Abstract
Some studies suggested the effects of inflammatory cytokines in reducing muscle mass and muscle strength and, performance. This study aimed to compare pro-inflammatory cytokines in sarcopenic and non-sarcopenic subjects. 120 men and women were selected out from the cross-sectional study ‘sarcopenia and its determinants among Iranian elders’ (SARIR). Sarcopenia was defined based on the first ‘European Working Group on sarcopenia in older people’ (EWGSOP1) guidelines. A fasting blood sample was taken from each participant to measure serum high-sensitivity C-reactive protein (hs-CRP), Interleukin 6 (IL-6), and tumor necrosis factor α (TNFα). A total of 120 participants were included in this study. Mean age was 66.7 ± 7.7 years and mean body mass index (BMI) was 27.3 ± 4.2 kg/m2. Forty participants had the criteria of EWGSOP1 sarcopenia. A statistically significant difference was seen between normal and abnormal groups of muscle strength in hs-CRP (P-value = 0.04). Furthermore, we did not observe any remarkable association between inflammatory biomarkers including IL-6 (OR 1.15; 95% CI 0.31–4.28), TNF-α (OR 0.68; 95% CI 0.17–2.77), and hs-CRP (OR 2.39; 95% CI 0.87–6.55) and the presence of sarcopenia even after controlling for plausible confounders. We found that inflammatory biomarkers level was not associated with odds of sarcopenia. The lack of correlation between inflammatory cytokines and sarcopenia could be due to the participants’ age and genetics. Future studies are required to confirm these findings.
Similar content being viewed by others
Introduction
The proportion of elderly is rapidly increasing in World’s population1. Old age is associated with reduced physical function with negative impacts on quality of life and activities of daily living2. Sarcopenia is characterized by skeletal muscle loss, low muscle strength and/or physical performance and is one of the critical issues in geriatric medicine3. Based on the definition provided by the European Working Group on sarcopenia (EWGSOP1), sarcopenia is the presence of both low muscle mass and low muscle function (including strength or performance)4. Besides being common among elderly people, sarcopenia can also occur in early life as a consequence of other inflammatory conditions and systemic diseases which is called secondary sarcopenia, due to the involvement of causal factors other than (or in addition to) aging5. Other factors that potentially may play a role in the development of sarcopenia are poor nutrition and physical inactivity5. Sarcopenia can result in disabilities and an increased risk of frailty, falls, fractures, and other consequences that burden society and health care services6,7,8.
Some physiological changes related to aging, including oxidative stress, abnormal anabolic hormone production, neuromuscular atrophy, and inflammatory factors, are associated with decreased muscle mass and strength9. High levels of inflammatory factors have been associated with an increased odds of disability10, 11 and mortality12 in older adults. On the other hand, increased plasma levels of pro-inflammatory cytokines and acute-phase proteins are often observed in older sarcopenic populations13. There are various hypotheses proposed to examine the effect of inflammation on physical performance and muscle strength2. Previous studies have suggested that inflammatory mediators could induce muscle wasting by affecting muscle protein metabolism14, 15. In line with this, other studies have shown that increasing levels of inflammatory markers such as IL-6, TNF-a, and CRP in older adults are associated with increased muscle loss and progression of sarcopenia16,17,18. Schaap et al. in the ‘Longitudinal Aging Study Amsterdam’ indicated that higher levels of serum IL-6 and CRP were associated with an increased risk of muscle strength loss during 3 years of follow-up17. Moreover, Taaffe et al. in a cross-sectional and prospective study, indicated a reverse relationship between CRP level and handgrip strength, though high baseline concentrations did not predict a 7-year later decrease in performance and grip strength19. Tuttle et al. showed in a recent systematic review and meta-analysis that higher levels of inflammatory markers including IL-6, TNF-a, and CRP were associated with decreased skeletal muscle mass and muscle strength20. Most previous studies on the relation of inflammatory markers and sarcopenia have been conducted in Europe and the USA. There have been only few studies in Asian populations21,22,23. On the other hand, prevalence of sarcopenia among Asia population24 is higher than population in western coutries25 and number of elderly population in Iran is growing. In this cross-sectional study, we aim to compare inflammatory factors levels, including IL6, CRP, and TNFα, between sarcopenia and non-sarcopenia in Iranian adults. In addition we evaluated the relationship between inflammatory factors and sarcopenia components including muscle performance, strength, and muscle mass in the Iranian elder population.
