Abstract
Terlipressin with albumin, the recommended treatment for hepatorenal syndrome-acute kidney injury (HRS-AKI), is associated with adverse events. Furthermore, the course of AKI in patients with acute-on-chronic liver failure (ACLF) is unknown. We aimed to analyze the safety and efficacy of terlipressin infusion and AKI course in patients with ACLF. We prospectively enrolled consecutive adult patients with ACLF with HRS-AKI (satisfying EASL criteria) treated with terlipressin infusion between 14 October 2019 and 24 July 2020. The objectives were to assess the incidence of adverse events, response to terlipressin, course of HRS-AKI and predictors of mortality. A total of 116 patients were included. Twenty-one percent of patients developed adverse effects. Only 1/3rd of patients who developed adverse events were alive at day 90. Sixty-five percent of the patients responded to terlipressin. Nearly 22% developed recurrence of HRS, and 5.2% progressed to HRS-chronic kidney disease. TFS was 70.4% at day 30 and 57.8% at day 90. On multivariate stepwise Cox regression analysis terlipressin non-response (hazard ratio [HR], 3.49 [1.85–6.57]; P < 0.001) and MELD NA score (HR,1.12 [1.06–1.18]; P < 0.001) predicted mortality at day-90. Patients with ACLF who develop terlipressin related adverse events have dismal prognoses. Terlipressin non-response predicts mortality in patients with ACLF and HRS-AKI.
Similar content being viewed by others
Introduction
Acute kidney injury (AKI) is common in acute-on-chronic liver failure (ACLF). Organ failures, especially AKI, form the diagnostic criteria for ACLF according to the European Association for the Study of the Liver (EASL) definition1. Furthermore, the presence of AKI determines the outcome of patients with ACLF2,3,4. The cause of AKI in ACLF is multi-factorial3,5. Apart from the pre-existing profound systemic inflammation in ACLF, diuretics, sepsis, and cholestasis may impair the renal function by exacerbating hypovolemia, worsening inflammation, macrovascular dysfunction, or promoting bile salt-related direct tubular damage3,6,7.
Vasoconstrictor therapy (specially terlipressin) and volume expansion (with albumin) is the recommended treatment of choice for the hepatorenal syndrome (HRS)-AKI to counteract systemic arterial vasodilation and hypovolemia. However, there are limited studies assessing the role of terlipressin in patients with ACLF and HRS-AKI8,9,10,11.
Terlipressin is associated with adverse events in about 25–40% of patients with ACLF, and approximately 40% of those patients require treatment discontinuation9,11. In addition, ischemic complications and volume overload are common in patients with ACLF and high model for end-stage liver disease (MELD) score12,13. Continuous infusion of terlipressin leads to sustained suppression of portal pressure with a lower total dose than intermittent bolus therapy7. This low dose infusion protocol maintains a high mean arterial pressure (MAP) with a concomitant reduction in adverse events due to terlipressin7,14,15. However, a detailed evaluation of adverse events with continuous infusion and the outcomes and implications of the adverse events have not been studied in a prospective real-world study7,16. Furthermore, the incidence of HRS recurrence and progression to chronic kidney disease (CKD) is unknown in patients with ACLF. Here we aimed to analyze the outcomes and course of HRS-AKI in patients with ACLF treated with continuous terlipressin infusion in a real-world cohort.
Methods
This was a prospective cohort study conducted at the Asian Institute of Gastroenterology hospital, Hyderabad, India, from 14 October 2019 and completed on 24 July 2020. We included consecutive ACLF patients aged 18–75 years treated with terlipressin infusion for HRS-AKI. We recorded the baseline clinical, demographic, and biochemical data. We excluded AKI patients treated with other vasoconstrictors (octreotide/midodrine or noradrenaline), patients with CKD, hepatocellular carcinoma (HCC), and those who refused to participate.
Written informed consent was obtained from each patient, and the study protocol conformed to the ethical guidelines of the Declaration of Helsinki. The institutional human research ethics committee (Institutional Ethics Committee-Asian Institute of Gastroenterology [IEC-AIG]) approved the study vide letter number AIG/IEC34/07.19-16 and was registered at clinical trials registry-India (CTRI/2019/10/021737). All authors had access to the study data and reviewed and approved the final manuscript.
