Abstract
This paper explores the associations between sex, age and hospital health care pressure in the context of the COVID-19 pandemic in Portuguese mainland municipalities. To represent the impact of sex and age, we calculated COVID-19 standardised incidence ratios (SIR) in Portuguese mainland municipalities over fourteen months daily, especially focusing on the Porto metropolitan area. A daily novel indicator was devised for hospital health care pressure, consisting of an approximation to the ratio of hospitalisations per available hospital medical doctor (HPI). In addition, 14-day incidence rates were also calculated daily (DIR14), both as an approach and an alternative to the current national pandemic surveillance indicator (which is not calculated with such regularity). Daily maps were first visualised to evaluate spatial patterns. Pearson's correlation coefficients were then calculated between each proposed surveillance indicator (SIR and DIR14) and the HPI. Our results suggest that hospital pressure is not strongly associated with SIR (r = 0.34, p value = 0.08). However, DIR14 bears a stronger correlation with hospital pressure (r = 0.84, p value < 0.001). By establishing the importance of tackling sex and age through the inclusion of these factors explicitly in an epidemiological monitoring indicator, and assessing its relationship with a hospital pressure indicator, our findings have public policy implications that could improve COVID-19 incidence surveillance in Portugal and elsewhere, contributing to advancing the management of potential pandemics in the near future, with a particular focus on local and regional territorial scales.
Similar content being viewed by others
Introduction
By the end of 2019, a large number of pneumonia cases was reported in the Wuhan region of China, expanding rapidly to the rest of the world. This disease was named COVID-19 and its causative agent (SARS-CoV-2) was soon identified as belonging to the family of coronaviruses.
Transmission of SARS-CoV-2 between people occurs mostly through the respiratory tract (droplets and aerosols) and by contact as well.
The incubation period varies between 2 and 14 days, and patients with COVID-19 may experience a wide range of symptoms. Some of these are mild, like fever, cough, fatigue, diarrhoea, or muscle pain. Yet, much more serious symptoms appear as the disease gets worse, with people of all ages at risk of developing an intense fever and/or a cough linked with breathing difficulties or shortness of breath. Pain related to chest pressure or loss of speech or movement may also happen.
The severity of symptoms depends on a range of factors such as ethnicity, sex, pregnancy, certain medical conditions and the use of certain drugs, poverty and overcrowding, and certain professional occupations1.
Furthermore, patients with certain pre-existing medical conditions are at higher risk of developing severe outcomes of COVID-19, defined as hospitalization, admission to the intensive care unit (ICU), intubation or mechanical ventilation, or death. Some types of Chronic Lung disease, Heart conditions (such as heart failure, coronary artery disease, or cardiomyopathies), Cerebrovascular disease, Chronic kidney disease and Cancer are amongst these medical conditions2.
Several types of Epidemiological surveillance have been proposed and applied to control the COVID-19 pandemic. This study focuses on applying comprehensive routine surveillance, a form of surveillance that assumes complete testing of all suspected cases. For this purpose, the number of newly confirmed cases/100,000 inhabitants is used, an indicator that depicts the rate at which new events occur in a population and can also be designated as the Incidence Rate3,4. This form of epidemiological surveillance provides the most accurate approximation to the intensity of COVID-19. In addition, it provides the capability of measuring the geographic evolution and severity of this disease. Furthermore, it is also helpful to keep track of temporal trends and compare areas within the same country3, such as municipalities.
As the COVID-19 situation evolved in Portugal, from March 2020 up to the present, the preferred indicator for this type of surveillance (and to report the status quo to the population) stabilised mainly on the Incidence Rate per 100,000 inhabitants over 14 days, reported at the municipality level5 with a 14-day interval. However, this strategy has been criticised as being inefficient for decision-making due to the considerable time lag between incidence reports which may lead to delays in pandemic management decisions at the local level, and provide the general public with non-updated information. Daily reporting is released at the NUTS (Nomenclature of Territorial Units for Statistics) level 2, though, but these are basic regions for the application of regional policies6 that encompass a large number of municipalities, thus providing a generalized regional overview, with low territorial granularity. For instance, NUT2 Centro (Center of mainland Portugal) comprises 100 municipalities7.