Materials and methods
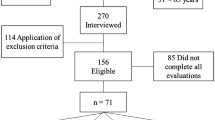
Phase one of this population-based cross-sectional study was carried out between May and October 2011 in Tehran, Iran. Three hundred men and women were invited randomly from the 6th district of Tehran. The details of the sampling method and data collection procedure of the SARIR study were reported previously26. After assessment of muscle mass, strength and performance, 54 participants were identified as sarcopenic.
In phase two, 40 sarcopenic and 80 non-sarcopenic individuals were randomly selected out—due to the limited budget—based on participant’s codes. Then inflammatory markers were measured for them.
This study was performed according to the Declaration of Helsinki. The study protocol was accepted by the TUMS (Tehran University of Medical Sciences) ethics committee. All subjects confirmed the written informed consent before being included in the study.
Assessment of muscle mass
DXA scanner (Discovery W S/N 84430), which can measure fat, muscle, and bone mass of limbs, was used to determine body composition for each person. According to DXA results, we calculated the appendicular skeletal muscle for each participant as the sum of upper and lower limb muscle mass (in kg)27.
We calculated the skeletal muscle mass index by dividing the sum of muscles’ limbs to height square28. Appendicular skeletal muscle index less than 7.26 kg/m2 for men and less than 5.45 kg/m2 for women were considered abnormal4.
Assessment of muscle strength
Handgrip test was done using a pneumatic instrument—a squeeze bulb dynamometer (manufactured by Jamar, Inc. USA: c7489-02 Rolyan) calibrated in pounds per square inch (psi)—to measure the muscle strength. Participants were requested to sit in a straight-backed chair, with their shoulders adducted in a neutral position, arms unsupported, and elbows flexed at 90°. The measurement of maximum voluntary contraction (handgrip strength) was done three times with 30 s rest in between measurements for each hand. The average measurements of the subjects’ hands were defined as muscle strength. To identify low muscle strength for each participant, age and sex-specific thresholds suggested by Merkies et al. were used29.
Assessment of muscle performance
We asked each individual to walk at his/her usual pace to measure the muscle performance using a 4-m walk gait speed test. Subjects with gait speeds lower than 0.8 m/s were identified as having abnormal muscle performance.
Sarcopenia determination
The current study used European Working Group on Sarcopenia in Older People (EWGSOP1) guidelines for sarcopenia definition4.
A sarcopenic person is an individual with abnormal hand grip strength or abnormal gait speed test in addition to abnormal muscle mass.
Assessment of biomarkers
Blood samples were taken after 12-h fasting. Serum samples were kept at − 80 °C until performing the ELISA test in the Endocrinology and Metabolism Research Center of Tehran University in specialized laboratories. The ELISA method was used to measure serum IL-6 and TNF-α levels using commercial kits (I.D. labs Canadian company), and the hs-CRP test was performed using Pars Azmoon kit. All the assessments were done in duplicate for all inflammatory cytokine measures. More details were reported previously26.
Assessment of other variables
A short form of the International Physical Activity Questionnaire (IPAQ) was used to assess the physical activity level based on metabolic equivalent hours per week (MET-h/week). A digital scale was used for the body weight of the participants only wearing minimal clothing. Height was assessed using a wall tape measure with the participants in a standing position without shoes. Body mass index (BMI) was computed by dividing weight (kg) by height squared (m2).