Objectives
The primary objective was to assess the incidence of adverse effects and its predictors. The secondary objectives were to evaluate the response to terlipressin, determine the course of AKI, assess the predictors of terlipressin non-response, and lastly, determine transplant-free survival (TFS) at day 30, 90 in patients with ACLF and HRS-AKI.
Definitions
We included the EASL definition of ACLF17. Patients with HRS only were graded as ACLF I; patients with HRS and one extrarenal organ failure were graded as ACLF II, and patients who had HRS with ≥ 2 organ failures were graded as ACLF III9.
AKI was defined as a rise in serum creatinine (sCr) by 0.3 mg/dl or ≥ 50% rise in sCr, which is presumed or known to have occurred in the previous seven days. Patients satisfying the International Club of Ascites (ICA) criteria of HRS were classified as HRS-AKI18. Responders (reversal of AKI) were classified as either complete or partial responders. Complete response was defined as a reversal in the stage of AKI with a final sCr value of ≤ 0.3 mg/dL of the baseline. Partial response was defined as regression in the stage of AKI with a final sCr > 0.3 mg/dL above the baseline and non-responder if the sCr did not decrease or increased from the baseline18. Patients with glomerular filtration rate (GFR) < 60 ml/min per 1.73 m2 for three months were considered CKD patients. Sepsis was defined as per SEPSIS-3 criteria19.
Management of AKI
Standard therapy was initiated, i.e., withdrawal of diuretics and volume expansion with intravenous 20% albumin infusion at a maximum dose of 1 g/kg for all patients. Terlipressin infusion was initiated at 2 mg/day in the absence of shock and response to volume expansion at 48 h, provided the renal ultrasonography, urinary protein-creatinine ratio, urine examination were normal, as per the ICA diagnostic criteria of HRS. The clinical and biochemical data after 48 h of volume expansion (at the time of initiation of terlipressin therapy) was considered baseline data and enrolled for the study. The dose of terlipressin was doubled every 48-h in case the sCr did not decrease by 25%18. Terlipressin 2 mg (10 ml = 1 mg) was diluted in 30 ml of normal saline and infused over 24 h at a rate of 2.1 ml/h. Electrocardiogram and echocardiography were done for all patients before initiating terlipressin. Daily clinical evaluation of the enrolled patients was done by two independent investigators (STR and AVK). Patients were followed up for 90 days to observe the recurrence of HRS and outcome (transplant-free survival).
Withdrawal of terlipressin therapy was decided based on the response to treatment and the development of adverse events. Standard care of management as per institution protocol was provided to all patients. For mild adverse events, the drug dose was reduced and monitored. Severe adverse effects requiring the withdrawal of terlipressin therapy were defined as previously suggested by Cavallin et al.15. The second line of therapy included either octreotide with midodrine or noradrenaline. The timing and modality of renal replacement therapy (RRT) was planned as per the multidisciplinary team’s (involving hepatologists, nephrologists, and intensivists) decision on a case-to-case basis.
Statistical analysis
The data is analyzed using SPSS version 25.0 (IBM Corp, NY, USA). Descriptive statistics will be expressed as mean (standard deviation [SD]) or median (interquartile range [IQR]) for parametric or non-parametric continuous data, respectively, and number (%) for categorical data. We used the student’s t-test for comparison of means between the two groups. The categorical data are compared using Pearson’s Chi-square test (or Fisher’s exact test when required). The predictors of adverse events and terlipressin non-response are derived using stepwise multivariate logistic regression analysis involving parameters that have P < 0.1 on univariate logistic regression analysis and is expressed as odds ratio (OR). The predictors of mortality are derived using stepwise multivariate Cox regression analysis involving parameters that have P < 0.1 on univariate analysis and is expressed as hazard ratio (HR). Further, to find the different cut-off points for terlipressin non-response and mortality, receiver operating characteristic (ROC) curve analysis was also carried out. All statistical tests with P < 0.05 were considered significant.