A different insight on the evolution of COVID-19 municipal incidence may be obtained by the Standardised Incidence Ratio (SIR). This indicator represents the ratio of the incident number of cases of a health condition within the study population to the expected incident number if the study population experienced the same incidence rate as a standard population (or other) for which the incidence rate is known4,8.
Regardless of the applied indicator, it is essential to benchmark its accuracy. Towards this purpose, one of the most critical consequences of increasing incidence is the risk of proportionally increasing pressure over national health systems, more specifically over hospital response capacity. In effect, the concentration of large numbers of severely ill patients in a narrow period nearly overwhelmed hospital capacity, and the impact of the novel coronavirus on the health and safety of patients, staff, and healthcare organizations, has not yet been accurately assessed. As more significant numbers of patients infected with COVID-19 are admitted to healthcare premises, conventional practises for providing safe and quality care face challenges with patient isolation protocols, lack of family presence, and staff limitations regarding the availability of personal protective equipment9,10.
As per our knowledge, there are not many studies analyzing the correlation between the number of cases of COVID-19 and the utilisation of health care services, but there are many studying the correlation between death rates and health care services availability or the lack of it. As an example, in France11, analised data from COVID-19 hospitalizations, severity (admission to intensive care units for reanimation or endotracheal intubation) and mortality, from 19 March to 8 May 2020, corresponding to the first French lockdown (all rates were age-standardized to eliminate differences in districts age structure). These authors show that the higher case-fatality rates observed by districts are mostly related to the level of morbidity. In metropolitan France, the higher case-fatality rates were generally related to the higher level of hospitalization, then potentially connected to the overload of healthcare system. In Spain12, showed that the Spanish regions with more rapid and extensive spread of SARS-CoV-2 had higher infection fatality rates. These findings are compatible with the theory that slowing down the spread of COVID-19 reduces the infection fatality rate and case fatality rate via preventing hospitals from being overrun, and thus allowing better and lifesaving care.
Ápropos the evolution of COVID-19 in Portugal throughout this study (March 2020–April 2021), the first wave started at the beginning of March 2020 and lasted just over a month. A state of emergency was in effect from 19 March 2020 to 2 May 2020. This period included several measures aimed at controlling the spread of the disease, such as the obligatory adoption of remote work, and for more than a year, progresses and setbacks were observed in some of these measures. Despite the lockdown measures it implicated, the first wave was minor in incidence compared to later ones, with the worst day registering 1516 cases on 10 April. Beyond May 2020, Portugal experienced a flattening curve and a plateau with few cases until the beginning of October. The second wave then started to unravel and reached its peak by 4 November 2020, with 7497 cases registered. The evolution of cases and deaths in Fig. 1 suggests that the third wave began without the second one having really ended.
COVID-19 cases and deaths in Portugal, 1st—3rd waves. Source:13, adapted.
The situation escalated after December 2020, and a new state of emergency was declared on 15 January 2021, lasting until 30 April 2021. On 28 January 2021, Portugal registered 16,432 new cases, the highest national count attained during the course of the pandemic until then. Along with the second state of emergency, a new set of control measures was deployed, and after January 2021 the incidence gradually subsided, with only 375 new cases confirmed on 25 May 20215,13.
At the very beginning of this research, we asked ourselves if, besides the contribution of co-morbidities already established in the literature, the combination of patients' sex and age would contribute to hospital pressure, in other words if higher numbers of infected patients belonging to either sex, or to (putatively) higher age segments of the population would lead to increased pressure on hospital units. Hence, it was possible to hypothesize that it would.
