Abstract
We aimed to explore factors associated with prognosis in patients with metastatic small bowel adenocarcinoma (SBA) as well as to develop and validate nomograms to predict overall survival (OS) and cancer-specific survival (CSS). Relevant information of patients diagnosed between 2004 and 2016 was extracted from the Surveillance, Epidemiology, and End Results (SEER) database. Nomograms for predicting 1- and 3-year OS and CSS were established with potential risk factors screened from multivariate cox regression analysis. The discrimination and accuracy of the nomograms were assessed by concordance index (C-index), calibration plots, and the area under receiver operating characteristic curve (AUC). In total, 373 SBA patients with M1 category were enrolled. Multivariate analysis revealed that age, size and grade of primary tumor, primary tumor surgery, and chemotherapy were significant variables associated with OS and CSS. The C-index values of the nomogram for OS were 0.715 and 0.687 in the training and validation cohorts, respectively. For CSS, it was 0.711 and 0.690, respectively. Through AUC, decision curve analysis (DCA) and calibration plots, the nomograms displayed satisfactory prognostic predicted ability and clinical application both in the OS and CSS. Our models could be served as a reliable tool for prognostic evaluation of patients with metastatic SBA, which are favorable in facilitating individualized survival predictions and clinical decision-making.
Similar content being viewed by others
Background
Small intestine malignancies are rare and fatal, accounting for approximately 3% of all gastrointestinal cancers1,2,3, of which small bowel adenocarcinoma (SBA) is the most-common histopathological type, followed by neuroendocrine tumors, lymphomas, and sarcomas4,5. Despite its rarity, SBA is on the rise around the world, with an annual estimated 5,300 new cases and 1,100 cancer deaths in the United States6,7. Advancements in capsule endoscopy, enteroscopy, cross-sectional imaging techniques, and therapeutic strategies have markedly improved detection and survival rates in recent decades8. However, approximately one-third of patients present with advanced disease at diagnosis, and overall survival (OS) of metastatic SBA patients remains unsatisfactory, reported 8–22 months in limited, multicenter series9,10. Surgical excision remains the mainstay of treatment for SBA manifesting as localized disease, while treatment for SBA patients with M1 category is at a standstill, for whom the rationales for the choice of therapeutic modalities are extrapolated from colon adenocarcinoma11. Therefore, it has become imperative to estimate clinicopathologic characteristics related to prognosis, thus facilitating individualized and optimal patient treatment.
To date, the American Joint Committee on Cancer (AJCC) TNM staging system has been periodically updated for prognostic evaluation of SBAs, which only takes the depth of tumor invasion (T), number of metastatic lymph nodes (N), and distant metastasis (M) into consideration. Other independent factors, however, such as age12,13, gender13, race14, performance status15, carbohydrate antigen 19-9 (CA 19-9)16, carcinoembryonic antigen (CEA)15, tumor site17,18, metachronous or synchronous metastasis19, and treatment20,21 can also influence the survival of SBA patients significantly. Additionally, tumor biological behavior including tumor size22, histological type20, the degree of differentiation21, growth pattern18, and metastasis pathway18 can affect the prognosis of SBA patients to a certain extent. Likewise, a lack of emotional support, family care, and medical insurance implicates that this category of patients with tumors would miss the optimal treatment opportunity and have a poor prognosis23. Consequently, the role of sociomedical support covering marital and insurance status cannot be neglected in the clinical outcome of SBA patients24,25. Under this circumstance, the traditional TNM staging system needs further improvement and validation, which is inadequately formulated for the prognostic prediction and may not deficiently encompass the tumor biology26. And a more effective and accurate model is under requirement for predicting the prognosis of patients with metastatic SBA.
Of the available and effective decision-making tools, nomogram, as an integrative graphical calculation or algorithm that incorporates biological and clinical variables, is the most widely used to predict individual prognosis in clinical investigations currently. It has been proved to be favorable and accurate compared to the TNM staging system in diverse cancers, highlighting their application as new alternatives or even new standards27,28,29,30, with the advantage of quantification and visualization for clinicians. To the best of our knowledge, although a prior study has reported a nomogram about cancer-specific survival (CSS) in the whole patients with SBA13, it did not refer to the populations with substantially poorer prognosis, nor did it conduct a subgroup analysis on this. Therefore, the present study aimed to identify demographic and tumor characteristics that influence the particular outcomes of patients with metastatic SBAs and subsequently develop nomograms to predict 1- and 3-year OS and CSS based on the Surveillance, Epidemiology, and End Result (SEER) database, which could provide more informative and representative evidence.
