Abstract
SPATA7, an early onset LCA3 retinal disease gene, encodes a putative scaffold protein that is essential for the proper assembly of the connecting cilium (CC) complex in photoreceptors. Previous studies have shown that SPATA7 interacts with other photoreceptor-specific ciliary proteins, such as RPGR and RPGRIP1, and maintains the integrity of CC integrity. However, although it is known that Spata7 is required for early formation of the CC, it is unclear if Spata7 is also required for the maintenance of the CC. To investigate Spata7 function in the retina at the adult stage, loss of function was induced in the adult retina upon tamoxifen induction of an inducible Spata7 knockout allele (Spata7flox/−; UbcCreERT2/+). The phenotype of mutant retina was characterized by a combination of histology, immunobiochemistry, and electroretinography (ERG). Our results demonstrated that Spata7 is also essential for maintaining the integrity of the mature retinal CC. Loss of Spata7 in adults caused phenotypes similar to those seen in germline mutant mice, including photoreceptor cell degeneration and defective ERG responses. Close examination of the CC revealed significantly shortened NPHP1 length as a result of Spata7 deletion. Furthermore, mislocalization of rhodopsin, leading to ER stress-mediated apoptosis, was observed in the retinal layers. Our results indicate that Spata7 is required not only for the establishment but also for the maintenance of the CC of photoreceptors.
Similar content being viewed by others
Introduction
Retinal photoreceptor cells contain a primary cilium known as the connecting cilium (CC)1. The CC is a slender structure that connects the outer (OS) and inner segments (IS) of photoreceptors and mediates a critical protein trafficking function2,3. Mutations in several photoreceptor-specific and common cilia genes can lead to deficient morphogenesis and/or dysfunction of the CC and result in retinal ciliopathies, a group of inherited retinal degenerative diseases including Retinitis pigmentosa (RP)4 and Leber congenital amaurosis (LCA)1.
Proper formation and maintenance of the CC is essential for proper function of photoreceptor cells. Many genes that are important for CC development are also essential for CC maintenance. For example, both early and late loss of function of intraflagellar transport factors KIF3A or IFT88 result in CC defects and photoreceptor degeneration5. In contrast, it has been observed that although NPHP1 is crucial during OS development, once the CC is established, a low level of NPHP1 is sufficient to maintain the proper function of the CC6.
We previously identified SPATA7 (spermatogenesis-associated protein 7) as a disease gene in the LCA3 locus7,8. Mechanistic studies indicate that Spata7 is critical for the integrity of microtubule filaments of the CC and physically interacts with multiple members of the RPGR and NPHP complexes9. Loss-of-function mutations in SPATA7 cause rapid photoreceptor degeneration and lead to LCA in humans8,10,11. Similarly, severe early-onset photoreceptor degeneration is observed in Spata7 germline knockout (Spata7−/−) mouse retinas with 50% of photoreceptors actively undergoing apoptosis by one month of age12,13. Loss of SPATA7 protein disrupts the protein trafficking process, leading to ectopic accumulation of proteins within the inner segment of photoreceptor cells and subsequently triggering photoreceptor apoptosis9,12.
However, it is unclear if SPATA7 is continuously required for CC maintenance or if its requirement diminishes in mature photoreceptors similar to NPHP1. Using a tamoxifen inducible Spata7 conditional knockout mouse model (tamoxifen injected Spata7flox/−; UbcCreERT2/+, or Spata7 iKO) to delete Spata7 in adult mouse retinas, we show that Spata7 is continuously required for the integrity of the CC of photoreceptor cells. Conditional knockout of Spata7 in the adult retina causes rapid retinal degeneration defects. In addition, consistent with observations in germline Spata7−/− mice, photoreceptor cells of tamoxifen-induced Spata7 iKO mice exhibit rhodopsin mislocalization in the IS and outer nucleus layer (ONL) and ER stress activation. Taken together, our results indicate that Spata7 is required for both the initial establishment and continued maintenance of the retinal CC.