By tackling the research issues placed above, our study aims to contribute to the improvement of comprehensive COVID-19 routine surveillance at the municipality level by testing with incidence indicators that diverge from those currently used, and benchmark the accuracy of the chosen indicators to depict incidence in the current COVID-19 surveillance context, therefore contributing to increasing the accuracy of decision-making in the context of COVID-19 crisis management.
Materials and methods
Research area selection
All municipalities in the Porto metropolitan area (Area Metropolitana do Porto—AMP), and with its second-order adjacent neighbours, were selected as the research area (Fig. 2). The comprehensive list of chosen municipalities can be consulted in Table 1. Porto (41° 9′ 0″ N, 8° 36′ 37″ W) is the second largest metropolitan area in Portugal. AMP bears an area of 2040 km2, a total population of 1,722,374 inhabitants and a population density of 844 inhab./km2, close to that of Lisbon Metropolitan Area, with 954 inhab./km214.
In early March 2020, the AMP represented the starting point of the COVID-19 pandemic in Portugal and was also an important focus in mainland Portugal during the first three waves of this disease which are covered by the period of data used in our research. Given the importance of commuting between the centre of the metropolitan area (particularly Porto municipality) and its surrounding area, this research area was expanded to include its second-order neighbour municipalities too. Furthermore, to obtain a broader picture to compare with the research area, all Portuguese mainland municipalities were also tested within the scope of this study.
Data acquisition
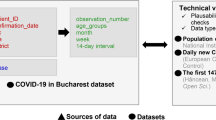
A temporal series (from 3 March 2020 to 22 April 2021) of Covid-19 daily incidence per municipality was obtained from the General Directorate of Health (Direção-Geral de Saúde—DGS)15, which releases it to authorised researchers periodically (although not daily). This data was further cleaned to include complete new cases only. Complete new cases are the ones that have the following information: date, municipality (Portuguese mainland), age and sex, totalling 740,103 cases.
Demographic data were obtained from the National Statistics institute (Instituto Nacional de Estatística—INE)16, namely 2019 estimates of total population per municipality and the corresponding age and sex structure.
The monthly count of hospitalizations per Portuguese National Health Service (Sistema Nacional de Saúde—SNS) hospital unit was obtained from March 2020 to February 2021, from the SNS Transparency Portal17, as well as the hospital location (latitude and longitude coordinates) and monthly availability (count) of health professionals, per hospital unit of the SNS. The national daily count of hospitalizations was also collected from DGS for the same study period.
Cartographic datasets with the administrative boundaries of Portuguese mainland municipalities were obtained from the General Directorate of the Territory (Direção-Geral do território—DGT)18.
Epidemiological indicators and spatial analysis
A municipal Covid-19 accumulated 14-day incidence rate per 100,000 inhabitants (DIR14) was first calculated daily by applying a moving window to the temporal series (Eq. 1). This calculation aimed to provide the 14-day accumulated incidence rate with a much higher frequency than the indicator currently provided by DGS. For each calendar day, the incidence of new cases was calculated for the previous 14-days.
with \(i=1, \dots , 278\) municipalities, \(y=\) confirmed new cases, \(p=\) population and \(t=0, \dots , 403\), representing respectively (1) the calendar day of 16 March 2020, the first day with 13 previous days of new cases available and (403) the last day, 22 April 2021.
Alternatively, the 14-day standardised incidence ratio (SIR) was calculated daily by applying a moving window to the temporal series (Eq. 2).
Accordingly, the following notations and/or definitions are introduced. For simplicity we omit the time index. For the aforementioned period, all calculations below are done at the t = day level, with the variables represented as follows:
-
(a)
\({Y}_{kg}\) The number of observed cases in each k age and g sex group.
-
(b)
\({p}_{kg}\) The number of people at risk in each k age and g sex group.
-
(c)
\({r}_{kg}= \frac{{y}_{kg}}{{n}_{kg}}\) The observed prevalence proportion for each k age and g sex group.
