Abstract
Prediabetes and not just diabetes can cause kidney damage. This study assess the association of prediabetes with development of impaired renal function (IRF). We used data from PREDAPS prospective study a cohort of 1072 subjects with prediabetes and another cohort of 772 subjects without prediabetes were follow-up from 2012 to 2017. Prediabetes was defined according to American Association of Diabetes criteria. IRF was defined as having a glomerular filtration rate < 60 mL/min/1.73 m2. Incidence rates of IRF in both cohorts and in different categories of prediabetes, based on impaired glycosylated hemoglobin (HbA1c) and/or fasting plasma glucose (FPG), were calculated. Hazard ratios (HR) for the association of the prediabetes with IRF, adjusting for potential confounders, were estimated by Cox regression models. Incidence rates of IRF per 100 person-years were 1.72 (95% confidence interval [CI]: 1.34–2.21) and 1.79 (95%CI: 1.45–2.20) for those without and with prediabetes, respectively .The HR of IRF in subjects with prediabetes with respect to subjects without prediabetes was 0.76 (95% CI: 0. 54–1.07). Corresponding HRs for type of prediabetes was 0.68 (95%CI: 0.40–1.15) for those with both altered parameters, 0.68 (95%CI: 00.40–1.15) for those with only impaired HbA1c and 1.12 (95%CI: 0.68–1.85) for those with only impaired FPG. The present study reflects an overall trend towards a slightly decreased risk of IRF onset associated to prediabetes except for individuals with only isolated impaired FPG. Further studies are warranted to fully assess the renal progression of each group.
Similar content being viewed by others
Introduction
Approximately 422 million people globally suffer from diabetes globally and 1.6 million deaths are directly attributed to this each year1,2. Type 2 diabetes (T2D) is the most common, resulting as a result of increased insulin resistance. Diabetes is among the leading causes of chronic kidney failure around the world2. Impaired renal function (IRF) in patients with diabetes impose a significant health burden3. Deterioration of the renal function in combination with diabetes can lead to poorer health prognosis4.
It has been reported that up to 40% of patients in early stage of T2D demonstrate some degree of microvascular complication5. In addition, a high proportion of patients with diabetes are found to have non-diabetic renal disease (NDRD), being nephroangiosclerosis (NAS) the most frequent cause6,7. Metabolic changes associated with diabetes lead to glomerular hypertrophy, glomerulosclerosis, and tubulointerstitial inflammation and fibrosis5. In addition, according to various studies, one-third of adults with newly diagnosed diabetes mellitus already have kidney damage, suggesting that IRF may occur in pre-diabetic state8. The effect of hyperglycemia on the occurrence of IRF may start before glucose levels reaches diabetic ranges. Other diabetes-related microvascular complications, such as retinopathy and neuropathy have been described in some previous studies, in subjects with prediabetes9,10. On the other hand, it is known the high risk of kidney disease in the presence of microalbubnuria and the prevalence of microalbuminuria is higher in subjects with prediabetes than in subjects without alterations in glucose metabolism11,12.
Several authors have emphasized the early detection of IRF as an important component of its strategies for prevention of noncommunicable diseases9. However, the long-term influences of prediabetes on kidney function remains unknown. In order to fill major gaps in the role of prediabetes on IRF onset, the aim of this study was to evaluate the association between prediabetes and three diagnostic categories of prediabetes and incidence rate of IRF using a prospective cohort of individuals with prediabetes followed up by primary care physicians from Spain.