Statistical analysis
Normally distributed variables were described using mean and standard deviation. The general characteristics of the study individuals were compared using one-way analysis of variance and chi-squared tests. The distribution of inflammatory markers was examined using the Kolmogorov–Smirnov test. Since plasma levels of inflammatory markers were not normally distributed, the analyses were performed using log-transformed values. T-test and Mann Whitney test were used to compare the hs-CRP, IL6, and TNFα levels between normal and abnormal muscle mass, handgrip strength, and gait speed. Multivariate logistic regression was applied to find the relationship between inflammatory cytokines and the presence of sarcopenia. In the first statistical model, we adjusted for age (years) and sex (male/female). Further controlling was performed for physical activity smoking (yes/no), (MET-h/week), alcohol consumption (yes/no), medication use (yes/no), and history of the disease (yes/no) in the second model. hs-CRP, IL-6, and TNF-α were categorized based on detection limits (5 mg/l for hs-CRP, 10 pg/ml for IL-6, and 8 pg/ml for TNF-α), which levels above the detection limits identified as an abnormal. Our statistical analyses were performed using SPSS software Version 16 (SPSS Inc., Chicago, IL, USA). P values less than 0.05 were considered statistically significant.
Research ethics and patient consent
The study protocol was approved by the Tehran University of Medical Sciences ethics committee. We explained the study’s aims to the participants at first and then requested all participants to complete a written informed consent before data collection.
Results
A total of 120 participants were included in this study. General characteristics of participants are presented in Table 1. About 50% of the participants in both the sarcopenia group and the non-sarcopenia group were female. Only 28% of the sarcopenia group had a medical history (including diabetes, myocardial infarction, cerebrovascular accident, asthma and arthritis) versus 40% in the non-sarcopenia group. Regarding taking medication, 5% of sarcopenia and 2.5% of the non-sarcopenia group were using sexual hormones, and 2.5% of both groups were taking corticosteroids. In the sarcopenia group, handgrip strength was significantly lower than the control group.
Comparison of inflammatory cytokines between normal and abnormal components of sarcopenia, including handgrip strength, muscle mass, and gait speed test, is shown in Table 2. These results showed a statistically significant difference in serum hs-CRP between normal and abnormal handgrip strength groups (P-value = 0.04). There was no statistically significant difference in other groups. Logistic regression analysis of the relationship between inflammatory biomarkers and presence of sarcopenia is represented in Table 3. In the crude model, there was no association between hs-CRP levels and risk of sarcopenia (OR 1.02; 95% CI 0.32, 3.24). In addition, after adjustment for plausible confounders, this association remained non-significant (OR 1.15; 95% CI 0.31, 4.28). Moreover, we did not observe any association between IL-6 and TNF-α and presence of sarcopenia neither before (OR 0.90; 95% CI 0.25, 3.13, OR 1.60; 95% CI 0.73, 3.48) nor after controlling for potential confounders (OR 0.68; 95% CI 0.17, 2.77, OR 2.39; 95% CI 0.87, 6.55).
Discussion
In the current study, we investigated the association between the presence of sarcopenia and inflammatory markers including TNF-a, IL-6, and hs-CRP. No association was found. In addition to this, we investigated the levels of TNF-a and IL-6 between normal and abnormal groups of muscle mass and muscle performance and found no significant interaction. However, a significant difference in hs-CRP level between normal and abnormal muscle strength groups was observed. Several previous studies showed that inflammation plays a role in the developing of sarcopenia16, 17, 20. A longitudinal aging study in Amsterdam revealed a positive association between higher levels of IL-6 and CRP and elevated risk of muscle strength loss but not muscle mass17. Moreover, in another prospective cohort study, the researchers found that increased levels of inflammatory markers were significantly correlated with a more 5-year decrease in the thigh muscle area16. Higher TNF-α levels and its soluble receptors investigated the most consistent relationship with lower grip strength and muscle mass16. In support of these findings, a systematic review and meta-analysis that evaluated the association of markers of inflammation with muscle strength and muscle mass found a significant relationship between increased levels of circulating TNF-α, CRP, and IL-6 with a decline in muscle mass, handgrip and knee extension strength20. On the other hand, consistent with our findings, a systematic review and meta-analysis of cross-sectional studies investigated that subjects with sarcopenia compared with controls have higher levels of serum hs-CRP. However, levels of IL-6 and TNF-α were not significantly different30. This could be due to the small sample size of the present study. In addition, differences in methodological approach such as control of confounders in the analyses might also contribute.