Results
UbcCreERT2 successfully deletes Spata7 in adult mouse retinas
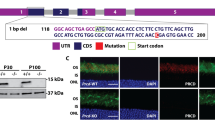
Previous studies have focused on investigating the role of SPATA7 in the developing retina using germline Spata7 knockout mice9,12. To determine the role of SPATA7 in maintaining the photoreceptor CC in the mature mammalian retina, we generated an inducible Spata7 knockout mouse (Spata7flox/−; UbcCreERT2/+) by cross-breeding conditional Spata7 knockout mice (Spata7flox/flox)14 with germline Spata7 knockout mice harboring the UbcCreERT2 transgene15,16 (Spata7−/−; UbcCreERT2/+) (Fig. 1A). Cre expression was induced by injecting tamoxifen at 3 months of age, when mouse retinas have fully matured17. Previous studies have shown that Spata7 localizes to the CC, which connects the IS and the OS12. UbcCreERT2-mediated Spata7 deletion was evaluated by staining for Spata7 protein in the CC of Spata7flox/−; UbcCreERT2/+ mice one month after tamoxifen induction. Immunostaining showed the absence of Spata7 in the CC of tamoxifen-injected Spata7flox/−; UbcCreERT2/+ mice, whereas Spata7 protein was readily detected in control mice, including Spata7flox/−; UbcCreERT2/+ mice without tamoxifen induction and Spata7flox/− mice without the UbcCreERT2 allele (Fig. 1B–D). We performed PCR on genomic DNA extracted from isolated mouse retinas using primers flanking the Spata7 allele14, and no band corresponding to was detected for tamoxifen-injected Spata7flox/−; UbcCreERT2/+ mice compared with control mice (Fig. 1E). These data indicate that the Spata7 conditional allele was completely deleted in adult mouse retinal cells upon tamoxifen induction, making the Spata7flox/−; UbcCreERT2/+ mouse a suitable model for studying Spata7 function in adult retina.
Loss of SPATA7 expression in retinas of Spata7flox/−; UbcCreERT2/+ mice. (A) Schematic of the Spata7 flox allele and breeding scheme for generating Spata7flox/−; UbcCreERT2 mice. Numbered rectangles represent Spata7 exons. (B–D) Anti-SPATA7 staining (pink) of retinal cryosections showing absence of Spata7 protein in 4-month-old Spata7flox/−; UbcCreERT2/+ mice with tamoxifen (injected at 3 months of age) (C) compared to non-injected Spata7flox/−; UbcCreERT2/+ mice (B) and tamoxifen-injected Spata7 flox/− mice (D). Nuclei are stained with DAPI (blue). INL inner nuclear layer, IS inner segment, ONL outer nuclear layer, OS outer segment, CC connecting cilium. (E) PCR amplification of genomic DNA shows complete deletion of the floxed portion of Spata7 following tamoxifen induction in Spata7flox/−; UbcCreERT2 mice. (1) Non-injected Spata7flox/−; UbcCreERT2 and (2) tamoxifen injected Spata7flox/−; UbcCreERT2. Scale bar = 20 µm.
Conditional knockout of Spata7 in adult mouse retina leads to photoreceptor degeneration
Germline loss of SPATA7 leads to photoreceptor degeneration in both humans and mice8,9,12. To determine the effects of Spata7 deletion in adult mice, retinas of tamoxifen-induced Spata7flox/−; UbcCreERT2/+ mice were characterized by functional and morphological studies.
Hematoxylin and eosin (H&E) staining was performed on retinal sections of Spata7flox/−; UbcCreERT2/+ mice three months after induction. H&E results indicated that the induced conditional knockout mice showed approximately 70% reduction in ONL thickness compared to control mice (Fig. 2A). Photoreceptor degeneration across the entire retina was measured by retinal morphometry. Similar to Spata7−/− mice, tamoxifen-injected Spata7flox/−; UbcCreERT2/+ mice also presented with severely reduced ONL thickness (70% in tamoxifen-injected Spata7flox/−; UbcCreERT2/+ mice and 65% in Spata7−/− mice) compared to wildtype and non-injected Spata7flox/−; UbcCreERT2/+ mice (Fig. 2B).