Methods
Study design
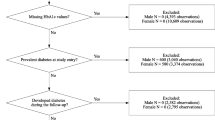
The Cohort Study in Primary Health Care on the Evolution of Patients with Prediabetes (PREDAPS), is a prospective study conducted by 125 Primary Care physicians at their practices from different provinces of Spain. The details of the cohort have been described elsewhere13,14. The age range of patients was between 30 and 74 years. The study period started in 2012 and continued up to the fifth annual follow-up visit in 2017. All individuals with the following criteria at baseline were excluded: diagnosis of diabetes, terminal disease, pregnancy, surgery, hospital admissions in the previous 3 months at study entry or any hematologic disease which could alter glycated hemoglobin A1c (HbA1c) values. The estimate glomerular filtration rate (eGFR) at baseline was calculated according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI). (10) A total of 92 subjects did not have measures eGFR at baseline and an additional 66 patients had values below 60 mL/min/1.73 m2 min which is already considered as a reduction; therefore, they were excluded due to this pre-existing condition. Final population included a total of 1844 subjects.
Study population was subdivided into two mutually exclusive cohorts according to glycemic parameters following American Diabetes Association (ADA) criteria: cohort of subjects with prediabetes (n = 1072) and cohort of subjects without prediabetes (N = 772). To define prediabetes, individuals met the ADA criteria for prediabetes: Considering having a Fasting Plasma glucose levels of 100–125 mg/dL and/or HbA1c range levels from 5.7 to 6.4%. Individuals were subdivided into three mutually exclusive diagnostic categories based on impaired glycemic parameters, collected at baseline. First category, included all subjects with only impaired fasting plasma glucose (FPG range levels: 100–125 mg/dL [5.6–6.9 mmol/L]), second category included all subjects with isolated impaired HbA1c (HbA1c range levels: 5.7–6.4% [39–47 mmol/mol]) and third category included subjects with both impaired glycemic parameters (FPG range levels: 100–125 mg/dL [5.6–6.9 mmol/L]) and HbA1c range levels: 5.7–6.4% [39–47 mmol/mol]))15.
Study period started on 2012 up to the fifth follow-up visit. Once meeting the eligibility criteria, individuals were followed up from baseline until the occurrence of one of the following end points: (i) IRF (ii) death, (iii) loss of follow up or (iv) end of study period (2017), whichever came first. In each subject, baseline serum creatinine was measured the day of enrolment in the study and additionally, each annual visit. IRF occurrence was measured at each annual visit and considered when a subject presented an eGFR < 60 mL/min/1.73 m2 during the follow up. IRF patients were immediately censored within the follow up (Fig. 1).
Subjects gave their written informed consent for participation. The study was classified by the Spanish Agency of Medicines and Medical Devices as a Non-Interventional (Observational) Post-Authorization Study, and the study protocol was approved by the Parc de Salut Mar Clinical Research Ethics Committee in Barcelona (2011-4274-I).
Assessment of covariables
Data were collected at the first visit (baseline period). Information on biographical data, family history, comorbidities, demographical data (i.e. including social support and socio-economic position), lifestyle factors, and drug use were obtained from medical records of study subjects as well as a personal interview conducted by the physicians.
Comorbidities were categorized as follows. Hypertension (HTN) classified as systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg, being treated by antihypertensive drugs or previous diagnosis of HTN. Hypercholesterolemia, as serum total cholesterol ≥ 250 mg/dL, HDL-C as < 40 mg/dL in men or < 50 mg/dL in women, and hypertriglyceridemia (HTG) as serum triglycerides ≥ 200 mg/dL.
Lifestyle factors were categorized as follows: Body mass index (BMI) between 25.0 and 29.9 kg/m2 classified as overweight, general obesity defined as BMI ≥ 30 kg/m2; abdominal obesity defined as waist circumference ≥ 102 cm in men and ≥ 88 cm in women. Smoking categories into smokers, ex-smokers and non-smokers; Alcohol consumption as: daily drinkers, occasional drinkers, and non-drinkers (never or former) which included ex-drinkers and teetotalers. Physical activity was classified according to World Health Organization (WHO) recommendations. Subjects followed the recommendations if they practiced more than 150 min per week of moderate aerobic physical activity, more than 75 min each week of vigorous aerobic physical activity or an equivalent combination16. For the adherence to Mediterranean diet (MedDiet), it was used as reference the definition followed in ATTICA study and their designed Panagiotakos score17. For each twenty types of studied nutrients, subjects responded the frequency of consumption: every day, more than three times a week, two times each week, once a week, less than once a week, never or rarely. Zero as a score in each meal was considered if the subject was having a less healthy diet and 4 was considered if the subject was having a very healthy diet. There were no missing values on demographic and clinical characteristics of participants at baseline.