Overall, TNF-α, CRP, and IL-6 are inflammatory markers that are considered suitable indicators of inflammatory status for investigating the association with sarcopenia and its components30. CRP is an indicator of acute and chronic phase inflammation and increases chronic diseases such as type 2 diabetes, cardiovascular disease, and sarcopenia. Muscle mass, muscle strength, and physical performance decline in chronically high level of inflammatory markers such as CRP30,31,32. Increased levels of CRP influences muscle cell size by suppression of the muscle protein synthesis pathway33. Furthermore, high levels of CRP are associated with obesity and insulin resistance34. It seems that there is a direct relation between the mechanical and metabolic performance of aged muscles35. Increasing CRP levels can increase insulin resistance, which disturbs muscles' metabolic function, and as a result, mechanical function is also impaired35. Although the reason is unknown, the mechanism of cellular and molecular changes in sarcopenia and insulin resistance are the same36. In both cases, there is an accumulation of fat in muscle fibers which can affect the insulin pathway36. Furthermore, disruption of a critical protein in muscles, such as myosin heavy chain, can be seen in insulin resistance and aging muscles37, 38. However, the exact effect of CRP on muscle atrophy is still unknown20.
IL-6 is an Inflammatory cytokine secreted by immune cells in tissue damage or infection conditions20. Also, IL-6 is known as a myokine, which is produced by skeletal muscle and regulates muscle contraction and metabolism39, 40. Some studies showed that temporal and low levels of IL-6 could be beneficial41; however, it is well understood that chronic exposure to IL-6 may result in muscle atrophy and facilitate muscle catabolism42. TNF-α is a pro-inflammatory cytokine and has been associated with muscle pathology43. Muscle catabolism in various inflammatory diseases, including congestive heart failure44, cancer45, and chronic obstructive pulmonary disease (COPD)46, has been attributed to TNF-α. Studies investigate that TNF-α interferes with the muscle differentiation process and can elevate catabolism in mature cells47. Also, TNF-α, through another pathway mediated by reactive oxygen species (ROS) and nuclear factor-kappa B, promotes muscle wasting47. Verghese et al. showed in a cross-sectional study that higher levels of IL-6 but not TNF-α were associated with gait speed decline in elderly48. Renner et al. showed in another cross-sectional study a negative association between inflammatory markers including IL-6 as well as CRP and functional decline49. Along with the catabolic effect of inflammatory markers on muscle mass, as discussed earlier17, 18, the negative effect of inflammatory cytokines on the aging brain might lead to motor function disorder and is another possible mechanism in this regard48, 50.
Recently, researchers have presented interesting data concerning the hypothesis of a “gut-muscle axis” and sarcopenia. They suggest that disruption of intestinal epithelial tight junctions and gut dysbiosis which especially occur in IBD (inflammatory bowel disease) patients through inflammatory markers could lead to muscle wasting and sarcopenia51. Higher levels of inflammatory markers make skeletal muscle fibers initiate an overproduction of reactive oxygen species (ROS)52 and finally cause oxidative stress and consequently sarcopenia53. This supports the crucial association of inflammatory markers with muscle mass/strength and sarcopenia.
In the present study, we found no significant association between inflammatory markers and sarcopenia and its components, including muscle mass and muscle performance. We have only seen a significant association between normal and abnormal muscle strength groups evaluated based on handgrip strength. The difference between our findings and previous studies could be related to the limited number of participants in this study, and more longitudinal studies are needed to clarify our findings. Also, this inconsistency between our results and previous studies may be due to racial and age differences in the current study.
