Abstract
Immunocompromised patients, especially those who undergo kidney transplantation, have lower antibody levels following SARS-CoV-2 mRNA vaccination. The situation of transplant treatment, such as transplant source and immunosuppressive drugs, is different in Japan than that in other countries. Therefore, it is necessary to clarify whether antibody acquisition rates differ between Japan and other countries. This retrospective study included patients with post-kidney transplant who were attending at the Nagoya University Hospital. The anti-SARS-CoV-2 IgG antibody titers were measured between 3 weeks and 3 months after vaccination. Seventy-three patients (45 men and 28 women) were included. Of these, 23 (31.5%) showed antibody presence, and the rates of antibody acquisition were very low than those in the control group (100.0% vs. 31.5%, P < 0.05). Antibody acquisition rates were associated with body mass index (odds ratio [OR]: 1.21, 95% confidence interval [CI]: 1.04–1.39, P < 0.05) and the duration between transplantation and vaccination (OR: 1.01, 95% CI: 1.00–1.02, P < 0.05). The immunosuppressive drugs used were: prednisolone in all cases, tacrolimus in 89.0%, cyclosporine in 9.6%, and mofetil mycophenolate in 97.3%. None of the patients were excluded from receiving two doses of the vaccine due to adverse effects. The study indicated that vaccination-induced antibody acquisition rates against SARS-CoV-2 were extremely low in Japanese patients who underwent post-kidney transplantation. Thus, despite two doses of vaccination, it is necessary to closely monitor infection control in such patients.
Similar content being viewed by others
Introduction
The COVID-19 pandemic that started in 2019 has killed many patients1. It has been reported that immunocompromised patients, such as those with underlying diseases, are at a high risk for developing severe disease and need to be careful about infection2. The SARS-CoV-2 mRNA vaccines have shown high antibody acquisition rates in healthy individuals, indicating that they are useful in preventing infections and severe diseases3,4. However, immunocompromised patients, especially those after kidney transplantation, have low rates of antibody acquisition after SARS-CoV-2 mRNA vaccination than immunologically healthy individuals5,6. The antibody acquisition rates are higher with two doses of vaccination than with one dose in immunocompromised patients6, but they are still lower than those in healthy individuals.
In case of kidney transplantation, many living-donor kidney transplantation and ABO-incompatible kidney transplantation have been performed to overcome the donor shortage issue7.
The kidney transplant situation is different in Japan from that in other countries, as ABO-incompatible kidney transplants require blood purification procedures, such as plasma exchange and stronger immunosuppression than ABO-compatible transplants. Furthermore, due to a shortage of donors, kidney transplants are performed in some cases when weak positive donor-specific HLA antibodies (DSA) are considered acceptable7. Therefore, in addition to ethnic differences, the strength of immunosuppression in kidney transplants may also vary; thus, it is necessary to obtain Japan-specific data. In this study, we measured SARS-CoV-2 antibody titers in post-kidney transplant patients between 3 weeks and 3 months after vaccination to investigate the rates of antibody acquisition.
Materials and methods
Patients
Post-kidney transplant recipients who attended Nagoya University Hospital between June and September 2021 were included in this study. Patients who underwent kidney transplantation at other hospitals, but were subsequently attended Nagoya University Hospital, were also included. At the time this study was conducted, two types of vaccines were used in Japan, namely BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). Whenever possible, we investigated which of them was used by obtaining the inoculation forms. Patients who failed to receive SARS-CoV-2 mRNA vaccine due to a history of anaphylaxis or allergy were excluded. Patients already on dialysis, post–SARS-CoV-2 infection, and those who failed to provide consent were also excluded. The control group comprised patients (n = 8) with chronic kidney disease (CKD) stage G3 or better, including transplant donors and patients with mild IgA nephropathy not requiring steroid therapy. The estimated glomerular filtration rate (eGFR) was calculated using the Japanese equation8. Data regarding patient background, past history, comorbidity, medication, and laboratory data were collected. The study was approved by the Institutional Review Board of the Nagoya University Hospital (approval number: 2010–1135 and 2020–0486), and all participants provided written informed consent. All methods were conducted in compliance with the Declaration of Helsinki and the relevant guidelines.