Significant photoreceptor degeneration due to loss of Spata7 in adult mouse retina. (A) Hematoxylin and eosin staining of paraffin-embedded retinal sections shows reduced ONL thickness in 6-month-old Spata7flox/−; UbcCreERT2 mice following tamoxifen induction at 3 months of age. Tamoxifen non-injected Spata7flox/−; UbcCreERT2 mice and 3-month-old wild-type and Spata7−/− mice act as controls. ONL outer nuclear layer, INL inner nuclear layer, GCL ganglion cell layer. (B) Spider plot showing retinal morphometry reveals reduced ONL thickness resulting from loss of Spata7 either in the germline or in adults. The thickness of the ONL was measured at 20 equally spaced positions along the vertical meridian of the retina. Position 0 corresponds to the optic nerve head. Each point represents the mean ± SEM obtained for each group (n ≥ 3 mouse retinas). (C) 6-month-old Spata7flox/−; UbcCreERT2 mice with tamoxifen induction at 3 months of age exhibit reduced ERG scotopic a-wave and b-wave and photopic b-wave amplitudes compared to non-injected controls. Statistical analysis was performed using the Mann–Whitney test (***p < 0.001). N = 10 and N = 8 for Spata7flox/−; UbcCreERT2 mice without or with tamoxifen induction, respectively.
Consistent with the morphological changes described above, electroretinograms (ERGs) performed on tamoxifen-injected Spata7flox/−; UbcCreERT2/+ mice 3 months after induction showed a significant reduction in the amplitudes of both scotopic and photopic waves in tamoxifen-induced Spata7flox/−; UbcCreERT2/+ mice compared to controls (Fig. 2C). These data indicate that the loss of both cone and rod photoreceptors in the induced adult Spata7 knockout mice causes impaired photoresponsiveness. Collectively, these results demonstrate that, similar to what has been observed in germline mutant retinas, loss of Spata7 in adult mouse retinas leads to photoreceptor cell degeneration.
Protein mis-localization and ER-tress activation in Spata7 adult knockout mouse retina
Previous studies have indicated that the integrity of the CC is lost in germline Spata7 mutants, resulting in defective protein trafficking, ectopic accumulation of protein in the IS of photoreceptor cells, and ER stress-mediated apoptosis12. To test if a similar phenotype is observed in adult Spata7 knockout mice, immunofluorescence staining was performed to assess the levels of rhodopsin mis-localization and ER stress activation. Substantial mislocalization of rhodopsin to the IS and ONL was observed in the induced Spata7flox/−; UbcCreERT2/+ mice compared to the control mice (Fig. 3A). Moreover, elevated expression of C/EBP homologous protein (CHOP)18,19, activating transcription factor 6 (ATF6)20,21, and phospho-pancreatic ER kinase (P-PERK)22, markers for ER stress, were detected in Spata7 iKO mouse retinas (Fig. 3B, C), including an increase in CHOP-positive cells (Supplementary Fig. 1). These results show that loss of Spata7 in adult mouse retinas causes a very similar set of phenotypes to those observed in germline Spata7−/− mice.
Rhodopsin mislocalization and ER stress activation in Spata7flox/−; UbcCreERT2 mice. (A) Mislocalized rhodopsin is observed in retinal sections of tamoxifen injected Spata7flox/−; UbcCreERT2 and Spata7−/− mice compared with tamoxifen non-injected Spata7flox/−; UbcCreERT2 retinas. Scale bar = 5 µm. (B) Elevated ATF6, CHOP, and P-PERK signals were detected in the retina of tamoxifen injected Spata7flox/−; UbcCreERT2 mice, while little to no signal was detected in tamoxifen non-injected Spata7flox/−; UbcCreERT2 mouse retina. Scale bar = 20 µm. (C) Quantification of the fluorescence intensities of ATF6, CHOP, and P-PERK from the whole retina indicates increased ER stress activation in tamoxifen injected Spata7flox/−; UbcCreERT2 mice and Spata7−/− mice. Fluorescence intensity is reported relative to the signal of each marker in non-injected Spata7flox/−; UbcCreERT2 retinas. Error bars represent SEM (N = 5 mice per genotype). OS outer segment, IS inner segment, ONL outer nuclear layer, INL inner nuclear layer, GCL ganglion cell layer.