Statistical analysis
First of all, a descriptive analysis was conducted showing the distribution of the baseline characteristics among those who were classified in prediabetic cohort and those in the cohort without prediabetes. Continuous and count variables were described using mean (± standard deviation [SD]), median (quartiles) and 95% confidence intervals (95%CI). Incidence rate of IRF per 100 person-years together with 95%CI were calculated in each cohort. Incidence rate of IRF according to prediabetes categories was also calculated. Kaplan Meier survival functions with log rank test were performed to compare the survival distributions across each group. Cox proportional hazards analyses were used to estimate the hazard ratios (HR) with 95%CI for the association of covariables with incidence rate of IRF and for the association of prediabetes and diagnostic categories of prediabetes with reduction of incidence rate of IRF. Results of the association of prediabetes and diagnostic categories of prediabetes with incidence rate of IRF onset were shown for four levels of adjustment: model 1 (adjusted by age and sex), model 2 (model 1 plus adjusted by lifestyle variables such as smoking status, regular physical activity, high-risk alcohol consumption, adherence to MedDiet score, model 3 (model 2 plus adjusted by metabolic risk factors such as waist circumference, BMI, hypertension (HTN), total cholesterol, low HDL-cholesterol, triglycerides), and model 4 (model 3 plus adjusted by use of angiotensin converting enzyme inhibitors [ACEIs] or angiotensin II receptor blockers [ARBs]). Cox proportional hazards models assume that the HR is constant over time. It was verified graphically that this assumption was not violated since the observed and predicted value curves were similar. Likewise, the proportional-hazards assumption on the basis of Schoenfeld residuals test confirmed the findings obtained graphically. Interval censoring strategy was uses when information on time to event was not available due to loss to follow-up or non-occurrence of outcome event before the l end of the study. However, if the periodicity of examination is at a justified frequency, interval censored data were dealt with as point censor. Statistical analyses were performed using the STATA package version 12.0 (StataCorp LP, College Station, TX, USA).
Ethics approval and consent to participate
The study was classified by the Spanish Drug and Health Product Agency as a Non-Interventional (Observational) Post-Authorization Study, and the protocol was approved by the Parc de Salut Mar Clinical Research Ethics Committee in Barcelona. Informed consent was obtained from all participants and/or their legal guardians.
Method and Declaration Section
Authors confirm that all methods were performed in accordance with the relevant guidelines and regulations.
Guarantor’s
Dr. Regidor is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Results
Baseline characteristics
A total of 1,844 patients (95% Spanish origin) were included in the present study, from whom 1072 (58.1%) had prediabetes according to the ADA guidelines the mean age of prediabetic and normoglycemic groups were 59.1 (SD 9.3) and 56.6 (SD 10.3) years, respectively. At baseline, lifestyle factors were similarly distributed among patients with prediabetes compared with normoglycemia group. In terms of comorbidities, more than half of patients with prediabetes presented metabolic syndrome compared with 12.4% in the normoglycemic group. Likewise, the prevalence of HTN was higher in the prediabetic group. In particular, 36.9% of patients with prediabetes had treatment with ACEIs or ARBs drugs versus 23.6% in the normoglycemia group. For all metabolic parameters measured, prediabetic group presented a higher mean (p value < 0.01) with the exception of total cholesterol which the distribution was almost the same (210 mg/dL). Finally, the mean value of eGFR (mL/min/1.73 m2) was very similar across groups: 89.1 (SD: 13.7) for prediabetic cohort and 90.4 (SD:13.4) for normoglycemic cohort (Table 1).