Methods
Database and patient selection
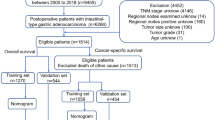
Information of patients with a histological confirmation of SBA with M1 category were obtained between 2004 and 2016 after receiving permission to access the SEER research files (accession number: 18892-Nov 2018). The SEER program is a national collaboration cancer registry, which comprehensively accumulates demographic and clinical information on associated prevalence, treatment and prognosis of various cancer types, covering up to 34% of the US population31. We used the following SEER variables to identify primary SBA: “Primary Site-labeled” (C17.0-duodenum, C17.1-jejunum, C17.2-ileum, or C17.9-small bowel not otherwise specified) and “Histologic Type International Classification of Diseases for Oncology, Third edition (ICD-O-3)” (histology codes: 8140, 8144, 8210, 8211, 8220, 8221, 8255, 8260, 8261, 8262, 8263, 8480, 8481, 8490, 8574, or 8576). The detailed inclusion criterias were as follows: (a) patients pathologically diagnosed with metastatic SBA from 2004 to 2016; (b) age ≥ 18 years; (c) no history of other types of malignancy; (d) survival months and follow-up information were available; (e) complete data on tumor location and size, grade, T classification, status of nodal metastasis, marital and insurance status, and treatment. Besides, patients diagnosed at the time of autopsy and death certificates were excluded. Finally, 373 SBA patients with M1 category were deemed eligible, and they were randomly divided into the training set and the validation set at a ratio of 7:3. The flowchart of patient selection was presented in Fig. 1.
Data extraction
Individual data retrieved in our analysis included race, gender, marital and insurance status of patients, age at diagnosis, year of diagnosis, tumor location, histology, tumor size and grade, T classification of 8th AJCC staging scheme, nodal metastasis, and treatment (surgery, chemotherapy, and radiation therapy status) of primary tumor, distant metastasis site, vital status, cause-specific death classification, and survival time. For patients with no tumor resection, the T classification, nodal metastasis, and tumor size were assessed by endoscopy and imaging, such as esophagogastroduodenoscopy with endoscopic ultrasound, double balloon endoscopy, capsule endoscopy, CT, MRI, PET-CT and so on, which could evaluate the extent of local tumor invasion and lymph node involvement32. To maximize predictive ability, continuous age variable was further categorized into three groups based on the optimal cut-off values generated by X-tile program. In the same way, tumor size was transformed into dichotomous categorical variable. According to the best cut-off values, the age of diagnosis was grouped into three categories: ≤ 59 years old (150 cases), 60–75 years old (159 cases), and ≥ 76 years old (64 cases). Tumor size was stratified into two sets: ≤ 48 mm (218 cases,), and ≥ 49 mm (155 cases) (Fig. S1). Analogously, the categorical variables were also classified accordingly for some clinical reasons. Divorced, separated, widowed, and single patients were converted into unmarried category. Surgery was defined as two styles, including primary radical surgery (total removal of the primary site with an en bloc resection of other organs) and primary palliative surgery (excisional biopsy, laser ablation, simple or partial surgical removal of primary site, etc.) according to the SEER surgery codes for the small intestine. Treatment was classified as primary tumor surgery alone, primary tumor surgery plus chemotherapy or radiation, chemotherapy or radiation alone and none (receive no therapy). The principal outcome of interest was the probability of 1-year and 3-year OS, whereas CSS was the secondary endpoint of our study. The reason why 1-year and 3-year outcomes were chosen was that the majority of patients experienced death within 3 years. We defined OS as the duration between first diagnosis of SBA and death or the last follow-up control. CSS was measured as the interval from a positive diagnosis to death attributed to SBA (or the most recent contact data).
Statistical analysis
A descriptive analysis of the basic characteristics of patients was conducted. Continuous variables were presented as the mean and all categorical data were reported as the number of cases with proportions. The Chi-square test and Student's t-test were used to compare the demographic and clinical parameters between the training cohort and the validation cohort. Univariate and multivariate Cox regression analysis were applied to analyze risk factors on OS and CSS along with hazard ratios (HRs) and corresponding 95% confidence intervals (CIs). Afterwards, a predictive model was constructed to predict 1-, 3-year OS and CSS on the basis of the independent prognostic variables identified from the multivariate analysis in the training cohort. It is worth mentioning that treatment did not enter into the univariate and multivariate analysis because of the collinearity between the treatment and variables such as primary tumor surgery, chemotherapy, and radiation.
Both discrimination and calibration were measured to assess the performance of the nomogram using the training set and internal validation set. The ability to discriminate between observed and predicted outcome was evaluated by Harrell's concordance index (C-index)33. A higher C-index indicated a superior capacity to separate patients with different survival outcomes. Similarly, the area under receiver operating characteristic (ROC) curve (AUC) was further utilized to appraise the prediction efficiency of the prognostic models. The larger the area is, the more precise the model’s predictive ability is. The calibration curves were performed according to a bootstrapped resample with 1000 iterations. A calibration plot in the 45-degree line implied a perfect model, with great concordance between the predicted and actual survival. Furthermore, the clinical value of the predictive models was reckoned with decision curve analysis (DCA) by quantifying the net benefit at distinct threshold probabilities34. Additionally, the total scores of each patient were calculated based on the established Cox regression model, and then patients were assigned into the low-, and high-risk groups using the X-tile program. Survival curves among two groups of patients with different prognostic risk were delineated by the Kaplan–Meier method and log-rank test.