Measurement of SARS-CoV-2 antibody titers
Serum samples collected on the day of routine medical checkups from 3 weeks to 3 months after two doses of vaccination were used for the measurement. A completely automated immunoassay analyzer (Alinity i; Abbott Laboratories, IL, USA) was used as the analytical instrument. SARS-CoV-2 IgG II Quant Reagent Kit (Lot; 28531FN00, Abbott Japan Co., Ltd.) was used for the SARS-CoV-2 IgG antibody assay. The antibody-positive cutoff was set as at least 50 AU/mL according to the previous reports9,10.
Statistical analysis
Baseline characteristics are presented descriptively. They were tested using the Mann–Whitney U test and χ2 test. Univariate and multivariate logistic regression analyses were used to evaluate the rates of antibody acquisition. Differences were considered statistically significant at P < 0.05. The R software (R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org/) was used to perform all statistical analyses in this study11. We also used gglot2 package (https://ggplot2.tidyverse.org.) to draw violin plots12.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Results
Patient background and antibody acquisition rates
We investigated 73 transplant recipients (45 men and 28 women) who received two doses of SARS-CoV-2 mRNA vaccine (Fig. 1). The baseline characteristics of the patients are shown in Table 1. The median age at the time of vaccination was 61 years (interquartile range [IQR], 50–69 years), and the median age at the time of transplantation was 53 years (IQR, 41–61 years). The median duration between transplantation and vaccination was 74 months (IQR, 30–131 months). The median body mass index (BMI) was 22.5 (IQR, 21.0–25.7). Chronic glomerulonephritis (CGN) was the most common primary kidney disease (35.6%), followed by diabetic nephropathy (DMN) (13.7%), and hereditary disease (12.3%). The coexisting diseases were diabetes mellitus (26.0%) and hypertension (68.5%). Living-donor kidney transplants showed a high rate (91.8%). Preemptive kidney transplantation was performed in 31 patients (42.5%); and of 42 patients who underwent renal replacement therapy (RRT), 32 underwent hemodialysis (HD) and 10 underwent peritoneal dialysis (PD) as pre-transplant RRT. The median pre-transplant RRT period in patients who underwent HD and PD was 23 (IQR, 9–76) months. The blood test immediately before vaccination was 6.7 × 103/μl (IQR, 5.4–7.7) for white blood cells and 1.6 × 103/μl (IQR, 1.2–2.1) for lymphocytes. The immunosuppressive drugs administered were prednisolone in all cases, tacrolimus in 89.0%, cyclosporine in 9.6%, and mofetil mycophenolate (MMF) in 97.3%. Twenty-three (31.5%) patients in the post-kidney transplant group tested positive for acquired antibodies that was a very low percentage than in control group (Fig. 2). Moreover, antibody titer was low in patients who showed acquired antibodies. None of the patients failed to receive the second vaccine dose due to adverse reactions, such as anaphylaxis.
Comparison between antibody-acquired and non-acquired groups
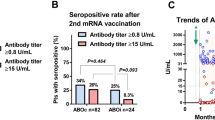
Further, we compared the characteristics in the antibody-acquired group with those in non-acquired group of patients who underwent post-kidney transplantation. The characteristics of the patients in each group are presented in Table 2. Patients’ age at transplantation, BMI, eGFR, duration between transplantation and vaccination, and lymphocyte counts immediately prior to vaccination were significantly different between the groups. For instance, in the antibody-acquired group, the shortest duration between transplantation and vaccination was 16 months. There was no significant difference in the antibody acquisition rate between ABO-incompatible and ABO-compatible kidney transplant recipients (Fig. 3).
Examination of factors associated with antibody acquisition
Next, we performed a logistic regression analysis to examine the factors involved in antibody acquisition. Factors found significant in previous analysis (Table 2) were considered in this analysis, by excluding multicollinearity. The analyses suggested that BMI and duration between transplantation and vaccination significantly correlated with antibody acquisition rates, even after adjustment for various factors (Fig. 4).
Additionally, we attempted to confirm the type of vaccine (BNT162b2, Pfizer-BioNTech or mRNA-1273, Moderna) by obtaining details with an inoculation form, but failed to determine all of patients. In patients for whom the vaccine type was known, administration of mRNA-1273 vaccine (Moderna) correlated with a higher rate of antibody acquisition than BNT162b2 (Pfizer-BioNTech) (Fig. S1).