In addition, the urinary albumin could not be obtained in a large percentage of subjects (41%) and for this reason this variable was excluded from the analyses. However, the analysis of subjects with prediabetes (591) and subjects with normoglycemia (400) in whom this parameter was obtained, revealed no significant differences in the prevalence of microalbuminuria, whose magnitude was 7.5 and 5.8%, respectively (p = 0.297).
Incidence rate of IRF overall and by prediabetes categories
A total of 88 incident cases of IRF occurred in the prediabetic group and 61 cases occurred in the normoglycemic group. Incidence rates of IRF among the two study groups, overall and by prediabetes categories, are shown in Table 2. The overall incidence rate of IRF per 100 person-years was 1.72 (95%CI: 1.34–2.21) and 1.79 (95%CI: 1.45–2.20), log rank test p = 0.84. Focusing on the prediabetic group, the incidence of IRF was lower in the HbA1c 5.7–6.4% group (IR: 1.40 [95%CI: 0.89–2.19]) and highest among those with isolated FPG 100–125 mg/dL (IR: 2.06 [95%CI: 1.36–3.13]) log rank test p = 0.74. Figure 2 shows the Kaplan Meier survival function of IRF by type of cohort and Fig. 3 by prediabetes categories. To tests of proportional-hazards assumption, we estimated the Kaplan–Meier observed survival curves and compares them with the Cox predicted curves for the same variable. Supplemental Fig. 1 displays lines that the observed values and predicted values are close together.
Covariables and incidence rate of IRF
Table 3 shows the risk factors associated to IRF onset sex- and age-adjusted. There was a trend towards and increase risk of IRF according to age, for example those aged 50–64 years had a HR of 5.11 (95%CI: 1.85–14.12) and 16.48 (95% CI: 6.06–44.85) for those aged ≥ 65 years. Adherence to MedDiet showed a protective effect against reduction of eGFR (HR: 0.75 (95%CI: 0.54–1.04). On the contrary, metabolic conditions such as waist circumference ≥ 102 cm in men and ≥ 88 cm in women or having a BMI ≥ 30 were associated with an increased risk of reduction of eGFR (HR: 1.39 [95%CI: 0.54–1.04] and 1.21 [95%CI: 0.87–1.68], respectively). History of HTN showed a HR of 2.08 (95%CI: 1.38–3.12) and 1.82 (95% CI: 1.31–2.52) for use of ACEIs/ARBs but there was not association with metabolic syndrome (1.14 [95%CI: 0.82–1.59]). Having levels of HDL-cholesterol (mg/dL) as < 40 mg/dL in men or < 50 mg/dL was associated with a HR of IRF of 1.28(95%CI: 0.86–1.91).
Association of prediabetes and diagnostic categories of prediabetes with incidence rate of IRF
Results are shown in Table 4. Using the cohort of subjects without prediabetes as reference, prediabetes was associated with a HR of IRF onset of 0.89 (95%CI: 0.64–1.24) when adjusting by age and sex. This estimate remained the same when adding lifestyle variables to the model and HR decreased to 0.76 (95%CI: 0.54–1.07) when adding metabolic conditions together with lifestyle factors. Of note, the estimate remained constant when adding on top of this model use of ACEIs or ARBs (Table 4). When evaluating the risk of IRF onset according to prediabetes diagnostic categories, a trend towards a decreased risk of IRF onset was observed in subjects with both parameters altered (FPG and HbA1c) and those with only impaired HbA1c levels, corresponding HR estimates were 0.68 (95%CI: 0.40–1.15) and 0.68 (95%CI: 0.40–1.15), respectively. However, subjects with only impaired FPG did not show any association (HR: 1.12 [95%CI: 0.68–1.85]).
Discussion
Findings of this prospective cohort study reflect an overall trend towards a slightly decreased risk of IRF onset associated to prediabetes with an adjusted HR of 0.76. This finding is restricted to subjects who only had impaired HbA1c and those who had both parameters impaired: both groups represent 80% of the subjects with prediabetes and their adjusted HR was 0.76. Instead, subjects with only impaired FPG levels had a slightly increased risk (adjusted HR = 1.12).