SPSS software version 25.0 (IBM Corp, Armonk, NY), X-tile software version 3.6.1 (Rimm Laboratory, Yale School of Medicine, New Haven, CT, USA), and R software version 3.3.0 (Institute for Statistics and Mathematics, Vienna, Austria) were used for above statistical analysis, and statistical significance would be observed when P value was below 0.05 in a two-tailed test.
Ethics statement
The current study was based on available SEER database in which data contained unidentifiable patient information and were publicly retrieved. Therefore, the study approval was exempted by the institutional review board review. This article does not contain any studies with human participants performed by any of the authors. All methods were performed in accordance with relevant guidelines and regulations.
Ethics approval and consent to participate
Institutional review board approval was not needed for this study as it utilized publically available data.
Results
Clinicopathologic characteristics of patients
The demographic and clinicopathological features of the total cohort are presented in Table 1, and there was no statistically significant difference between the training and validation sets. Among the eligible patients, the mean age was 62 years (20–91 years). The majority of patients were white (65.1%), married (61.9%) and insured (85.0%) individuals, with a greater percentage of smaller tumor size (≤ 48 mm). The distribution of different tumor locations was not even. 58.2% of patients were located in the distal site (jejunum and ileum), with lower prevalence of duodenum (41.8%). Overall, the most frequent organ of metastasis was the liver (27.1%). For tumor histology, conventional adenocarcinoma (87.9%) was the most common, followed by mucinous adenocarcinoma (6.4%) and signet ring cell carcinoma (5.7%). Besides, the cohorts were also in unequally distribution of T classification: T1/T2 (9.9%), and T3/T4 (90.1%). In both sets, they were far more likely to happen nodal metastasis. Generally, 268 patients performed primary tumor surgery while 105 have not in the whole cohort, of whom 55 underwent primary radical surgery and 213 had primary palliative surgery. As to adjuvant treatment, there were nearly two thirds people receiving chemotherapy (263/373, 70.5%).
Development and construction of the nomogram
As shown in Tables 2 and 3, age at diagnosis (P = 0.000, both), race (P = 0.013, P = 0.018, respectively), primary tumor site (P = 0.004, P = 0.016, respectively), tumor size (P = 0.001, P = 0.000, respectively), metastatic site (P = 0.003, P = 0.004, respectively), T classification (P = 0.007, P = 0.019, respectively), grade (P = 0.001, P = 0.002, respectively), primary tumor surgery (P = 0.000, both), chemotherapy (P = 0.004, P = 0.003, respectively), and radiation (P = 0.016, P = 0.010, respectively) were significantly connected with OS and CSS by univariate analysis for the training cohort. Meanwhile, taking the results of multivariate analysis into account, the following five independent predictive variables were integrated into the prognostic nomogram of 1- and 3-year OS (Fig. 2A) and CSS (Fig. 2B), including age at diagnosis (P = 0.017, P = 0.042, respectively), tumor size (P = 0.005, P = 0.004, respectively), grade (P = 0.002, P = 0.003, respectively), primary tumor surgery (P = 0.002, P = 0.005, respectively), and chemotherapy (P = 0.004, P = 0.002, respectively). Each risk factor was assigned a score on a points scale, and by projecting the total scores to the bottom scale, the probability of 1-, and 3-year OS and CSS can be easily predicted.
Validation of the nomogram
The training set manifested that the C-index values to appraise OS and CSS were 0.715 (95% CI, 0.711–0.719) and 0.711 (95% CI, 0.707–0.715), respectively. Analogously, for the internal validation cohort, the predictive value of OS was 0.687 (95% CI, 0.680–0.694), and the C-index for prediction of CSS was 0.690 (95% CI, 0.683–0.697). The 1- and 3-year AUC values for OS were 0.800 and 0.689, respectively, in the training cohort, and 0.700 and 0.640 in the validation cohort (Fig. S2). Similarly, the 1- and 3-year AUC values for CSS were 0.800, 0.685, 0.700, and 0.638 in the training and validation sets, respectively (Fig. S3). These results exhibited favorable survival predictive ability of nomograms. Then we conducted the calibration of the nomograms with a bootstrap sampling for 1000 times, and the calibration plots in both training and validation cohorts displayed an excellent correlation between the predicted and observed survival probability (Figs. 3 and 4). Specially, the DCA also presented that the developed models in predicting OS and CSS showed a larger net benefit with a wider range of threshold probabilities in the analysis (Figs. S4 and S5). In summary, the nomograms for metastatic SBA showed considerable discriminative and calibrating abilities. Moreover, we divided all 373 patients into low-risk group, and high-risk group according to individual scores, and plotted the Kaplan–Meier curves. As shown in Fig. 5, the median OS of patients among two groups were 18, and 4 months (P < 0.0001), respectively, in the training set, and 16, and 6 months (P < 0.0001), respectively, in the validation set. While for CSS (Fig. 6), compared with lower risk group, patients who presented with higher risk had worse survival outcomes in both cohorts (19 and 4 months in the training set and 16 and 6 months in the validation set; P < 0.0001 and P = 0.0024, respectively), illustrating that there was no apparent difference in utilization of the models between the training and validation groups.