-
(d)
\({n}_{ikg}\) The number of people at risk in each k age and g sex group in the ith municipality.
-
(e)
\({E}_{ikg}\) and \({y}_{ikg}\) The expected and observed number of cases for the k age and g sex group in the ith municipality, respectively, where \({E}_{ikg}= {r}_{kg}* {n}_{ikg}\).
-
(f)
\({E}_{i}={{\sum }_{kg}r}_{kg}* {n}_{ikg}\) and \({y}_{i}^{* }= {\sum_{kg}y}_{ikg}\) The total number of expected and observed cases in the ith municipality, respectively.
$$SIR_{i} = \frac{{y_{i}^{*} }}{{E_{i} }}$$(2)
Equation (2) depicts the standardised incidence ratio, representing the risk of each ith municipality. A value of SIR greater (less) than one indicates that the area i has a higher (lower) than average disease risk. If the SIRi = 1.15, it can be said that area i has a 15% increased risk of the disease.
In this case, \(k=1, \dots , 9\), with each k representing a decade, so, k = 1 is for people up to 9 years of age and k = 9 is for people 80 years or older and \(g=1, 2\), representing the sex group, males or females.
The accumulated 14-day incidence rate, as calculated above, was finally multiplied by 100,000 inhabitants, to facilitate direct comparison with the one currently calculated by DGS.
An index of daily pressure on SNS hospitals (Hospital Pressure Index—HPI) was developed, to benchmark the above-mentioned epidemiological indicators' relative success.
First, it was essential to establish a correspondence between hospital and municipal area. Within the context of the SNS, every hospital has an area of influence (usually specified on its website) that defines to which hospital an inhabitant is expected to be referred in case of need. This area of influence may include one or more municipalities. Therefore, the territorial boundaries of municipalities were dissolved to obtain an area of influence for each Portuguese mainland SNS Hospital, and all mainland municipalities were assigned the code of their respective area of influence. Since some municipalities of main Portuguese cities, such as Lisbon and Porto, include more than one hospital within its area, it was decided to aggregate all hospitals within the municipality area to a single influence area. Through this procedure, 37 influence areas were obtained (figure in supplementary materials), covering all mainland municipalities, and a direct match between hospital influence area and the municipality was achieved, each municipality belonging to one hospital influence area. All geoprocessing and spatial analysis operations were executed in ESRI ArcGIS Pro 2.719.
The second step consisted of calculating daily HPI values. Monthly hospitalizations were aggregated by hospital influence area and then summed to obtain the total hospitalisations for each month in the Portuguese mainland. A monthly ratio was then established between the area of influence hospitalizations and the total count for the Portuguese mainland, allowing to establish the proportion of hospitalizations attributable to each area of influence. Next, this proportion of hospitalisations was multiplied by the daily national count of hospitalisations from COVID-19, consequently allowing an approximation to the daily hospitalisation count per area of influence. Finally, a ratio was established between each daily count of hospitalisations per area of influence and the available monthly count of medical doctors per area of influence (this count assumed, for the purposes of this study, to be constant along each day of the month). This final ratio is the HPI value, its rationale being that as hospitalisations rise (during the study period, primarily due to the correspondent rise in COVID-19 incidence), the number of available medical doctors to assist patients in hospitals tends to be progressively lower, and the pressure on the available medical human resources increases accordingly. HPI values were then mapped to municipalities through spatial overlay20, in other words by attributing an HPI value to municipalities that fall within a hospital influence area.
Accordingly, the following notations are introduced:
-
(a)
\(H= \sum {h}_{j}\) The number of hospitalisations per month for Portugal mainland, each month, during the considered period, with \(j=1, \dots , 37\) hospital influence areas.
-
(b)
\({w}_{j}=\frac{{h}_{j }}{H}\) The monthly proportion of each hospital area, compared to the national.
