A systematic review of global drivers of ant elevational diversity. PLoS ONE 11, e155040 (2016).
McCain, C. M. & Grytnes, J.-A.A. Elevational gradients in species richness. In Encyclopedia of Life Sciences (ed. Wiley, J.) (Wiley, 2010). https://doi.org/10.1002/9780470015902.a0022548.
Silva, R. R. & Brandão, C. R. F. Morphological patterns and community organization in leaf-litter ant assemblages. Ecol. Monogr. https://doi.org/10.1890/08-1298.1 (2010).
Dunn, R. R. et al. Climatic drivers of hemispheric asymmetry in global patterns of ant species richness. Ecol. Lett. 12, 324–333 (2009).
Warren, R. J. & Chick, L. Upward ant distribution shift corresponds with minimum, not maximum, temperature tolerance. Glob. Chang. Biol. 19, 2082–2088 (2013).
Cerdá, X. & Retana, J. Alternative strategies by thermophilic ants to cope with extreme heat: Individual versus colony level traits. Oikos 89, 155–163 (2000).
Kadochová, Š & Frouz, J. Thermoregulation strategies in ants in comparison to other social insects, with a focus on red wood ants (Formica rufa group). F1000 Res. 2, 280 (2013).
Moreau, C. S., Bell, C. D., Vila, R., Archibald, S. B. & Pierce, N. E. Phylogeny of the ants: diversification in the age of angiosperms. Science 312, 101–104 (2006).
Rabeling, C., Brown, J. M. & Verhaagh, M. Newly discovered sister lineage sheds light on early ant evolution. Proc. Natl. Acad. Sci. 105, 14913–14917 (2008).
Ward, P. S., Brady, S. G., Fisher, B. L. & Schultz, T. R. The evolution of myrmicine ants: Phylogeny and biogeography of a hyperdiverse ant clade (Hymenoptera: Formicidae). Syst. Entomol. 40, 61–81 (2015).
Pie, M. R. The macroevolution of climatic niches and its role in ant diversification. Ecol. Entomol. 41, 301–307 (2016).
Smith, M. R. Revision of the genus Stenamma Westwood in America north of Mexico (Hymenoptera, Formicidae). Am. Midl. Nat. 57, 133–174 (1957).
Herbers, J. M. & Johnson, C. A. Social structure and winter survival in acorn ants. Oikos 116, 829–835 (2007).
Kaspari, M. & Weiser, M. D. Ant activity along moisture gradients in a neotropical forest1. Biotropica 32, 703–711 (2006).
Flores, O., Seoane, J., Hevia, V. & Azcárate, F. M. Spatial patterns of species richness and nestedness in ant assemblages along an elevational gradient in a Mediterranean mountain range. PLoS ONE 13, 1–16 (2018).
Almeida, R. P. S. et al. Induced drought strongly affects richness and composition of ground-dwelling ants in the eastern Amazon. BioRxiv (2020).
Le Breton, J., Chazeau, J. & Jourdan, H. Immediate impacts of invasion by Wasmannia auropunctata (Hymenoptera: Formicidae) on native litter ant fauna in a New Caledonian rainforest. Austral Ecol. 28, 204–209 (2003).
Vonshak, M., Dayan, T., Ionescu-Hirsh, A., Freidberg, A. & Hefetz, A. The little fire ant Wasmannia auropunctata: A new invasive species in the Middle East and its impact on the local arthropod fauna. Biol. Invasions 12, 1825–1837 (2010).
Wheeler, W. M. Ants: Their Structure, Development and Behavior (Columbia University Press, 1910).
Cavender-Bares, J., Ackerly, D. D., Baum, D. A. & Bazzaz, F. A. Phylogenetic overdispersion in Floridian oak communities. Am. Nat. 163, 823–843 (2004).
Parr, C. L., Sinclair, B. J., Andersen, A. N., Gaston, K. J. & Chown, S. L. Constraint and competition in assemblages: A cross-continental and modeling approach for ants. Am. Nat. 165, 481–494 (2005).
Retana, J. & Cerdá, X. Patterns of diversity and composition of Mediterranean ground ant communities tracking spatial and temporal variability in the thermal environment. Oecologia 123, 436–444 (2000).
Hawkins, B. A. et al. Energy, water, and broad-scale geographic patterns of species richness. Ecology 84, 3105–3117 (2003).
Graham, C. H., Parra, J. L., Rahbek, C. & McGuire, J. A. Phylogenetic structure in tropical hummingbird communities. Proc. Natl. Acad. Sci. 106, 19673–19678 (2009).