Loss of Spata7 leads to microtubule destabilization in the DCC
To further assess if loss of Spata7 in the adult mouse retina leads to disruption of the CC, we performed immunostaining against acetylated α-tubulin and NPHP1. The retinal CC can be partitioned into two regions: the proximal CC (PCC), which is homologous to the transition zone (TZ) of primary cilia, and the distal CC (DCC), a photoreceptor-specific extension of the ciliary TZ9. Acetylated α-tubulin localizes to the tubulin-based cytoskeletal core along the entire CC9,23. NPHP1, a member of the NPHP–MKS complex24 that normally localizes along the entire CC, only localizes to the PCC in germline Spata7 mutants9,25. As shown in Fig. 4A, consistent with the phenotype of germline mutants, NPHP1 signal was significantly reduced in the DCC in induced Spata7flox/−; UbcCreERT2/+ mice compared to controls (Fig. 4A). Both the germline knockout and adult-specific knockout mouse models showed more than a 60% reduction in NPHP1 signal length (Fig. 4B). Interestingly, significantly shortened acetylated α-tubulin signal was observed in tamoxifen-injected Spata7flox/−; UbcCreERT2/+ retinas (Fig. 4C). These results indicate that adult-specific knockout of Spata7 leads to axoneme microtubule destabilization in the DCC.
Conditional loss of Spata7 in adult mouse retinas leads to collapse of the distal region of the CC. (A) Retinal cryosections of 6-month-old Spata7flox/−; UbcCreERT2 mice with or without tamoxifen induction and 3-month-old wildtype and Spata7−/− mice were costained for NPHP1 (red) and acetylated α-tubulin (green). A single-cilium image of NPHP1 localized to the distal CC is shown on the right. Bars for single-cilium images are 1 µm. (B and C) Histogram displaying NPHP1 (B) and acetylated α-tubulin (C) signal length from 6-month-old Spata7flox/−; UbcCreERT2 mice with or without tamoxifen induction and 3-month-old wild-type and Spata7−/− mice. Statistical analysis was performed using two-way ANOVA analysis (***p < 0.001). N = 5 mice per genotype, 10 CC per animal.
Discussion
In this study, we showed that inducible conditional knockout of Spata7 in adult mouse retinas results in a rapid reduction in ONL thickness and decreased response to light, indicating that Spata7 is essential for maintaining adult mouse retinal integrity and function. Combined with our previous reports, this study indicates that Spata7 is required for both establishment and maintenance of the CC. Our findings suggest that SPATA7 is continuously required during retinal development and post-maturation. This study provides a more detailed understanding of the CC and helps clarify the overlooked roles of SPATA7 in matured retina.
In our previous report, we generated tissue-specfic Spata7 knockout mouse models by crossing Spata7flox/flox mice with photoreceptor-specific Crx-Cre mice or retinal pigment epithelium (RPE)-specific Best1-Cre mice, demonstrating sufficient and pathogenic roles of Spata7 during photoreceptor development14. However, the roles of Spata7 in matured photoreceptor cells remained to be elucidated. We applied the UbcCreERT2/+ mouse to generate the conditional knockout of Spata7 in adult mouse retinas. Our results suggest that rhodopsin mislocalization and ER stress induction likely contributed to the photoceptor degeneration observed in Spata7 iKO mice. These phenomena are consistent with phenotypes observed in Spata7 germline mutant mouse retinas, suggesting that the mechanism of photoceptor degeneration in Spata7 iKO mice involves the disruption of correct protein trafficking and induction of ER stress-mediated photoreceptor apoptosis and is similar to that of germline mutant mice. Additionally, the induction of ER stress was not limited to the photoreceptor layer. Rather, both the inner nuclear layer (INL) and the ganglion cell layer (GCL) also showed elevated ATF, P-PERK and CHOP expression. The elevated expression of ER stress markers indicates that many cell types in the retina were under stress when Spata7 was deleted, likely a secondary effect of photoreceptor degeneration.