Conference presentation
The abstract was presented as a poster at IDDF 2020, Hong Kong.
Results
During the study period, 141 patients with ACLF were diagnosed with HRS-AKI. Of them, twenty-five patients were excluded (reasons: received midodrine and octreotide-9; CKD-8; HCC-4, refusal to participate-4). Thus, a total of 116 patients with ACLF received terlipressin therapy for HRS-AKI. The mean age in the cohort was 48.31 ± 9.01 years. Ninety-four percent of patients were males. Alcohol was the most common cause of ACLF. The mean MELD-sodium (MELD NA) was 31.37 ± 7.36, and baseline sCr was 3.07 ± 1.36 mg/dl. Baseline characteristics of the included patients are shown in Table 1.
The most common source of sepsis was urinary tract infection, bloodstream infection (BSI), and spontaneous bacterial peritonitis (Supplementary Table 1). Culture was positive in 68% of patients. The most common organisms isolated were Escherichia coli and Klebsiella pneumonia (Supplementary Table 2).
Drug therapy
The mean dose of albumin infused (per day) for the first 2 days was 44.48 ± 17.56 g/day. The mean dose of terlipressin was 2.75 ± 0.93 mg/day for a mean duration of 5.28 ± 3.51 days.
Primary endpoint: incidence of adverse events
A total of 20.7% (95% CI, 13.76–29.2) developed adverse effects to terlipressin (Supplementary Fig. 1). Twelve percent of patients had to discontinue terlipressin due to adverse events. (Table 2) Diarrhea and abdominal pain were the most common adverse events. Of the 24 patients who developed adverse effects, 54.2% expired, 12.5% underwent liver transplantation, and 33.34% were alive at day 90 in the whole cohort (P = 0.03). (Supplementary Table 3) The incidence of mortality at day 90 in patients who developed ischemic adverse events was 91.7% (11/12). MELD NA score was higher in patients who developed adverse events (MELD NA adverse events group-34.46 ± 5.49 vs. no adverse events-30.57 ± 7.59; P = 0.02). Presence of sepsis at baseline (OR, 4.2 [1.41–12.4]; P = 0.01) and baseline serum bilirubin (OR, 1.07 [1.02–1.12]; P = 0.002) were predictors of adverse events to terlipressin on multivariate stepwise logistic regression analysis. (Table 3).
Secondary endpoints
Efficacy of terlipressin in patients with ACLF and HRS-AKI and the course of AKI
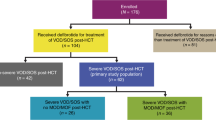
Sixty-five percent (95%CI, 55.23–73.3) of the patients responded to terlipressin. Complete response was noted in 39.7% (46/116) and partial response in 25% (29/116). The mean time to reversal of AKI was 4.8 ± 2.64 days. Terlipressin increased the mean arterial pressure (MAP) on day 3 by 5.23 ± 3.13 mmHg and urine output by 193.41 ± 145.65 ml/day. Twenty-six percent of patients (30/116) required RRT. All the patients requiring RRT succumbed. Nearly 22% developed recurrence of HRS, and 5.2% progressed to HRS-CKD (Fig. 1: flow of patients in the whole cohort).
The course of HRS-AKI in the whole cohort. Of the 116 patients, 75 were responders, and 41 were non-responders. In the responder group, 46 patients achieved a complete response, and 29 patients achieved a partial response. Of the 41 non-responders, 29 required renal replacement therapy, and those requiring RRT succumbed. Nine patients of the non-responders were treated with octreotide and midodrine, and the rest were treated with noradrenaline. HRS-AKI hepatorenal syndrome acute kidney injury, CR complete response, PR partial response, LT liver transplantation, TFS transplant free survival, TIPS transjugular intrahepatic portosystemic shunt, OCT/MID octreotide with midodrine, CKD chronic kidney disease.