Camacho, G. P., Loss, A. C., Fisher, B. L. & Blaimer, B. B. Spatial phylogenomics of acrobat ants in Madagascar—Mountains function as cradles for recent diversity and endemism. J. Biogeogr. 1, 1706–1719. https://doi.org/10.1111/jbi.14107 (2021).
Lobo, J. M. & Halffter, G. Biogeographical and ecological factors affecting the altitudinal variation of mountainous communities of coprophagous beetles (Coleoptera: Scarabaeoidea): A comparative study. Ann. Entomol. Soc. Am. 93, 115–126 (2000).
Halffter, G., Favila, M. & Arellano, L. Spatial distribution of three groups of Coleoptera along an altitudinal transect in the Mexican Transition Zone and its biogeographical implications. Elytron 9, 1–10 (1995).
Blaimer, B. B. et al. Phylogenomic methods outperform traditional multi-locus approaches in resolving deep evolutionary history: a case study of formicine ants. BMC Evol. Biol. 15, 1–14 (2015).
Longino, J. T., Branstetter, M. G. & Colwell, R. K. How ants drop out: ant abundance on tropical mountains. PLoS ONE 9, e104030 (2014).
Longino, J. T. & Branstetter, M. G. The truncated bell: An enigmatic but pervasive elevational diversity pattern in Middle American ants. Ecography 42, 272–283 (2019).
Branstetter, M. G. Origin and diversification of the cryptic ant genus Stenamma Westwood (Hymenoptera: Formicidae), inferred from multilocus molecular data, biogeography and natural history. Syst. Entomol. 37, 478–496 (2012).
Prebus, M. Insights into the evolution, biogeography and natural history of the acorn ants, genus Temnothorax Mayr (hymenoptera: Formicidae). BMC Evol. Biol. 17, 1–22 (2017).
Kluge, J. & Kessler, M. Phylogenetic diversity, trait diversity and niches: Species assembly of ferns along a tropical elevational gradient. J. Biogeogr. 38, 394–405 (2011).
Janzen, D. H. Why mountain passes are higher in the tropics. Am. Nat. 101, 233–249 (1967).
Fernandes, G. W. et al. Cerrado to rupestrian grasslands: Patterns of species distribution and the forces shaping them along an altitudinal gradient. in Ecology and Conservation of Mountaintop Grasslands in Brazil 345–378 (2016). https://doi.org/10.1007/978-3-319-29808-5_15.
Leibold, M. A. et al. The metacommunity concept: A framework for multi-scale community ecology. Ecol. Lett. 7, 601–613 (2004).
Perrigo, A., Hoorn, C. & Antonelli, A. Why mountains matter for biodiversity. J. Biogeogr. 47, 315–325 (2020).
Myers, N., Mittermeier, R. A., Mittermeier, C. G., Da Fonseca, G. A. B. & Kent, J. Biodiversity hotspots for conservation priorities. Nature 403, 853–858 (2000).
Colwell, R. K., Brehm, G., Cardelus, C. L., Gilman, A. C. & Longino, J. T. Global warming, elevational range shifts and lowland biotic attrition in the wet tropics. Science 322, 258–261 (2008).
Moreau, C. S. & Bell, C. D. Testing the museum versus cradle tropical biological diversity hypothesis: Phylogeny, diversification, and ancestral biogeographic range evolution of the ants. Evolution 67, 2240–2257 (2013).
Borowiec, M. L. Generic revision of the ant subfamily Dorylinae (Hymenoptera, Formicidae). Zookeys 1, 280 (2016).
Lapolla, J. S., Brady, S. G. & Shattuck, S. O. Phylogeny and taxonomy of the Prenolepis genus-group of ants (Hymenoptera: Formicidae). Syst. Entomol. 35, 118–131 (2010).
Schmidt, C. A. & Shattuck, S. O. The higher classification of the ant subfamily Ponerinae (Hymenoptera: Formicidae), with a review of ponerine ecology and behavior. Zootaxa 3817, 1–242 (2014).
Revell, L. J. phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223 (2012).
Arnan, X., Arcoverde, G. B., Pie, M. R., Ribeiro-Neto, J. D. & Leal, I. R. Increased anthropogenic disturbance and aridity reduce phylogenetic and functional diversity of ant communities in Caatinga dry forest. Sci. Total Environ. 631, 429–438 (2018).
Divieso, R., Silva, T. S. R. & Pie, M. R. Morphological evolution in the ant reproductive caste. BioRxiv https://doi.org/10.1101/2020.07.18.210302 (2020).
Paradis, E. et al. Package ‘ape’. Anal. Phylogenet. Evol. 2, 1–10 (2019).
Faith, D. P. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 61, 1–10 (1992).
Tucker, C. M. et al. A guide to phylogenetic metrics for conservation, community ecology and macroecology. Biol. Rev. 92, 698–715 (2017).