Discussion
In view of the epidemiological facts, the annual incidence of SBA is steadily on the rise although it is a relatively rare tumor. However, it was noteworthy that far fewer studies on its prognosis was reported for patients with distant metastases of SBA in comparison to their counterparts without metastasis, while distant metastasis is extremely essential for treatment selection and survival assessment. Traditional TNM staging system is commonly used for the prognostication of metastatic SBA, but it solely considers the anatomical scope of the disease without taking biofunctional heterogeneity into account, leading to an imprecise evaluation of prognosis, particularly in patients with incurable tumors35. Recent years, clinicians have been continually wrestling with obstacles regarding the way to optimally incorporate established and novel prognostic variables alongside anatomic stage into personalized estimation of clinical events. Accordingly, the nomogram, a graphical presentation of a mathematical model, is developed by combining available baseline clinical and laboratory information for the identification of the possibility of outcomes. It has been reported that nomograms achieve more superior predictive precision and prognostic value than the existing tumor staging system for numerous cancers30,36,37. Hence, it is of great significance to conduct an efficient nomogram model, which will facilitate survival predictions of SBA patients with M1 category and enable the administration of individualized therapies.
In this study, a novel nomogram model was established by incorporating these putative prognostic factors to predict the 1-, and 3-year OS and CSS rates of SBA patients with M1 category. The final parameters incorporated in the predictive model were age, size and grade of primary tumor, primary tumor surgery, and chemotherapy, which are easily available and measurable during diagnosis and treatment. Additionally, the nomogram indicated excellent discrimination and showed superior clinical usability throughout the survival as assessed by DCA.
It is widely known that age is an important variable related with different prognosis in malignancies30,38. As demonstrated in the nomogram, patients older than 76 years would have an increasing risk of death in comparison to younger patients, and this result is consistent with the previous study11. The potential mechanism of the correlation we found might be that some factors associated with age, including lower immune response and higher levels of chronic inflammation, may affect the survival of metastatic patients39,40. Moreover, the current study found that patients with a duodenal primary tumor location suffered worse survival than patients with distal adenocarcinoma in univariate analysis, but not in multivariate analysis. Meanwhile, tumor size and tumor grade were identified as predictors for the OS and CSS of metastatic SBA patients, with survival being worse in patients with lager tumor size or higher tumor grade. It has been reported that this two factors play crucial roles in the prognosis of SBA by several studies3,25. Surprisingly, univariate analysis indicated that a lower T category was associated with a worse prognosis in our study. Generally, tumors with higher T categories mean deeper infiltration depth and they are more likely to experience a poorer outcome. As reported in the literature, the T category served as an independent prognostic factor for SBA, and advanced T category was correlated with significantly inferior survival12,41. The differences exist in sample size and characteristics of study population between studies might be underlying explanations for the disparate findings. For example, compared to T3/T4 category, there were more elderly patients and fewer patients who subjected primary tumor surgeries in T1/T2 category (data not shown), which could lead to the risk of confounding and selection bias. To eliminate any potential confounders, the current study also excluded the confounding effects of these factors by using multivariate analysis. Notably, research in the form of randomized controlled trials with balanced characteristics is desperately required to fill this knowledge gap.
Accumulating evidence revealed that sociomedical support, including marital and insurance status, can impact the mental health and prognosis of patients with tumors23,42. Several retrospective studies have demonstrated that uninsured cancer patients were correlated with a higher risk of all-cause mortality and cancer-specific mortality in Eastern countries43,44. And a SEER-based study conducted by Wang et al. has also shown that patients with SBA with insurance coverage had a more favorable survival compared with medicaid and uninsured patients in the United States24. Analogously, another recent study of 6747 SBA patients has observed that married patients enjoyed a significantly better OS and CSS compared with unmarried patients25. In contrast, we were not able to detect any such association in the present study. This seemingly paradoxical phenomenon might be related to the different study populations. This study was limited to a cohort of SBA patients with M1 category, which is the most aggressive and malignant subtype of SBA with the worst outcome. One possible explanation is that auxiliary sociomedical support has little effect on the prognostic outcome of advanced disease. Thus, the relationship between marital and insurance status and metastatic SBA survival remains to be further explored.
Some clinical research has indicated that the liver is the commonest organ for metastatic spread from SBA45,46, which corresponded to our observations. Furthermore, in line with a prior report10, we confirmed that the metastatic site was not an independent prognostic factor for OS and CSS based on multivariable analysis. Due to the inherent defects of SEER database, we could not evaluate the prognostic value of the size of the metastasized tumor in the setting of metastatic SBA. Consequently, further studies with more representative samples are warranted to add this knowledge in the future.