Previous studies indicated that SPATA7 might act as a scaffold protein that interacts with other CC proteins, such as RPGRIP1 and NPHP1, members of the RPGR and NPHP complexes9. RPGR and NPHP complexes act as a ciliary gate that controls access of both membrane and soluble proteins to the photoreceptor outer segment26. Protein–protein interactions are thought to play critical functions to maintain the integrity of microtubule filaments of the CC. The absence of SPATA7 leads to mislocalization of DCC proteins and destabilized axonemal microtubules9. Shortened DCC length as inferred from NPHP1 expression is observed in both Spata7−/− and tamoxifen-injected Spata7flox/−; UbcCreERT2/+ mice. Similarly, the lengths of expression of other DCC proteins are predicted to be reduced in both germline knockout mice and adult-specific knockout mice. However/Interestingly, tamoxifen-injected Spata7flox/−; UbcCreERT2/+ mice and Spata7−/− mice showed different microtubule patterns. In contrast to the thin and elongated axonemal microtubules observed in Spata7−/− mice, axonemal microtubules in the adult Spata7 conditional knockout mouse model were almost completely collapsed (Fig. 4A, C). The biological molecular behind this remains unknown and is worth future investigations. These data suggest that SPATA7 is continuously required for the proper structure and function of the CC, and newly synthesized SPATA7 is necessary to replenish the protein pool.
In summary, we propose that Spata7 is also required for maintenance of the retinal CC post-maturation. Loss of Spata7 in mature retinas results in a degeneration phenotype similar to what is observed due to loss of Spata7 during retina development, including disruption of CC integrity and protein trafficking along microtubule tracks and activation of ER stress-mediated cell death. Additionally, given that the loss of Spata7 in the adult retina results in more widespread ER stress in inner retina layers and more severe axonemal microtubule collapse, Spata7 may have a larger number of requirements in mature retinas.
Methods
Mouse strain generation, breeding, and genotyping
This study is reported in accordance with ARRIVE guidelines (https://arriveguidelines.org). All animals were handled in accordance with the policies on the treatment of laboratory animals’ protocol (AN-4175) approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine. Mice were housed on a standard diet and on a 6 am–6 pm light cycle, 20 ± 2° with 50 ± 5% relative humidity. UbcCreERT2/+ mice were purchased from the Jackson Laboratory (Stock No: 007001). Spata7 iKO (Spata7flox/−; UbcCreERT2/+) mice were generated by crossing Spata7flox/flox mice with Spata7−/−; UbcCreERT2/+ mice. Spata7 iKO mice were obtained at the expected frequency (50%), and UbcCreERT2 mice are homozygous embryonic lethal15. The genotyping protocols for Spata7−/− and Spata7 flox/flox alleles were described previously12,14. We used a genomic PCR assay to detect the Spata7 allele using the following primers: P1 (5′-CACATTCATTCCCGATCTTTTTA-3) and P2 (5′-CTGACTAGGGGAGGAGTAGAAGG-3′). Spata7 deletion was assessed by the presence of a 500 bp band after running an agarose gel.
Tamoxifen induction
Tamoxifen (Sigma‐Aldrich, CAS#10540‐29‐1) dissolved in corn oil at a concentration of 25 mg/ml was intraperitoneally injected into adult mice (3-month-old) for 5 consecutive days. Mice were then maintained for another 3 months before retinal assays were conducted.
Immunohistochemistry and immunostaining
Immunofluorescent staining with paraffin sections were conducted as previously described (Reference). Briefly, eyes were enucleated and fixed in modified Davidson’s fixative overnight for paraffin embedding. Eye sections (7 µm) were deparaffinized, and antigen retrieval was performed by boiling sections in 0.01 M Tris, EDTA buffer (pH 9.0) or 10 mM sodium citrate buffer (pH 6.0) for 30 min. Cooled slides were then washed in PBS, incubated for 1 h in hybridization buffer (10% normal goat serum, 0.1% Triton X-100, in PBS), and incubated with primary antibody overnight. Primary antibodies used were anti-SPATA7 (1:100, custom antibody made by Bethyl Laboratories), anti-Rhodopsin (1D4) (1:200, Santa Cruz Biotechnology sc-57432), anti-CHOP (1:200, Santa Cruz Biotechnology sc-575), anti-ATF6 (1:200, Novus Biologicals NBP1-40256), anti-P-PERK (Thr980) (1:100, Invitrogen MA5-15033), anti-NPHP1 (aa 394–687) (1:100, from Greg Pazour lab)27, and anti-acetylated-alpha-tubulin (1:200, Santa Cruz Biotechnology sc-23950). The next day, slides were washed in PBS, incubated with secondary antibody for 2 h, incubated with DAPI at room temperature, then mounted with anti-fade medium (Prolong; Invitrogen) and coverslipped. Fluorescent images were captured with a Zeiss Apotome.2 microscope (Zeiss Axio Imager). Relative fluorescent intensities were measured by ImageJ. All immunostaining experiments were performed independently using five biological replicates.