A recent meta-analysis, including a total of eight cohort studies with subjects with impaired FPG as prediabetes criteria, has also reported a modest increased risk of IRF associated to impaired FPG18. It is known that hyperglycemia increases the production of reactive oxygen species, which lead to the accumulation of advanced glycation end products. This, in turn, activate intracellular signaling pathways such as protein kinase C and intensify the effects of the renin-angiotensin system5. This effect may lead to early onset of glomerular hyperfiltration and subsequently a decreased of IRF onset. In addition, eGFR has been reported to decrease faster in patients with hyperfiltration which might lead to kidney damage occurrence19,20. Although there is still controversy towards if hyperfiltration occurs in the early stages of hyperglycemia, several studies have found significant associations between hyperfiltration and prediabetes21,22. Specifically, it has been suggested how the prevalence of hyperfiltration increases with worsening stages of prediabetes21..
Several previous population-based studies of follow up have no found association of prediabetes with chronic kidney diseases nor with decreased GFR when using eGFR, after adjusting for risk factors20,23,24. Even in one of those studies prediabetes was associated with increased risk of hyperfiltration, but with reduced risk of having an mGFR < 60 ml/min/1.73 m2 at follow-up24. Our study presents as a novelty that the reduced risk of kidney damage can be observed only in some types of prediabetes. The reduced risk of IRF was concentrated among subjects who only had impaired HbA1c and those who had both parameters, while subjects with isolated impaired FPG showed a slightly increased risk of IRF, suggesting that these subjects might still preserve the renal function, and this is not via hyperfiltration. These results are consistent with a prior study using the same study population where it was found how individuals with impaired of both FPG and HbA1c had an OR of hyperfiltration of 1.69 (95% CI: 1.05–2.74) while there was no association among individuals with solely impaired FPG levels (25). Hyperfiltration was defined as an eGFR above the age and sex-specific 95th percentile25.
The early detection of IRF has been emphasized as an important component of its strategies for prevention of noncommunicable diseases9, as this has proven to improve outcomes for both individual and national healthcare economy. Since hyperfiltration is thought to be an early and proxy to reversible stage of kidney damage26 monitoring and identifying high risk prediabetic patients might result as an effective and cost-efficient preventive strategy. For example, both FPG and HbA1C levels can serve as chemical marker to identify early deterioration of IRF and avoid nephropathy. Another advantage of intensive blood glucose control in the prediabetic state can be seen in the long-term protective effect known as metabolic memory27. Thus, early intensive glycemic control could prevent irreversible damage that has been associated with hyperglycemia through closer monitoring of patients26. A 24% reduction in microvascular complications, including IRF, compared with tight glycemic control has been found in another study that followed up subjects with T2D for up to ten years27. Intensive glycemic control resulted in a 33% reduction in the risk of microproteinuria, proteinuria. Also a significant reduction in the proportion of patients with a doubling of the blood creatinine level (0.9% versus 3.5%) relative to the conventional therapy group was observed27.
This study reflects that it is possible to carry out a prospective study, with data obtained at the national level by primary care physicians during clinical practice. However, analytical determinations were made in different laboratories, which could have led to some misclassification. Given that each subject was assigned to the same laboratory during follow-up, this limitation should be non-differential in relation to the result, since an association between the methods used by specific laboratories and the development of IRF is unlikely.
This study used the IRF-EPI creatinine-based equation. This equation is more accurate and has less bias than the commonly used Diet Modification in Kidney Disease (MDRD) equation, especially at higher GFR levels10. In addition, GFR was estimated rather than using the gold standard of insulin clearance for this measure. Insulin clearance is more accurate than eGFR, but it is not cost-effective and it is an invasive method that is not used in clinical practice in Primary Care on a daily basis. However, analytical determinations were made in different laboratories, which could have led to some misclassification. Given that each subject was assigned to the same laboratory at baselines and during follow-up, this limitation should be non-differential in relation to the result, since an association between the methods used by specific laboratories and the development of IRF is unlikely. In addition, the urinary albumin could not be obtained in a large percentage of subjects (41%) and for this reason this variable was excluded from the analyses.