-
(c)
Assuming the daily hospitalisations follow the same spatial distribution as the monthly and knowing the national number of hospitalisations due to COVID-19 (Ht) in mainland Portugal, \({h}_{tj}={H}_{t}* {w}_{j}\) represents the number of hospitalisations per day and hospital area.
-
(d)
Assuming medical personnel is constant across the period, mj represents the number of available resources in terms of medical personnel in each of the j hospital areas.
-
(e)
\(HPI_{tj} = \frac{{h_{tj} }}{{m_{j} }}\) The daily hospital pressure index.
Regarding spatial and non-spatial data analysis, to obtain an initial comparative overview of the spatial distribution patterns of DIR14, SIR and HPI, daily choropleth maps20 were first produced for each of these variables for both the AMP study area and mainland Portugal, covering the entire temporal window of the study. These maps were also transformed into movie frames and a movie was then produced, allowing for a swifter visualization of the common spatiotemporal evolution of the three variables. A dashboard combining maps and line graphs for each variable was also produced using Microsoft Power BI21 for the Porto study area22 to add further data visualization analysis capabilities to our study.
After spatiotemporal visualisation, the Pearson correlation coefficient23 was calculated between each incidence indicator (DIR14 and SIR) and the HPI, for all mainland Portuguese municipalities, and its statistical significance was assessed. All non-spatial calculations were executed using the R statistical environment24.
Furthermore, the municipal population density was also calculated, based on the total municipality population in 2019 and the municipality area in square kilometres, to test the hypothesis of a potentially positive relationship between SIR and population density.
Ethical statement
This research does not involve the use of data at the individual level, only data from public sources aggregated spatially at the level of municipality and temporally at the daily level. Furthermore, it does not involve the realization of any laboratorial experiments, focusing only on the “natural experiment”. In addition, all methods were performed in accordance with the relevant guidelines and regulations.
Results and discussion
Visualising the combined spatiotemporal evolution of indicators for all Portuguese mainland suggests some alignment between indicators in time and space, but some discrepancies are also present. On the 10 April 2020 (Fig. 3), at the peak of the first wave, indicators suggested some coherence between themselves. The incidence rate (DIR14) shows a pattern of higher incidence in the northwest, with a focus on the Porto Metropolitan Area (Figs. 3, 4) but also extending to inland municipalities, and the standardised incidence rate (SIR) displays a similar pattern of excess incidence. However, the hospital pressure index (HPI) displays a different pressure pattern, with higher values occurring only in a cluster of municipalities north of Lisbon and to a much lesser extent, in the northwest near Porto. This aspect is hardly surprising, considering that the first wave exerted less pressure on the SNS, than the second and third waves.
On 9 August 2020 (figures in supplementary materials), with the disease experiencing a plateau of comparingly lower incidence between the first and second waves, the spatial patterns of DIR14 and SIR appear similar, especially in the area extending from Lisbon towards the northeast. As expected, pressure upon SNS hospitals (HPI) remained low due to the plateau of lower incidence, even considering the prevalence of active cases in need of extended hospitalization combined with the continuous incidence (albeit in relatively low numbers).
On 4 November 2020, when the peak of the second wave was attained, DIR14 and HPI displayed more similar patterns, while SIR signalled a strong cluster of higher risk municipalities around the Metropolitan Area of Porto and along the northeast border (figures in supplementary materials).
And in 28 January 2021, the height of the third wave was reached, the most critical moment in pandemic management for SNS Hospitals. As expected, with maximum pressure being exerted over the Portuguese hospital system, HPI displays all Portuguese mainland at extreme risk and DIR14 exhibits patterns of very high values in most of the territory. As SIR is a comparative measure, at this time, it can be mainly seen that the risk is across the whole of the Portuguese mainland and not concentrated in a specific area (Figs. 5, 6).