ERGs
Mice were dark-adapted overnight and anesthetized with Rodent-III (22 mg/kg ketamine, 4.4 mg/kg xylazine, and 0.37 mg/kg acepromazine; intraperitoneal injection). Both pupils were dilated using tropicamide (1.0%) and phenylephrine (2.5%) and corneas were anesthetized with proparacaine (1.0%). After removing excess fluid, a drop of Goniosoft (2.5%) was placed on each cornea to keep the eye moistened and improve contact between the cornea and the ERG electrode (N1530NNC). For the scotopic recordings, luminescence at 10−1, 100,101, 102 scot cd*s/m2 was used to record scotopic ERG a- and b-waves on a UTAS Visual Diagnostic System and EMWIN software (LKC Technologies, Gaithersburg, MD, USA). After scotopic ERG recordings, mice were light-adapted (30 cd*s/m2; white light) for 5 min, and then photopic ERGs were recorded with photopic luminescence of 5, 50, and 500 scot cd*s/m2 as previously described28. A minimum of 10 mice were used for each genotype for ERG analyses. Mann–Whitney tests were performed for statistical analysis (***p < 0.001).
Quantification and data analysis
Statistical analysis of immunostaining was performed using two-way ANOVA analysis (***p < 0.001). N = 5 mice per genotype, 3 staining slides per animal. CHOP + cells were counted in the 200um region adjacent to the optic nerve. Three retinal sections were counted per animal. N = 5 mice per genotype. Error bar is SEM.
Data availability
The datasets generated and/or analyzed during the current study are available in the [Jiaxiong] https://drive.google.com/drive/u/0/folders/12gzP93UuPV6BfDgnBt5FL3TzXwLQ9K_d.
References
Gerth-Kahlert, C. & Koller, S. Ciliopathies. Klin. Monbl. Augenheilkd. 235, 264–272 (2018).
Bachmann-Gagescu, R. & Neuhauss, S. C. The photoreceptor cilium and its diseases. Curr. Opin. Genet. Dev. 56, 22–33 (2019).
Roepman, R. & Wolfrum, U. Protein networks and complexes in photoreceptor cilia. Subcell. Biochem. 43, 209–235 (2007).
Bruninx, R. & Lepièce, G. Retinitis pigmentosa. Rev. Med. Liege 75, 73–74 (2020).
Jiang, L. et al. Heterotrimeric kinesin-2 (KIF3) mediates transition zone and axoneme formation of mouse photoreceptors. J. Biol. Chem. 290, 12765–12778 (2015).
Datta, P., Cribbs, J. T. & Seo, S. Differential requirement of NPHP1 for compartmentalized protein localization during photoreceptor outer segment development and maintenance. PLoS ONE 16, e0246358 (2021).
Kannabiran, C. The spermatogenesis-associated protein-7 (SPATA7) gene—An overview. Ophthalmic Genet. 41, 513–517 (2020).
Wang, H. et al. Mutations in SPATA7 cause Leber congenital amaurosis and juvenile retinitis pigmentosa. Am. J. Hum. Genet. 84, 380–387 (2009).
Dharmat, R. et al. SPATA7 maintains a novel photoreceptor-specific zone in the distal connecting cilium. J. Cell Biol. 217, 2851–2865 (2018).
Perrault, I. et al. Spectrum of SPATA7 mutations in Leber congenital amaurosis and delineation of the associated phenotype. Hum. Mutat. 31, E1241–E1250 (2010).