Finally, researchers were unable to determine a time-dependent variable. However, the vast majority of the factors considered in the present study are chronic conditions or long-term lifestyle factors not susceptible to a fast variation within the follow-up during the study period.
The current study did not show an increased risk of IRF onset associated to prediabetes, with the exception of those with isolated impaired FPG. Further studies are warranted to test the effect of these parameters can serve as chemical marker to identify early deterioration of IRF and avoid nephropathy. In any case, subjects with prediabetes could benefit from preventive measures that reduce their cardiovascular risk because cardiovascular and renal disease share common risk factors.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Abbreviations
- ACEIs:
-
Angiotensin converting enzyme inhibitors
- ARBs:
-
Angiotensin II receptor blockers
- ADA:
-
American diabetes association
- BMI:
-
Body mass index
- IRF:
-
Impaired renal function
- Cr:
-
Creatinine
- DBP:
-
Diastolic blood pressure
- FPG:
-
Fasting plasma glucose
- GFR:
-
Glomerular filtration rate
- HbA1c:
-
Glycated hemoglobin A1c
- HDL:
-
High-density lipoprotein
- MedDiet:
-
Mediterranean diet
- NS:
-
Not significant
- OGTT:
-
Oral glucose tolerance test
- SD:
-
Standard deviation
- SBP:
-
Systolic blood pressure
- T2DM:
-
Type 2 diabetes
- WC:
-
Waist circumference
References
World Health Organization. Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus. Available from:https://www.who.int/diabetes/publications/diagnosis_diabetes2011/en/.
Global report on diabetes. Available from:https://www.who.int/diabetes/global-report/en/.
Gorostidi, M. et al. Chronic kidney disease in Spain: Prevalence and impact of accumulation of cardiovascular risk factors. Nefrologia 38, 606–615 (2018).
Razeghi, E., Heydarian, P. & Heydari, M. The frequency of prediabetes and contributing factors in patients with chronic kidney disease. Rev. Diabet. Stud. 8(2), 276–281 (2011).
Alicic, R. Z., Rooney, M. T. & Tuttle, K. R. Diabetic kidney disease: Challenges, progress, and possibilities. CJASN 12(12), 2032–2045 (2017).
Bermejo, S., Pascual, J. & Soler, M. J. The current role of renal biopsy in diabetic patients. Minerva Med. 109, 116–125 (2018).
Bermejo, S. et al. Risk factors for non-diabetic renal disease in diabetic patients. Clin. Kidney J. 13(3), 380–388 (2020).
Olivarius Nde, F., Andreasen, A. H., Keiding, N. & Mogensen, C. E. Epidemiology of renal involvement in newly-diagnosed middle-aged and elderly diabetic patients. Cross-sectional data from the population-based study “Diabetes Care in General Practice”, Denmark. Diabetologia 36(10), 1007–1016 (1993).
White, S. L., Chadban, S. J., Jan, S., Chapman, J. R. & Cass, A. How can we achieve global equity in provision of renal replacement therapy?. Bull. World Health Organ. 86(3), 229–237 (2008).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern Med. 150(9), 604 (2009).
Bahar, A., Makhlough, A., Yousefi, A., Kashi, Z. & Abediankenari, S. Correlation between prediabetes conditions and microalbuminuria. Nephrourol. Mon. 5, 741–744 (2013).
Choi, J. W., Oh, I. H., Lee, C. H. & Park, J. S. Effect of synergistic interaction between abnormal adiposity-related metabolism and prediabetes on microalbuminuria in the general population. PLoS ONE 12, e0180924 (2017).