It is noteworthy that lymph node status is an important prognostic indicator of SBA47. However, the reliability of positive lymph nodes staging scheme has been questioned in recent years owing to the absent consideration of the numbers of negative and total lymph nodes retrieved48. Accumulating evidence has declared that adequate lymph nodes histopathological assessment would translate into more dependable pathologic staging22,49. Subsequently, lymph node ratio (LNR) and log odds of positive lymph nodes (LODDS) were proposed, which have shown a better performance than the numbers of positive lymph nodes regarding the prognosis of patients with SBA50. Zhou et al. compared the impact of positive lymph nodes, LNR, and LODDS on SBA survival from the SEER database and international multicentre hospitals48. They concluded that LODDS scheme showed its prognostic superiority over the LNR or positive lymph nodes schemes for SBA patients, suggesting the auxiliary of the LODDS scheme to lymph node staging systems in the future revisions of AJCC manual48. In the current study, we only evaluated the status of nodal metastasis without the specified N classification because there are too many missing values in the SEER database. The reason for missing values is complex and multifactorial. The first one stems from the fact that the SEER database only started recording information on the number of metastatic lymph nodes in 2010. In addition, this may be related to inadequate lymph node sampling in the earlier studies of SBA. Hence, our predictive models should be further consummated by prospective multicenter studies with detailed lymph node information.
Besides the factors mentioned above, we need to highlight the significant contribution of treatment, which plays an essential role in improving survival outcomes. With regard to the treatment patterns, patients receiving primary tumor surgery combined with chemotherapy or radiation made up a larger proportion in advanced SBA, followed by chemotherapy or radiation alone, and primary tumor surgery alone in the current research. However, Puccini et al. once pointed out that the management of advanced SBA (unresectable or metastatic) is mainly based on systemic treatment, although no randomized studies have been conducted to prove the benefit of systemic chemotherapy in patients with metastatic disease51. Of note, the predicted benefit of surgery on OS and CSS was noticed in SBA patients with M1 category. This is in accordance with the former study21, in which surgical resection of metastatic SBA conferred survival benefit in the whole cohort and majority of the subgroups. Nakanoko et al. reported that surgery, as a palliative treatment, might provide favorable prognosis even in the metastatic or recurrent setting52. Nevertheless, a previous study reported that primary tumor resection was generally not recommended in this metastatic setting except in cases of uncontrolled bleeding, perforation, or acute bowel obstruction51. As a result, it might be indispensable to validate the potential advantage of resection of primary tumor using a prospective study with a large population.
Although benefit of chemotherapy relied on lower level of evidence in our study, postoperative and definitive chemotherapy is still controversial. There is currently no standardized first-line chemotherapy scheme in advanced SBA, as a result of lacking randomized controlled trials comparing the different chemotherapy protocols9. In terms of survival advantage, Aydin et al. recommended chemotherapy in metastatic or locally advanced unresectable SBA9. This conclusion also requires multi-centered and prospective studies involving adequate sample sizes to suggest a therapy modality for advanced SBAs. French intergroup clinical practice guidelines suggested that fluoropyrimidine combination, such as 5-Fluorouracil or capecitabine plus oxaliplatin or cisplatin should be considered53. More recently, a published trial involved by a multi-institutional data registry tested the role of the combination of cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) in SBA patients with peritoneal metastases54. In this study, 152 patients obtained the 5-year survival rate of 30.8% associated to a median OS of 32 months and showed acceptable safety, proving that CRS plus HIPEC could be regarded as a new treatment option for some selected patients with peritoneal metastases from SBA. Still, the detailed information of specific chemotherapy regimen is not captured by this database. This covariate might impact on the prognosis of patients with metastatic SBA and our results could not be adjusted for these variables. More prospective well-defined cohorts are required to refine this.
There are several potential limitations that should be noted when expounding the results of our study. First, considering the retrospective nature of the SEER database, selection bias might be virtually brought in, which may lead to our observations. The second limitation stemmed from the lack of several clinicopathological parameters and treatment variables concerning comorbidities, performance status, LNR, LODDS, and biological data that have been reported as predictive factors for metastatic SBA patients. Moreover, some haematological indexes, such as elevated serum levels of lactate dehydrogenase, CA 19-9 and CEA as well as synchronous metastases were proven to be associated with poor prognosis16,19. Unfortunately, the SEER database did not have specific information about plasma assay and metachronous or synchronous metastasis. These variables could be an essential complement to the existing stage systems and this will be a major part of our future research. Therefore, we felt sorry that we were incapable to effectively evaluate these variables. Furthermore, we were also unable to conduct independent external validation which might strengthen the mathematical basis of predictions. In a word, the findings of the presented study could be more persuasive and instructive if the predictive model was performed by multicenter external validation with a greater amount of clinical sampling, which would efficaciously prove whether our results are more-widely acceptable and applicable in clinical practice.