It may be because of the way that cytokines were measured. The measurement of plasma inflammatory cytokines may not suffice to determine the differences, and cellular cytokines must be measured. Inflammation statuses such as disability, neurodegenerative processes, and aging-related hormonal changes can be other causes of sarcopenia54.
To the best of our knowledge, the present study is the first population-based study that evaluated the association of sarcopenia and its components with inflammatory markers in an Iranian population. Our study had some limitations that need to be considered: our study had a small sample size. The causal association between inflammatory markers and sarcopenia cannot be evaluated due to the study’s cross-sectional design. The usage of hydraulic dynamometer is recommended by EWGSOP1. However due the limits we used pneumatic dynamometer instead. Which have different measurement units. With regards to using IPAQ, although the validity of this assessment tool is generally considered adequate among elderly, some difficulties including non-repeatability and classification disagreement exist55. Finally, we conducted the present study on older adults, so generalization of these results to other age groups should be cautious.
Conclusion
There was no significant association between TNF-α, IL-6, and hs-CRP with the presence of sarcopenia, nor with muscle mass, and performance. However, we found a significant difference between serum level of hs-CRP and muscle strength. More prospective studies are needed to provide further insights into the association between these inflammatory markers and sarcopenia.
References
Loenneke, J. & Pujol, T. Sarcopenia: An emphasis on occlusion training and dietary protein. Hippokratia 15, 132 (2011).
Cesari, M. et al. Inflammatory markers and physical performance in older persons: The InCHIANTI study. J. Gerontol. A Biol. Sci. Med. Sci. 59, M242–M248 (2004).
Chen, L.-K. et al. Recent advances in sarcopenia research in Asia: 2016 update from the Asian Working Group for sarcopenia. J. Am. Med. Direct. Assoc. 17, 761–767 (2016).
Cruz-Jentoft, A. European Working Group on sarcopenia in older people: Sarcopenia: European consensus on definition and diagnosis. Report of the European Working Group on sarcopenia in older people. Age Ageing 39, 412–423 (2010).
Cruz-Jentoft, A. J. et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 48, 16–31. https://doi.org/10.1093/ageing/afy169 (2019).
Van Kan, G. A. Epidemiology and consequences of sarcopenia. J. Nutr. Health Aging 13, 708–712 (2009).
Janssen, I., Heymsfield, S. B. & Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 50, 889–896 (2002).
Rolland, Y. et al. Sarcopenia: Its assessment, etiology, pathogenesis, consequences and future perspectives. J. Nutr. Health Aging 12, 433–450 (2008).
Malafarina, V., Úriz-Otano, F., Iniesta, R. & Gil-Guerrero, L. Sarcopenia in the elderly: Diagnosis, physiopathology and treatment. Maturitas 71, 109–114 (2012).
Alexandraki, K. et al. Inflammatory process in type 2 diabetes: The role of cytokines. Ann. N. Y. Acad. Sci. 1084, 89–117 (2006).
Blake, G. J. & Ridker, P. M. Novel clinical markers of vascular wall inflammation. Circ. Res. 89, 763–771 (2001).
Harris, T. B. et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am. J. Med. 106, 506–512 (1999).
Dalle, S., Rossmeislova, L. & Koppo, K. The role of inflammation in age-related sarcopenia. Front. Physiol. https://doi.org/10.3389/fphys.2017.01045 (2017).
Beyer, I., Mets, T. & Bautmans, I. Chronic low-grade inflammation and age-related sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 15, 12–22 (2012).
Argiles, J. M., Busquets, S., Stemmler, B. & Lopez-Soriano, F. J. Cachexia and sarcopenia: Mechanisms and potential targets for intervention. Curr. Opin. Pharmacol. 22, 100–106 (2015).
Schaap, L. A. et al. Higher inflammatory marker levels in older persons: Associations with 5-year change in muscle mass and muscle strength. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 64, 1183–1189 (2009).
Schaap, L. A., Pluijm, S. M., Deeg, D. J. & Visser, M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am. J. Med. 119(526), e529 (2006).