Webb, C. O. Exploring the phylogenetic structure of ecological communities: An example for rain forest trees. Am. Nat. 156, 145–155 (2000).
Tucker, C. M. et al. Assessing the utility of conserving evolutionary history. Biol. Rev. 94, 1740–1760 (2019).
Kembel, S. W. et al. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26, 1463–1464 (2010).
R Core Team. A language and environment for statistical computing. R Found. Stat. Comput. 2, https://www.R-project.org (2021).
Baselga, A. & Orme, C. D. L. Betapart: An R package for the study of beta diversity. Methods Ecol. Evol. 3, 808–812 (2012).
Dobrovolski, R., Melo, A. S., Cassemiro, F. A. S. & Diniz-Filho, J. A. F. Climatic history and dispersal ability explain the relative importance of turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 21, 191–197 (2012).
Peixoto, F. P. et al. Geographical patterns of phylogenetic beta-diversity components in terrestrial mammals. Glob. Ecol. Biogeogr. 26, 573–583 (2017).
Körner, C. The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 22, 569–574 (2007).
Sundqvist, M. K., Sanders, N. J. & Wardle, D. A. Community and ecosystem responses to elevational gradients: Processes, mechanisms, and insights for global change. Annu. Rev. Ecol. Evol. Syst. 44, 261–280 (2013).
Cuervo-Robayo, A. P. et al. An update of high-resolution monthly climate surfaces for Mexico. Int. J. Climatol. 34, 2427–2437 (2014).
Hijmans, R. J., Phillips, S., Leathwick, J., Elith, J. & Hijmans, M. R. J. Package ‘dismo’. Circles 9, 1–68 (2017).
Schwarz, G. Estimating the dimension of a model. Ann. Stat. 6, 461–464 (1978).
Guthery, F. S., Burnham, K. P. & Anderson, D. R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach Vol. 67 (Springer, 2003).
Mazerolle, M. J. Improving data analysis in herpetology: Using Akaike’s information criterion (AIC) to assess the strength of biological hypotheses. Amphib. Reptil. 27, 169–180 (2006).
Ferrier, S., Manion, G., Elith, J. & Richardson, K. Using generalized dissimilarity modelling to analyse and predict patterns of beta diversity in regional biodiversity assessment. Divers. Distrib. 13, 252–264 (2007).
Fitzpatrick, M. C. et al. Environmental and historical imprints on beta diversity: Insights from variation in rates of species turnover along gradients. Proc. R. Soc. B Biol. Sci. 280, 20131201 (2013).
Manion, G. et al. gdm: Generalized dissimilarity modeling. R Packag. version (2018).
Wickham, H. Ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 3, 180–185 (2011).
Acknowledgements
Gibran R. Pérez-Toledo acknowledges the scholarship and financial support provided by the Consejo Nacional de Ciencia y Tecnología (CONACyT No. 487739) for doctoral research grant and the Doctoral Program of the Instituto de Ecología, A.C (INECOL). We acknowledge the technical support of MSc. Emanuel Villafán and the resources for high-performance computing that the Institute of Ecology (INECOL) made available for this research. G.R.P.-T. is particularly grateful with MSc. Victor Manuel Vásquez Reyes and MSc. Javier Tolome Romero for field assistance, Biol. Dora Luz Martinez Tlapa for ant processing and MSc. Sara M. Melo Merino for map design. This contribution was generated with funds from INECOL (Project 902-11-10204) and ADMAC project (National Science Foundation Grant DEB-1354739).
Author information
Authors and Affiliations
Contributions
G.R.P.-T., J.V.-G. and F.V. conceived the ideas; G.R.P.-T conducted all statistical analyses assisted by F.V., R.R.S. and C.E.M.; M.P. constructed phylogenetic trees used in all analyses; F.V., R.R.S. and C.E.M. suggested several methodological improvements. G.R.P.T. led the writing of the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pérez-Toledo, G.R., Villalobos, F., Silva, R.R. et al. Alpha and beta phylogenetic diversities jointly reveal ant community assembly mechanisms along a tropical elevational gradient. Sci Rep 12, 7728 (2022). https://doi.org/10.1038/s41598-022-11739-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-11739-y
This article is cited by
-
Long-term impact of an invasive predator on the diversity of fish assemblages in a neotropical reservoir
Biological Invasions (2024)
-
The diversity of moths (Erebidae: Arctiinae: Arctiini) from threatened mountain cloud forests in the Mesoamerican biodiversity hotspot
Journal of Insect Conservation (2023)
-
Ant communities in disturbed subtropical landscapes: is climate more important than stochastic processes?
Oecologia (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.