Conclusions
In summary, for patients with metastatic SBAs, we constructed nomograms to predict the OS and CSS for the first time. The validation process manifested that our models showed good discrimination and calibration, suggesting that it could be beneficial for clinicians to identify personalized treatment and survival in the metastatic population. However, the nomograms can be further optimized by exploiting potentially important factors unavailable in the database and performing external validation by independent, high-quality, and large-quantity cohort.
Availability of data and materials
The data were abstracted from the Surveillance, Epidemiology, and End Results (SEER) database. This is an open database. (https://seer.cancer.gov).
Abbreviations
- SBA:
-
Small bowel adenocarcinoma
- OS:
-
Overall survival
- CSS:
-
Cancer-specific survival
- SEER:
-
Surveillance, Epidemiology, and End Results
- C-index:
-
Concordance index
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the curve
- DCA:
-
Decision curve analysis
- AJCC:
-
American Joint Committee on Cancer
- T:
-
The depth of tumor invasion
- N:
-
Number of metastatic lymph nodes
- M:
-
Distant metastasis
- HRs:
-
Hazard ratios
- CIs:
-
Confidence intervals
- LNR:
-
Lymph node ratio
- LODDS:
-
Log odds of positive lymph nodes
- CA 19-9:
-
Carbohydrate antigen 19-9
- CEA:
-
Carcinoembryonic antigen
- CRS:
-
Cytoreductive surgery
- HIPEC:
-
Hyperthermic intraperitoneal chemotherapy
References
Makino, S. et al. A single institutional analysis of systemic therapy for unresectable or recurrent small bowel adenocarcinoma. Anticancer Res. 37, 1495–1500. https://doi.org/10.21873/anticanres.11476 (2017).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 70, 7–30. https://doi.org/10.3322/caac.21590 (2020).
Raghav, K. & Overman, M. J. Small bowel adenocarcinomas–existing evidence and evolving paradigms. Nat. Rev. Clin. Oncol. 10, 534–544. https://doi.org/10.1038/nrclinonc.2013.132 (2013).
Lu, Y., Frobom, R. & Lagergren, J. Incidence patterns of small bowel cancer in a population-based study in Sweden: Increase in duodenal adenocarcinoma. Cancer Epidemiol. 36, e158-163. https://doi.org/10.1016/j.canep.2012.01.008 (2012).
Bilimoria, K. Y. et al. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann. Surg. 249, 63–71. https://doi.org/10.1097/SLA.0b013e31818e4641 (2009).
Aparicio, T. et al. Small bowel adenocarcinoma: Epidemiology, risk factors, diagnosis and treatment. Dig. Liver Dis. 46, 97–104. https://doi.org/10.1016/j.dld.2013.04.013 (2014).
Chang, H. K. et al. Adenocarcinoma of the small intestine: A multi-institutional study of 197 surgically resected cases. Hum. Pathol. 41, 1087–1096. https://doi.org/10.1016/j.humpath.2010.01.006 (2010).
Barsouk, A., Rawla, P., Barsouk, A. & Thandra, K. C. Epidemiology of Cancers of the Small Intestine: Trends, Risk Factors, and Prevention. Med. Sci. (Basel) 7, 46. https://doi.org/10.3390/medsci7030046 (2019).
Aydin, D. et al. Evaluation of prognostic factors and treatment in advanced small bowel adenocarcinoma: Report of a multi-institutional experience of Anatolian Society of Medical Oncology (ASMO). J. BUON 21, 1242–1249 (2016).
Rompteaux, P. et al. Resection of small bowel adenocarcinoma metastases: Results of the ARCAD-NADEGE cohort study. Eur. J. Surg. Oncol. 45, 331–335. https://doi.org/10.1016/j.ejso.2018.11.012 (2019).
Young, J. I. et al. Treatment and survival of small-bowel adenocarcinoma in the United States: A comparison with colon cancer. Dis. Colon Rectum. 59, 306–315. https://doi.org/10.1097/DCR.0000000000000562 (2016).
Huffman, B. M. et al. Novel prognostic factors in resected small bowel adenocarcinoma. Clin. Colorectal. Cancer 18, 218–225. https://doi.org/10.1016/j.clcc.2019.05.002 (2019).
Wang, N. et al. A convenient clinical nomogram for predicting the cancer-specific survival of individual patients with small-intestine adenocarcinoma. BMC Cancer 20, 505. https://doi.org/10.1186/s12885-020-06971-6 (2020).
Colina, A. et al. Natural history and prognostic factors for localised small bowel adenocarcinoma. ESMO Open 5, e000960. https://doi.org/10.1136/esmoopen-2020-000960 (2020).
Sakae, H. et al. The characteristics and outcomes of small bowel adenocarcinoma: a multicentre retrospective observational study. Br. J. Cancer 117, 1607–1613. https://doi.org/10.1038/bjc.2017.338 (2017).
Zaanan, A. et al. Chemotherapy of advanced small-bowel adenocarcinoma: a multicenter AGEO study. Ann. Oncol. 21, 1786–1793. https://doi.org/10.1093/annonc/mdq038 (2010).