Comparing the visual patterns is not enough to draw conclusions and therefore the correlation coefficient between SIR and HPI, on the one hand, and DIR14 and HPI, on the other hand, was calculated. Table 2 shows the minimum, maximum, mean and median correlation coefficient between these pairs, calculated from the 278 municipalities in mainland Portugal. These values represent the linear relationship between a couple of indicators contemporaneously. Alternatively, we suspected that, given the timeline between exposure to infection, case confirmation, and the need for medical care (which might vary between one to 2 or 3 weeks), there might be a lag between those two events that exists and is not being accounted for here. We also calculated correlations with a 14-day lag25 to test this hypothesis, whose results can be seen in Table 3. However, as correlation values became lower, this hypothesis does not seem to hold, probably due to its (arguably corrective) effect being masked by diversity across municipalities regarding their epidemiological situation. Furthermore, the curves displaying the combined evolution of the average SIR and HPI for the Porto area display lesser adherence between themselves (Fig. 7) than DIR14 and HPI (Fig. 8), as demonstrated here from January to April 2021.
As mentioned above and concerning the benchmark of the tested indicators, the SIR does not seem to follow the peak moments of incidence and hospital pressure. On the other hand, the high correlation between DIR14 and HPI appears to support this conclusion. This result confirms the usefulness and good performance of DIR14 for the epidemiological surveillance of COVID-19 on a daily basis.
Conclusions
According to26, based on information from WHO for more than 183 countries, Portugal's hospital resources are at par with those in Spain and Italy. The authors concluded that global COVID-19 mortality rates are likely affected by multiple factors, including hospital resources, personnel, and bed capacity.
On another publication27, tried to explain why Spain and Italy suffered much more than France and Germany in the COVID pandemic and attributed that to the relevant large number of patients, in those two former countries, relying on a reduced number of both hospital beds and professionals, when compared with the patients in the latter countries. The lack of hospital beds in different European countries has been attributed to a set of important cuts in the financing of public health over the last years with reduction of healthcare personnel both in the community and in the hospital care. Knowing that Portugal has experienced the same type of cuts of other European countries, early indicators of the extra need of health care services are of upmost importance. According to OECD28 , the Health expenditure as a share of GDP in 2019 was, respectively for Portugal, Spain, Italy, France and Germany, 9.5%/9.1%/8.7%/11.1%/11.7%. The consciousness of this reality led this research.
This study demonstrates the need to strengthen epidemiological surveillance mechanisms, particularly concerning the need for faster availability of data and information on incidence; ideally, data and information should be made available to researchers and citizens, respectively, daily and at a sufficiently disaggregated territorial scale, such as the municipality (or even below if possible, e.g. at the urban scale) and not with a delay of two weeks as is currently the procedure for DGS concerning these elements. Our results at the municipal scale stress the need for improved management of the epidemiological situation at the local and regional levels. For instance, Portugal's Northern Regional Health Administration (Administração Regional de Saúde do Norte—ARSN), was involved in the current study and will benefit from its results concerning municipalities within its area of intervention).
The analysis of correlation values on Table 2 unveils the misalignment between SIR and HPI spatial patterns, confirming the previous observations through visual analysis of the maps. Nevertheless, the relatively low minimum value of 0.34 for the correlation between DIR14 and HPI suggests that the course of the epidemic is far from the pressure its reference hospital experienced for some municipalities. This result reinforces the idea that a single indicator such as DIR14 should be analysed jointly with others, as long as one wants to anticipate the very short-run demand for specialised medical care.
SIR is, as mentioned, a comparative measure. Given its mathematical characteristics, it represents elevated (reduced) risk areas if its value is above (below) one. In diseases with a linear relationship between the number of cases and a certain outcome (hospitalisation, for example), an elevated (lowered) risk corresponds linearly to that outcome. COVID-19 does not have that relationship because outcomes like hospitalisations are not the same per age and sex. An elevated risk can exist if more younger people get infected, which will not be translated into more hospitalisations. Therefore, to use SIR to predict the number of hospitalisations, a “reduced” version would need to be used. A direct comparison could be made if a SIR would be calculated using only the information for the most at-risk age groups. For the time being, the average SIR for the entire period displays a pattern of elevated risk in areas with higher population density (figures in supplementary materials).