Serrano, R. et al. Cohort study in primary health care on the evolution of patients with prediabetes (PREDAPS): Basis and methodology. Rev Esp Salud Publ. 87(2), 121–135 (2013).
Giráldez-García, C. et al. Cardiometabolic risk profiles in patients with impaired fasting glucose and/or hemoglobin A1c 5.7% to 6.4%: Evidence for a gradient according to diagnostic criteria: The PREDAPS study. Medicine (Baltimore) 94(44), e1935 (2015).
American Diabetes Association (ADA). Standards of med- ical care in diabetes—2020. Diabet. Care. 43(Suppl1), S14–S31 (2020).
Global Recommendations on Physical Activity for Health. Available from: https://www.who.int/dietphysicalactivity/global-PA-recs-2010.pdf.
Panagiotakos, D. B., Pitsavos, C. & Stefanadis, C. Dietary patterns: A Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr. Metab. Cardiovasc. Dis. 16(8), 559–568 (2006).
Echouffo-Tcheugui, J. B., Narayan, K. M., Weisman, D., Golden, S. H. & Jaar, B. G. Association between prediabetes and risk of chronic kidney disease: A systematic review and meta-analysis. Diabet. Med. 33(12), 1615–1624 (2016).
Landray, M. J. et al. Prediction of ESRD and death among people with CKD: The Chronic Renal Impairment in Birmingham (CRIB) prospective cohort study. Am. J. Kidney Dis. 56(6), 1082–1094 (2010).
Neves, J. S. et al. Association of prediabetes with CKD progression and adverse cardiovascular outcomes: An Analysis of the CRIC study. J. Clin. Endocrinol. Metab. 105(4), e1772–e1780 (2020).
Moriya, T. et al. Glomerular hyperfiltration and increased glomerular filtration surface are associated with renal function decline in normo- and microalbuminuric type 2 diabetes. Kidney Int. 81(5), 486–493 (2012).
Jones, S. L., Wiseman, M. J. & Viberti, G. C. Glomerular hyperfiltration as a risk factor for diabetic nephropathy: Five-year report of a prospective study. Diabetologia 34(1), 59–60 (1991).
Schottker, B., Brenner, H., Koenig, W., Muller, H. & Rothenbacher, D. Prognostic association of HbA1c and fasting plasma glucose with reduced kidney function in subjects with and without diabetes mellitus. Results from a population-based cohort study from Germany. Prev. Med. 57, 596–600 (1991).
Melsom, T. et al. Prediabetes and risk of glomerular hyperfiltration and albuminuria in the general nondiabetic population: A prospective cohort study. Am. J. Kidney Dis. 105, e1772-21780 (2020).
Rodríguez-Poncelas, A. et al. High levels of fasting glucose and glycosylated hemoglobin values are associated with hyperfiltration in a Spanish prediabetes cohort. The PREDAPS study. PLoS ONE 14(9), e0222848 (2019).
The Diabetes Control and Complications (DCCT) Research Group. Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int. 47(6), 1703–1720 (1995).
Mogensen, C. E. Early glomerular hyperfiltration in insulin-dependent diabetics and late nephropathy. Scand. J. Clin. Lab. Invest. 46(3), 201–206 (1986).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Consortia
Contributions
M.M., L.C.S. and E.R. analyzed and interpreted the data. L.C.S., E.R. and J.F.N. wrote the outline and edited the manuscript. A.R., A.G., R.V., J.D.-E., M.M.-C. and C.G.-G.- reviewed/edited the manuscript and contributed to discussion. As reviewed and edited the manuscript. All authors read and approved the final manuscript. E.R. serves as a nominated consortia representative of PREDAPS Study Group.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Manouchehri, M., Cea-Soriano, L., Franch-Nadal, J. et al. Heterogeneity in the association between prediabetes categories and reduction on glomerular filtration rate in a 5-year follow-up. Sci Rep 12, 7373 (2022). https://doi.org/10.1038/s41598-022-11392-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-11392-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.