Falcone, R. et al. Impact of tumor site on the prognosis of small bowel adenocarcinoma. Tumori 105, 524–528. https://doi.org/10.1177/0300891619839297 (2019).
Zhang, S. et al. Clinicopathological features, surgical treatments, and survival outcomes of patients with small bowel adenocarcinoma. Medicine (Baltimore) 96, e7713. https://doi.org/10.1097/MD.0000000000007713 (2017).
Aparicio, T. et al. Small bowel adenocarcinoma: Results from a nationwide prospective ARCAD-NADEGE cohort study of 347 patients. Int. J. Cancer 147, 967–977. https://doi.org/10.1002/ijc.32860 (2020).
Zhou, Y. W., Xia, R. L., Chen, Y. Y., Ma, X. L. & Liu, J. Y. Clinical features, treatment, and prognosis of different histological types of primary small bowel adenocarcinoma: A propensity score matching analysis based on the SEER database. Eur. J. Surg. Oncol. 47, 2108–2118. https://doi.org/10.1016/j.ejso.2021.03.260 (2021).
Liu, T., Wu, Y. & Jiang, T. Efficacy of surgery and chemotherapy for stage IV small bowel adenocarcinoma: A population-based analysis using Surveillance, Epidemiology, and End Result Program database. Cancer Med. 9, 6638–6645. https://doi.org/10.1002/cam4.3266 (2020).
Wu, S. et al. A new metastatic lymph node classification-based survival predicting model in patients with small bowel adenocarcinoma: A derivation and validation study. EBioMedicine 32, 134–141. https://doi.org/10.1016/j.ebiom.2018.05.022 (2018).
Arnold, M. et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol 20, 1493–1505. https://doi.org/10.1016/S1470-2045(19)30456-5 (2019).
Wang, N. et al. Insurance status is related to overall survival in patients with small intestine adenocarcinoma: A population-based study. Curr. Probl. Cancer 44, 100505. https://doi.org/10.1016/j.currproblcancer.2019.100505 (2020).
Chen, Z. et al. Influence of marital status on small intestinal adenocarcinoma survival: An analysis of the Surveillance, Epidemiology, and End Results (SEER) database. Cancer Manag. Res. 10, 5667–5676. https://doi.org/10.2147/CMAR.S177430 (2018).
Nicholl, M. B. et al. Small bowel adenocarcinoma: Understaged and undertreated?. Ann. Surg. Oncol. 17, 2728–2732. https://doi.org/10.1245/s10434-010-1109-x (2010).
Diao, J. D. et al. Construction and validation of a nomogram to predict overall survival in patients with inflammatory breast cancer. Cancer Med. 8, 5600–5608. https://doi.org/10.1002/cam4.2470 (2019).
Zhang, R. et al. Development and validation of a preoperative noninvasive predictive model based on circular tumor DNA for lymph node metastasis in resectable non-small cell lung cancer. Transl. Lung Cancer Res. 9, 722–730. https://doi.org/10.21037/tlcr-20-593 (2020).
Pan, Z. et al. Development and validation of a nomogram for predicting cancer-specific survival in patients with Wilms’ tumor. J. Cancer 10, 5299–5305. https://doi.org/10.7150/jca.32741 (2019).
Wu, J. et al. A nomogram for predicting overall survival in patients with low-grade endometrial stromal sarcoma: A population-based analysis. Cancer Commun. (Lond.) 40, 301–312. https://doi.org/10.1002/cac2.12067 (2020).
Surveillance, Epidemiology, and End Results Program Overview. https://seer.cancer.gov/about/factsheets/SEER_Overview.pdf. Accessed 28 Feb 2019.
Benson, A. B. et al. Small bowel adenocarcinoma, Version 1. 2020, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 17, 1109–1133. https://doi.org/10.6004/jnccn.2019.0043 (2019).
Wolbers, M., Koller, M. T., Witteman, J. C. & Steyerberg, E. W. Prognostic models with competing risks: methods and application to coronary risk prediction. Epidemiology 20, 555–561. https://doi.org/10.1097/EDE.0b013e3181a39056 (2009).
Fitzgerald, M., Saville, B. R. & Lewis, R. J. Decision curve analysis. JAMA 313, 409–410. https://doi.org/10.1001/jama.2015.37 (2015).
Mackillop, W. J. & Quirt, C. F. Measuring the accuracy of prognostic judgments in oncology. J. Clin. Epidemiol. 50, 21–29. https://doi.org/10.1016/s0895-4356(96)00316-2 (1997).
Iasonos, A., Schrag, D., Raj, G. V. & Panageas, K. S. How to build and interpret a nomogram for cancer prognosis. J. Clin. Oncol. 26, 1364–1370. https://doi.org/10.1200/JCO.2007.12.9791 (2008).