Taking into account the SIR calculation methodology, which implies indirect standardisation by age structure and sex of the population, results also seem to indicate the incidence of this disease is “democratic”, in the sense that susceptibility to infection does not vary in a pronounced way with age or sex of the infected persons (although this is no longer true after infection, within the clinical course of the disease). The distribution of cases per 100,000 inhabitants between sex and age groups during the whole period of this research seems to concur with this view (Fig. 9).
However, the response of the SIR to the incidence values may still be eventually helpful to establish a comparison between the severity at municipality and country levels. It should be noted that the SIR response seemed to deteriorate, at least visually, on the days of higher incidence, and in this sense, we hypothesise that in these situations, as there is some uniformity in terms of high incidence, the response of this index tends to “dilution”. It ceases to be helpful. This possibility can be deepened in further studies, considering the hypothesis of calibrating the SIR that allows overcoming this difficulty.
The HPI could still be improved, as it was calculated using the total number of daily hospitalisations throughout the country since it was impossible to obtain a segmentation between mainland Portugal and the islands. However, we firmly believe that, even at the risk of introducing a bias towards increasing the values of approximation to daily hospitalisations by area of influence, this bias will be systematic (insofar as it is distributed equally among the areas of influence of hospitals in Portugal). It will be of negligeable magnitude, considering the size of the island area population compared to mainland Portugal's population.
These and other improvements in epidemiological surveillance processes are critical, not only for managing the current pandemic, but also for managing potential pandemics in the near future.
Data availability
All data used sources are available as supplementary materials to this article.
References
Tadj, A. & Lahbib, S. S. M. Our overall current knowledge of covid 19: An overview. Microbes Infect. Chemother. 1, e1262–e1262 (2021).
Centers for Disease Control and Prevention, Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19: Information for Healthcare Providers, 2021. [Online]. Available: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html. [Accessed: 03-Feb-2022].
Ibrahim, N. K. Epidemiologic surveillance for controlling Covid-19 pandemic: Types, challenges and implications. J. Infect. Public Health 13(11), 1630–1638 (2020).
Porta, M. A Dictionary of Epidemiology 6th edn. (Oxford University Press, Oxford, 2014).
DGS.F21F23QAF Relatório de Situação—COVID-19, 2021. [Online]. Available: https://covid19.min-saude.pt/relatorio-de-situacao/. [Accessed: 26-Jul-2021].
Eurostat. NUTS—Nomenclature of territorial units for statistics, 2022. [Online]. Available: https://ec.europa.eu/eurostat/web/nuts/background. [Accessed: 03-Feb-2022].
INE, NUTS 2013: As novas unidades territoriais para fins estatísticos. Lisboa: Instituto Nacional de Estatística, 2015.
Baptista, H., Mendes, J. M., Caldas De Almeida, J. & Xavier, M. Alcohol abuse disorder prevalence and its distribution across Portugal. A disease mapping approach. REVSTAT-Stat. J. 13(1), 79–101 (2015).
Soria, A. et al. The high volume of patients admitted during the SARS-CoV-2 pandemic has an independent harmful impact on in-hospital mortality from COVID-19. PLoS ONE 16(1), e0246170 (2021).
Polancich, S. et al. Learning during crisis: The impact of COVID-19 on hospital-acquired pressure injury incidence. J. Healthc. Qual. 43(3), 137–144 (2021).
Souris, M. & Gonzalez, J.-P. COVID-19: Spatial analysis of hospital case-fatality rate in France. PLoS ONE 15(12), e0243606 (2020).
Kenyon, C. COVID-19 infection fatality rate associated with incidence—A population-level analysis of 19 Spanish autonomous communities. Biology (Basel) 9(6), 128 (2020).