Predictors of terlipressin non-response
On multivariate stepwise logistic regression analysis, baseline sCr (OR-2.24 [1.41–3.57]; P = 0.001), ACLF grade (Grade II-OR, 4.98 [1.5–16.5]; P = 0.009; Grade III-OR, 7.61 [1.91–30.16]; P = 0.004) and change in MAP at day 3 (OR, 0.73 [0.57–0.92]; P = 0.009) were predictors of terlipressin non-response (Table 4). Baseline sCr > 3.02 predicted terlipressin non-response with a sensitivity of 75.6%, specificity of 84% with an AUROC of 82.2 (74.1–90.4; P < 0.001) (Supplementary Fig. 2A). A change in MAP by 4.5 mmHg at day 3 predicted terlipressin response with a sensitivity of 62.7%, specificity of 67.6%, and an AUROC of 71.2 (61.5–80.9; P < 0.001) (Supplementary Fig. 2B).
Transplant-free survival at day 30, 90
TFS in the whole cohort was 70.4% (81/116) at day 30 and 57.8% (67/116) at day 90. Four patients underwent transjugular intrahepatic portosystemic shunt (TIPS) at day 90 for refractory ascites.
Predictors of mortality
On multivariate stepwise Cox regression analysis terlipressin non-response (HR, 3.49 [1.85–6.57]; P < 0.001) and MELD NA score (HR, 1.12 [1.06–1.18]; P < 0.001) predicted mortality at day-90 in the whole cohort (Table 5). Ischemic adverse events to terlipressin predicted mortality on univariate analysis but not on multivariate analysis.
Discussion
Terlipressin with albumin is an effective treatment for patients with ACLF and HRS-AKI9,11. This study has demonstrated that: (a) the overall incidence of adverse events due to terlipressin was 21% in patients with ACLF; (b) sepsis and baseline bilirubin levels predict the adverse events in patients with ACLF; (c) only 1/3rd of patients who developed adverse events were alive (without transplant) at day 90; (d) 65% of patients with ACLF responded to terlipressin; (e) the risk of recurrence of HRS was 21.5%; (f) TFS was 57.8% at day 90; (g) terlipressin non-response predicted mortality in patients with ACLF and HRS-AKI; (h) none of the patients treated with RRT survived.
The main strength of our study is the prospective collection of data on ACLF patients with HRS-AKI. Adverse events to terlipressin have been reported in earlier studies20,21. However, none of the studies have reported the implications and outcomes associated with it16. Our data is the first prospective study to report the outcomes related to adverse events due to terlipressin. MELD score was higher in patients who developed adverse events, and high MELD is known to be associated with ischemic adverse effects and, thereby, mortality12. Patients with adverse effects had poorer TFS. A small trial has reported fewer ischemic complications with terlipressin, even in the presence of sepsis8. But the study included only 18 patients with sepsis and HRS-AKI, and there was no comparator arm. Though sepsis was associated with higher adverse events, the response to terlipressin was unaltered by the presence of sepsis akin to the previous studies8,11. Hyperbilirubinemia is well known to be associated with poor prognosis and terlipressin response in patients with ACLF22,23. We noted hyperbilirubinemia to be predictive of adverse events, probably indicating the effect of cholemic injury in patients with ACLF6.
ACLF patients identified by EASL criteria might be diagnosed earlier and aid in prioritization for liver transplant/TIPS24. Terlipressin therapy is associated with a mortality benefit11. The adverse events of terlipressin are of great concern. Terlipressin increases the afterload and end-diastolic volume with a concurrent reduction in cardiac index7,25. This may unmask the pre-existing cardiac dysfunction in patients with cirrhosis and lead to volume overload and pulmonary edema, particularly if large amounts of albumin are administered concomitantly. The CONFIRM study recently highlighted the risk of pulmonary oedema in patients treated with terlipressin and albumin13. In contrast, we did not note any pulmonary overload due to a significantly lower dose of terlipressin and albumin than the dose used in the CONFIRM trial. Furthermore, continuous infusion of low-dose terlipressin may have prevented cardiac dysfunction in our patients.
Interestingly in our study, none of the ACLF patients treated with RRT survived at day 90. Previous studies have also reported that patients with cirrhosis requiring RRT have more than 85% mortality26,27. Similarly, patients with ACLF requiring RRT have high mortality. Furthermore, most of the patients included in our study had sepsis at baseline, which may have led to poor outcomes in these patients3.