Mackay, D. S. et al. Screening of SPATA7 in patients with Leber congenital amaurosis and severe childhood-onset retinal dystrophy reveals disease-causing mutations. Investig. Ophthalmol. Vis. Sci. 52, 3032–3038 (2011).
Eblimit, A. et al. Spata7 is a retinal ciliopathy gene critical for correct RPGRIP1 localization and protein trafficking in the retina. Hum. Mol. Genet. 24, 1584–1601 (2015).
Zhong, H. et al. AAV8(Y733F)-mediated gene therapy in a Spata7 knockout mouse model of Leber congenital amaurosis and retinitis pigmentosa. Gene Ther. 22, 619–627 (2015).
Eblimit, A. et al. Conditional loss of Spata7 in photoreceptors causes progressive retinal degeneration in mice. Exp. Eye Res. 166, 120–130 (2018).
Ruzankina, Y. et al. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell 1, 113–126 (2007).
Cox, A. R. et al. Extreme obesity induces massive beta cell expansion in mice through self-renewal and does not alter the beta cell lineage. Diabetologia 59, 1231–1241 (2016).
Völkner, M. et al. Mouse retinal organoid growth and maintenance in longer-term culture. Front. Cell Dev. Biol. 9, 645704 (2021).
Li, Y., Guo, Y., Tang, J., Jiang, J. & Chen, Z. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim. Biophys. Sin. (Shanghai) 46, 629–640 (2014).
Kasetti, R. B. et al. ATF4 leads to glaucoma by promoting protein synthesis and ER client protein load. Nat. Commun. 11, 5594 (2020).
Chan, P., Stolz, J., Kohl, S., Chiang, W.-C. & Lin, J. H. Endoplasmic reticulum stress in human photoreceptor diseases. Brain Res. 1648, 538–541 (2016).
Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 13, 89–102 (2012).
Yang, L.-P., Wu, L.-M., Guo, X.-J. & Tso, M. O. M. Activation of endoplasmic reticulum stress in degenerating photoreceptors of the rd1 mouse. Invest Ophthalmol Vis Sci 48, 5191–5198 (2007).
Arikawa, K. & Williams, D. S. Acetylated alpha-tubulin in the connecting cilium of developing rat photoreceptors. Investig. Ophthalmol. Vis. Sci. 34, 2145–2149 (1993).
Sang, L. et al. Mapping the NPHP-JBTS-MKS protein network reveals ciliopathy disease genes and pathways. Cell 145, 513–528 (2011).
Fliegauf, M. et al. Nephrocystin specifically localizes to the transition zone of renal and respiratory cilia and photoreceptor connecting cilia. J. Am. Soc. Nephrol. 17, 2424–2433 (2006).
Megaw, R. D., Soares, D. C. & Wright, A. F. RPGR: Its role in photoreceptor physiology, human disease, and future therapies. Exp. Eye Res. 138, 32–41 (2015).
Shi, X. et al. Erratum: Super-resolution microscopy reveals that disruption of ciliary transition-zone architecture causes Joubert syndrome. Nat. Cell Biol. 19, 1379 (2017).
Agrawal, S. A. et al. REEP6 deficiency leads to retinal degeneration through disruption of ER homeostasis and protein trafficking. Hum. Mol. Genet. 26, 2667–2677 (2017).
Acknowledgements
This project was funded by the Retina Research Foundation and NIH Grants R01EY022356, R01EY018571, and R01EY022356 (to R. Chen) and R01EY020540 to (G. Mardon and R. Chen).
Author information
Authors and Affiliations
Contributions
J.L., J.B., G.M., and R.C. built the experimental construct. J.L., K.X., and Y.S. performed all the assays. J.L., K.X., G.M., and R.C. conceived the study, interpreted the data, and wrote the manuscript. X.Q. and C.J. processed the data and assisted with their interpretation. All co-authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lu, J., Xiong, K., Qian, X. et al. Spata7 is required for maintenance of the retinal connecting cilium. Sci Rep 12, 5575 (2022). https://doi.org/10.1038/s41598-022-09530-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-09530-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.