Imai, K. et al. Nomogram for prediction of prognosis in patients with initially unresectable colorectal liver metastases. Br. J. Surg. 103, 590–599. https://doi.org/10.1002/bjs.10073 (2016).
Wang, C. Y. et al. Nomogram for predicting the survival of gastric adenocarcinoma patients who receive surgery and chemotherapy. BMC Cancer 20, 10. https://doi.org/10.1186/s12885-019-6495-2 (2020).
Hugo, H. J., Saunders, C., Ramsay, R. G. & Thompson, E. W. New insights on COX-2 in chronic inflammation driving breast cancer growth and metastasis. J. Mammary Gland Biol. Neoplasia 20, 109–119. https://doi.org/10.1007/s10911-015-9333-4 (2015).
Yang, J., Li, X., Liu, X. & Liu, Y. The role of tumor-associated macrophages in breast carcinoma invasion and metastasis. Int. J. Clin. Exp. Pathol. 8, 6656–6664 (2015).
Legue, L. M., Simkens, G. A., Creemers, G. M., Lemmens, V. & de Hingh, I. Synchronous peritoneal metastases of small bowel adenocarcinoma: Insights into an underexposed clinical phenomenon. Eur. J. Cancer 87, 84–91. https://doi.org/10.1016/j.ejca.2017.10.012 (2017).
Holt-Lunstad, J. Why social relationships are important for physical health: A systems approach to understanding and modifying risk and protection. Annu. Rev. Psychol. 69, 437–458. https://doi.org/10.1146/annurev-psych-122216-011902 (2018).
Xie, Y. et al. Public health insurance and cancer-specific mortality risk among patients with breast cancer: A prospective cohort study in China. Int. J. Cancer 148, 28–37. https://doi.org/10.1002/ijc.33183 (2021).
Phimha, S. et al. Health insurance and colorectal cancer survival in Khon Kaen, Thailand. Asian Pac. J. Cancer Prev. 20, 1797–1802. https://doi.org/10.31557/APJCP.2019.20.6.1797 (2019).
Dabaja, B. S., Suki, D., Pro, B., Bonnen, M. & Ajani, J. Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer 101, 518–526. https://doi.org/10.1002/cncr.20404 (2004).
Manguso, N. et al. Prognostic factors influencing survival in small bowel neuroendocrine tumor with liver metastases. J. Surg. Oncol. 120, 926–931. https://doi.org/10.1002/jso.25657 (2019).
Tran, T. B. et al. Prognostic relevance of lymph node ratio and total lymph node count for small bowel adenocarcinoma. Surgery 158, 486–493. https://doi.org/10.1016/j.surg.2015.03.048 (2015).
Zhou, Y. Y. et al. Comparison of three lymph node staging schemes for predicting the outcome in patients with small bowel adenocarcinoma: A population-based cohort and international multicentre cohort study. EBioMedicine 41, 276–285. https://doi.org/10.1016/j.ebiom.2019.02.043 (2019).
Ecker, B. L. et al. Lymph node evaluation and survival after curative-intent resection of duodenal adenocarcinoma: A matched cohort study. Eur. J. Cancer 69, 135–141. https://doi.org/10.1016/j.ejca.2016.09.027 (2016).
Scarinci, A. et al. The impact of log odds of positive lymph nodes (LODDS) in colon and rectal cancer patient stratification: A single-center analysis of 323 patients. Updates Surg. 70, 23–31. https://doi.org/10.1007/s13304-018-0519-3 (2018).
Puccini, A., Battaglin, F. & Lenz, H. J. Management of advanced small bowel cancer. Curr. Treat. Options Oncol. 19, 69. https://doi.org/10.1007/s11864-018-0592-3 (2018).
Nakanoko, T. et al. Characteristics and treatment strategies for small bowel adenocarcinoma in advanced-stage cases. Anticancer Res. 35, 4135–4138 (2015).
Locher, C. et al. Small bowel adenocarcinoma: French intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO). Dig. Liver Dis. 50, 15–19. https://doi.org/10.1016/j.dld.2017.09.123 (2018).
Liu, Y. et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy for peritoneal metastases from a small bowel adenocarcinoma: Multi-institutional experience. Ann. Surg. Oncol. 25, 1184–1192. https://doi.org/10.1245/s10434-018-6369-x (2018).
Acknowledgements
This publication is based on the SEER database.
Funding
This study was supported by the National Natural Science Foundation of China (Grant number 81873559, Grant number 81570506).
Author information
Authors and Affiliations
Contributions
H.Z. and S.Z. collected and analyzed the data, wrote the paper; M.J. and K.J. performed quality assessment and analyzed the data. L.M. and F.W. conceived and designed this study. H.Z. and T.Z. contributed with a critical revision of the manuscript. All authors reviewed the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, H., Zhao, S., Zhao, T. et al. Development and validation of prognostic nomograms for patients with metastatic small bowel adenocarcinoma: a retrospective cohort study. Sci Rep 12, 5983 (2022). https://doi.org/10.1038/s41598-022-09986-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-09986-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.