Martins, A. D., & Sobral, S. R. Working and learning during the COVID-19 confinement: An exploratory analysis with a small sample from Portugal. In Informatics (Vol. 8, No. 3, p. 44). Multidisciplinary Digital Publishing Institute (2021).
AMP. Área Metropolitana do Porto, 2021. [Online]. Available: http://portal.amp.pt/es/1/ampa/27. [Accessed: 28-Jul-2021].
DGS. General Directorate of Health—NHS, 2021. [Online]. Available: https://www.sns.gov.pt/entidades-de-saude/direcao-geral-da-saude/. [Accessed: 03-Sep-2021].
INE. Statistics Portugal—Web Portal, 2021. [Online]. Available: https://ine.pt/xportal/xmain?xpgid=ine_main&xpid=INE&xlang=en. [Accessed: 03-Sep-2021].
SNS. Transparência—SNS, 2021. [Online]. Available: https://www.sns.gov.pt/transparencia/. [Accessed: 26-Jul-2021].
DGT. Carta Administrativa Oficial de Portugal—CAOP, 2020. [Online]. Available: https://www.dgterritorio.gov.pt/Carta-Administrativa-Oficial-de-Portugal-CAOP-2020. [Accessed: 26-Jul-2021].
ESRI. ArcGIS Pro—2D and 3D GIS Mapping Software, 2022. [Online]. Available: https://www.esri.com/en-us/arcgis/products/arcgis-pro/overview. [Accessed: 24-Jun-2020].
de Smith, M. J., Goodchild, M. F., & Longley, P. A. Geospatial Analysis 6th Edition, 2020. [Online]. Available: https://www.spatialanalysisonline.com/HTML/index.html. [Accessed: 02-Oct-2020].
Microsoft. What is Power BI | Microsoft Power BI, 2021. [Online]. Available: https://powerbi.microsoft.com/en-us/what-is-power-bi/. [Accessed: 24-Feb-2022].
Microsoft. Microsoft Power BI, 2021. [Online]. Available: https://powerbi.microsoft.com/pt-pt/desktop/. [Accessed: 27-Jul-2021].
S. Stigler. Francis Galton’s Account of the Invention of Correlation on JSTOR, 1989.
R. R: The R Project for Statistical Computing, 2021. [Online]. Available: https://www.r-project.org/. [Accessed: 29-Jul-2021].
Makridakis, S., Wheelwright, S. C. & Hyndman, R. J. Forecasting Methods and Applications (Wiley, London, 1998).
Sen-Crowe, B., Sutherland, M., McKenney, M. & Elkbuli, A. A closer look into global hospital beds capacity and resource shortages during the COVID-19 pandemic. J. Surg. Res. 260, 56–63 (2021).
Pecoraro, F., Luzi, D. & Clemente, F. The efficiency in the ordinary hospital bed management: A comparative analysis in four European countries before the COVID-19 outbreak. PLoS ONE 16(3), e0248867 (2021).
OECD, Health Expenditure, 2021. [Online]. Available: https://www.oecd.org/els/health-systems/health-expenditure.htm. [Accessed: 03-Feb-2022].
Acknowledgements
This research was supported by project Data4Covid19 (project ID: 62821), funded by the European Regional Development Fund, through the Operational Competitiveness Programme—COMPETE 2020, in the framework of 15/SI/2020—R&D Companies and Testing and Optimization Infrastructures (COVID-19). The authors acknowledge the editors and reviewers, whose comments and suggestions helped to improve the presentation of the paper.
Author information
Authors and Affiliations
Contributions
J.M., H.B. and A.O. conceived and conducted the experiments, B.J. and M.N. analyzed the results. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mendes, J.M., Baptista, H., Oliveira, A. et al. Beyond comorbidities, sex and age have no effect on COVID-19 health care demand. Sci Rep 12, 7356 (2022). https://doi.org/10.1038/s41598-022-11376-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-11376-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.