Randomized trials with low-dose terlipressin or alternate day terlipressin therapy may be utilized to reduce the adverse effects of terlipressin in patients with ACLF. Endothelin-1/Nitric oxide ratio aid in predicting response to terlipressin therapy28. Serum endothelin levels, nitric oxide, lactate dehydrogenase levels may be used to monitor and predict the ischemic adverse events of terlipressin. Another unexplored area is a combination therapy of vasoconstrictors. Low-dose terlipressin, in addition to midodrine or noradrenaline, may be explored to assess the safety and efficacy in patients with ACLF and HRS-AKI.
There are certain limitations to our study. Most patients included were males with alcohol-related liver disease (ARLD). Whether males with ARLD are more prone to adverse outcomes of terlipressin in ACLF is unknown. Previous studies have also noted a higher number of male patients with ARLD developing adverse effects12. The use of intermittent bolus therapy was not assessed in this real-world cohort, and the results of infusion-based vs. bolus-based protocols in different study settings may skew results regarding adverse effects. Future data should also evaluate the role of cirrhotic cardiomyopathy as a confounder as this may also alter outcomes and renal response in HRS-AKI29.
In conclusion, our novel study described the course and outcomes of patients with ACLF and HRS-AKI. The outlying of adverse events and their effect on outcomes opens an avenue for future randomized trials, including patient selection, dosing, and alternatives.
Data availability
The datasets used during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACLF:
-
Acute-on-chronic liver failure
- AKI:
-
Acute kidney injury
- AARC:
-
APASL ACLF research consortium
- CLIF-C:
-
Chronic Liver Failure Consortium acute-on-chronic liver failure score
- EASL:
-
European Association for the Study of the Liver
- HR:
-
Hazard ratio
- HRS:
-
Hepatorenal syndrome
- IAC:
-
International ascites club
- OR:
-
Odds ratio
- sCr:
-
Serum creatinine
- TFS:
-
Transplant-free survival
References
EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 69(2), 406–460 (2018).
Khatua, C. R. et al. Acute kidney injury at admission is a better predictor of mortality than its persistence at 48 h in patients with acute-on-chronic liver failure. J. Clin. Transl. Hepatol. 6(4), 396–401 (2018).
Maiwall, R., Sarin, S. K. & Moreau, R. Acute kidney injury in acute on chronic liver failure. Hepatol. Int. 10(2), 245–257 (2016).
Maiwall, R. et al. Co-orchestration of acute kidney injury and non-kidney organ failures in critically ill patients with cirrhosis. Liver Int. 41(6), 1358–1369 (2021).
Davenport, A. et al. Acute kidney injury in acute-on-chronic liver failure: Where does hepatorenal syndrome fit?. Kidney Int. 92(5), 1058–1070 (2017).
Angeli, P., Garcia-Tsao, G., Nadim, M. K. & Parikh, C. R. News in pathophysiology, definition and classification of hepatorenal syndrome: A step beyond the International Club of Ascites (ICA) consensus document. J. Hepatol. 71(4), 811–822 (2019).
Kulkarni, A. V. et al. Terlipressin has stood the test of time: Clinical overview in 2020 and future perspectives. Liver Int. 40(12), 2888–2905 (2020).
Rodríguez, E. et al. Terlipressin and albumin for type-1 hepatorenal syndrome associated with sepsis. J. Hepatol. 60(5), 955–961 (2014).
Piano, S. et al. Association between grade of acute on chronic liver failure and response to terlipressin and albumin in patients with hepatorenal syndrome. Clin. Gastroenterol. Hepatol. 16(11), 1792–800.e3 (2018).
Jiang, Q. Q. et al. Acute kidney injury in acute-on-chronic liver failure is different from in decompensated cirrhosis. World J. Gastroenterol. 24(21), 2300–2310 (2018).
Arora, V. et al. Terlipressin Is superior to noradrenaline in the management of acute kidney injury in acute on chronic liver failure. Hepatology 71(2), 600–610 (2020).
Kulkarni, A. V., Kumar, P., Rao, N. P. & Reddy, N. Terlipressin-induced ischaemic skin necrosis. BMJ Case Rep. 13, 1 (2020).