Regression analysis between antibody-titers and the duration from vaccination to measurement
We measured antibody titers from 3 weeks to 3 months after two doses of vaccination, due to the timing of outpatient visits and vaccination. To investigate the possibility of antibody titer decline over time, we assessed for a correlation between the number of days from second vaccination to antibody titer measurement and the antibody titer values. Table 3 shows the results. No relationship was found between the number of days from vaccination to measurement and antibody titer.
Discussion
The study demonstrated that the rate of antibody acquisition in post-kidney transplant patients was extremely low. Moreover, the study indicated that a significant proportion of post-kidney transplant patients may remain at risk for COVID-19 even after two doses of the mRNA vaccination. A lower BMI and shorter duration between transplantation and vaccination correlated with lower rate of antibody acquisition. Additionally, despite incomplete data, the analyses indicated that there may be differences in antibody acquisition rates depending on the vaccine type.
The rates of antibody acquisition in immunocompromised patients, such as those after organ transplantation, reported in previous studies6,13 are higher than those found in the present study. This may be due to the high intensity of immunosuppression in Japan, where many ABO-incompatible transplants are performed. However, the results of Fig. 3 did not support this hypothesis. The lack of significant difference in antibody acquisition between ABO-incompatible and ABO-compatible transplants in the present results may be due to the fact that few patients were vaccinated immediately after transplantation, which has different immunosuppressive strengths. Taking the results into consideration, the low rate of antibody acquisition may be strongly influenced by other factors. Patients in the present study tended to be older and have a lower BMI than that observed in studies from other countries. Evidence suggests that people with a low BMI are more vulnerable to infections14, and underweight people tend to have a weakened immunity. The BMI of patients in this study was not extremely low, but it could be lower than that in patients in other countries; therefore, the antibody acquisition rates may have been lower than those in other countries. The duration between transplantation and vaccination remains controversial. In the antibody-acquired group, the shortest duration between transplantation and vaccination was 16 months. We hypothesized that patients are less likely to acquire antibodies immediately after transplantation even if they are vaccinated; thus, extending the duration between transplantation and vaccination may have become a trend and contributed to bias. The antibody acquisition rate may drop considerably for approximately one year after transplantation, although it is difficult to confirm this based on the small sample size in the present study. However, it is ethically incorrect that patients who have undergone transplantation and are receiving strong immunosuppressive therapy be excluded from vaccination for one year. Furthermore, evidence suggests that people who received the third booster vaccine dose have higher antibody titers than those who received only the second dose, which is also true for organ transplant patients15,16. Therefore, it is preferable to administer the third dose for infection control, especially in patients with weakened immune systems, such as those immediately after transplantation. Additionally, patients receiving MMF as immunosuppressant have difficulty in acquiring antibodies6. In this study, we failed to examine the relationship between immunosuppressive drugs, especially with or without MMF administration, and antibody acquisition rates as a majority of the patients were administering MMF. However, there was no significant difference in MMF dose between the two groups. We observed a difference in eGFR between the antibody-acquired and non-acquired groups, although it was not significant. It has been reported that renal failure is associated with decreased immunity17. Therefore, patients undergoing strong immunosuppressive therapy for graft rejection or other reasons, and with impaired renal function should be especially careful.
In the present study, patients vaccinated with mRNA-1273 (Moderna) had a higher rate of antibody acquisition than those vaccinated with BNT162b2 (Pfizer-BioNTech). This may be attributed to differences in the amounts of chemicals contained in the products. Studies suggest that the frequency of side effects varies with the vaccine type18,19, and allergic reactions may be suppressed in immunosuppressed patients. None of the patients in the present study canceled the second vaccine dose due to allergic reactions. Thus, considering these factors, interventions to improve vaccine response, such as vaccine type, timing of vaccination, additional booster dose administration, and adjustment of immunosuppressive drugs, need to be further investigated in post-kidney transplant patients.