Wong, F. et al. Terlipressin plus albumin for the treatment of type 1 hepatorenal syndrome. N. Engl. J. Med. 384(9), 818–828 (2021).
Gerbes, A. L., Huber, E. & Gülberg, V. Terlipressin for hepatorenal syndrome: Continuous infusion as an alternative to iv bolus administration. Gastroenterology 137(3), 1179 (2009) ((author reply-81)).
Cavallin, M. et al. Terlipressin given by continuous intravenous infusion versus intravenous boluses in the treatment of hepatorenal syndrome: A randomized controlled study. Hepatology 63(3), 983–992 (2016).
Satsangi, S. Noradrenaline for hepatorenal syndrome in patients with acute on chronic liver failure: Hope remains!. Hepatology 68(6), 2443–2444 (2018).
Moreau, R. et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 144(7), 1426-37.e1–9 (2013).
Angeli, P. et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: Revised consensus recommendations of the International Club of Ascites. J. Hepatol. 62(4), 968–974 (2015).
Singer, M. et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315(8), 801–810 (2016).
Moreau, R. et al. Terlipressin in patients with cirrhosis and type 1 hepatorenal syndrome: A retrospective multicenter study. Gastroenterology 122(4), 923–930 (2002).
Sanyal, A. J. et al. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology 134(5), 1360–1368 (2008).
López-Velázquez, J. A. et al. Bilirubin alone as a biomarker for short-term mortality in acute-on-chronic liver failure: An important prognostic indicator. Ann Hepatol. 13(1), 98–104 (2013).
Nazar, A. et al. Predictors of response to therapy with terlipressin and albumin in patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology 51(1), 219–226 (2010).
Trebicka, J., Gu, W., Ibáñez-Samaniego, L., Hernández-Gea, V. et al. Rebleeding and mortality risk are increased by ACLF but reduced by pre-emptive TIPS. J. Hepatol. (2020).
Krag, A. et al. Effects of a single terlipressin administration on cardiac function and perfusion in cirrhosis. Eur. J. Gastroenterol. Hepatol. 22(9), 1085–1092 (2010).
Allegretti, A. S. et al. Prognosis of patients with cirrhosis and AKI who initiate RRT. Clin. J. Am. Soc. Nephrol. 13(1), 16–25 (2018).
Wood, R. P., Ellis, D. & Starzl, T. E. The reversal of the hepatorenal syndrome in four pediatric patients following successful orthotopic liver transplantation. Ann. Surg. 205(4), 415–419 (1987).
Abdel-Razik, A. et al. Endothelin-1/nitric oxide ratio as a predictive factor of response to therapy with terlipressin and albumin in patients with type-1 hepatorenal syndrome. Front. Pharmacol. 11, 9 (2020).
Kaur, H., & Premkumar, M. Diagnosis and management of cirrhotic cardiomyopathy. J. Clin. Exp. Hepatol.
Author information
Authors and Affiliations
Contributions
Study concept and design by A.V.K. and P.N.R.; Data collection by S.T.R. and M.S.; Statistical analysis by A.V.K. and K.K.; compilation and initial drafting by A.V.K., K.K., and H.T. Figures by A.V.K. and M.S. Final editing and critical revision by A.V.K., M.P., P.N.R., and D.N.R. All members approved the final draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kulkarni, A.V., Ravikumar, S.T., Tevethia, H. et al. Safety and efficacy of terlipressin in acute-on-chronic liver failure with hepatorenal syndrome-acute kidney injury (HRS-AKI): a prospective cohort study. Sci Rep 12, 5503 (2022). https://doi.org/10.1038/s41598-022-09505-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-09505-1
This article is cited by
-
Pharmacological Profile of FDA-Approved Orphan Drugs in the Year 2022
Current Pharmacology Reports (2024)
-
The “dark” side of terlipressin: a case report of ischemic skin necrosis and review of literature
Egyptian Liver Journal (2023)
-
Human albumin infusion is safe and effective even in patients without acute kidney injury and spontaneous bacterial peritonitis
Indian Journal of Gastroenterology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.