The present study has a few limitations. First, it was a retrospective study. Second, the study had a limited number of participants and was conducted at a single center. Third, some of the participants failed to report the type of vaccine they had received. However, despite the limitation, it is extremely important to show the antibody acquisition rate after two doses of vaccinations in kidney transplant recipients because it provides basic information for clinical actions, such as infection control and the third vaccination.
Conclusion
The antibody response to two doses of SARS-CoV-2 vaccine in Japanese kidney transplant recipients was lower than that in healthy subjects. Thus, post-kidney transplant patients may remain at risk of infection with only two doses of vaccination, and it is necessary to closely monitor infection control.
Data availability
The datasets generated and analyzed during the current study are not publicly available because consent has not been obtained to make them available online to an unspecified number of people, but they are available from the corresponding author on reasonable request.
References
World Health Organization. WHO coronavirus disease (COVID-19) dashboard; 2021. https://covid19.who.int/.
US Centers for Disease Control and Prevention. Evidence used to update the list of underlying medical conditions that increase a person’s risk of severe illness from COVID-19; updated 2021. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/evidence-table.html.
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2021).
Boyarsky, B. J. et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA 325, 1784–1786 (2021).
Boyarsky, B. J. et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA 325, 2204–2206 (2021).
Fact Book 2020 on Organ Transplantation in Japan (http://www.asas.or.jp/jst/pdf/factbook/factbook2020.pdf).
Matsuo, S. et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 53, 982–992 (2009).
Grupper, A. et al. Humoral response to the Pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis. Clin. J. Am. Soc. Nephrol. 16(7), 1037–1042 (2021).
Dekervel, M. et al. Humoral response to a third injection of BNT162b2 vaccine in patients on maintenance haemodialysis. Clin. Kidney J. 14(11), 2349–2355 (2021).
Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 48, 452–458 (2013).
Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York. (https://ggplot2.tidyverse.org) (2016).
Deepak, P. et al. Effect of immunosuppression on the immunogenicity of mRNA vaccines to SARS-CoV-2: A Prospective Cohort Study. Ann. Intern. Med. 174, 1572–1585 (2021).
Fang, X. et al. Quantitative association between body mass index and the risk of cancer: A global Meta-analysis of prospective cohort studies. Int. J. Cancer 143, 1595–1603 (2018).
Hall, V. G. et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N. Engl. J. Med. 385, 1244–6 (2021).
Bar-On, Y. M. et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N. Engl. J. Med. 385, 1393–1400 (2021).
Kurts, C., Panzer, U., Anders, H. J. & Rees, A. J. The immune system and kidney disease: Basic concepts and clinical implications. Nat. Rev. Immunol. 13, 738–753 (2013).
Chapin-Bardales, J., Gee, J. & Myers, T. Reactogenicity following receipt of mRNA-based COVID-19 vaccines. JAMA 325, 2201–2202 (2021).
Blumenthal, K. G. et al. Acute allergic reactions to mRNA COVID-19 vaccines. JAMA 325, 1562–1565 (2021).
Acknowledgements
None.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
The manuscript was written by K.F. and A.T. The research idea and the study design were conceptualized by K.F., K.F., S.S., S.M., and A.T. Data acquisition and analysis were performed by K.F. and A.T. All authors performed data interpretation. All the authors treated the patients. S.M. provided supervision or mentorship. All the authors contributed with important intellectual content during the writing of the manuscript and its revisions. Thus, each author is personally accountable for their contributions. Additionally, all the authors agree to ensure that questions pertaining to the accuracy and integrity of any portion of the work will be appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fujieda, K., Tanaka, A., Kikuchi, R. et al. Antibody response to double SARS-CoV-2 mRNA vaccination in Japanese kidney transplant recipients. Sci Rep 12, 6850 (2022). https://doi.org/10.1038/s41598-022-10510-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-10510-7
This article is cited by
-
Anti-SARS-CoV-2 IgG antibody titer after BNT162b2 mRNA COVID-19 vaccination in Japanese patients who underwent renal replacement therapy, hemodialysis, peritoneal dialysis, and kidney transplantation
Clinical and Experimental Nephrology (2023)
-
Antibody acquisition after second and third SARS-CoV-2 vaccinations in Japanese kidney transplant patients: a prospective study
Clinical and Experimental Nephrology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.