Visser, M. et al. Relationship of interleukin-6 and tumor necrosis factor-α with muscle mass and muscle strength in elderly men and women: The Health ABC Study. J. Gerontol. A Biol. Sci. Med. Sci. 57, M326–M332 (2002).
Taaffe, D. R., Harris, T. B., Ferrucci, L., Rowe, J. & Seeman, T. E. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J. Gerontol. A Biol. Sci. Med. Sci. 55, M709–M715 (2000).
Tuttle, C. S., Thang, L. A. & Maier, A. B. Markers of inflammation and their association with muscle strength and mass: A systematic review and meta-analysis. Ageing Res. Rev. 64, 101185 (2020).
Zhao, W.-Y. et al. The association between systemic inflammatory markers and sarcopenia: Results from the West China Health and Aging Trend Study (WCHAT). Arch. Gerontol. Geriatr. 92, 104262 (2021).
Mu, Z.-J., Fu, J.-L., Sun, L.-N., Chan, P. & Xiu, S.-L. Associations between homocysteine, inflammatory cytokines and sarcopenia in Chinese older adults with type 2 diabetes. BMC Geriatr. 21, 1–9 (2021).
Lin, B., Bai, L., Wang, S. & Lin, H. The Association of systemic interleukin 6 and interleukin 10 levels with sarcopenia in elderly patients with chronic obstructive pulmonary disease. Int. J. Gen. Med. 14, 5893–5902. https://doi.org/10.2147/IJGM.S321229 (2021).
Chen, L.-K. et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 15, 95–101 (2014).
Cruz-Jentoft, A. J. et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 43, 748–759 (2014).
Hashemi, R. et al. Sarcopenia and its determinants among Iranian elderly (SARIR): Study protocol. J. Diabetes Metab. Disord. 11, 1–6 (2012).
Heymsfield, S. B. et al. Appendicular skeletal muscle mass: Measurement by dual-photon absorptiometry. Am. J. Clin. Nutr. 52, 214–218 (1990).
Baumgartner, R. N. et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 147, 755–763 (1998).
Merkies, I. et al. Assessing grip strength in healthy individuals and patients with immune-mediated polyneuropathies. Muscle Nerve 23, 1393–1401 (2000).
Bano, G. et al. Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas 96, 10–15 (2017).
Cesari, M. et al. Inflammatory markers and cardiovascular disease (the health, aging and body composition [health ABC] study). Am. J. Cardiol. 92, 522–528 (2003).
Nanri, A., Moore, M. A. & Kono, S. Impact of C-reactive protein on disease risk and its relation to dietary factors: Literature review. Asian Pac. J. Cancer Prev. 8, 167 (2007).
Wåhlin-Larsson, B. et al. Mechanistic links underlying the impact of C-reactive protein on muscle mass in elderly. Cell. Physiol. Biochem. 44, 267–278 (2017).
Forouhi, N. G., Sattar, N. & McKeigue, P. M. Relation of C-reactive protein to body fat distribution and features of the metabolic syndrome in Europeans and South Asians. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 25, 1327–1331. https://doi.org/10.1038/sj.ijo.0801723 (2001).
Janssen, I. & Ross, R. Linking age-related changes in skeletal muscle mass and composition with metabolism and disease. J. Nutr. Health Aging 9, 408 (2005).
Shulman, G. I. Cellular mechanisms of insulin resistance. J. Clin. Investig. 106, 171–176. https://doi.org/10.1172/jci10583 (2000).
Hickey, M. S. et al. The insulin action-fiber type relationship in humans is muscle group specific. Am. J. Physiol.-Endocrinol. Metab. 269, E150–E154 (1995).
Karakelides, H. & Nair, K. S. Sarcopenia of aging and its metabolic impact. Curr. Top. Dev. Biol. 68, 123–148. https://doi.org/10.1016/s0070-2153(05)68005-2 (2005).
Nieto-Vazquez, I., Fernández-Veledo, S., de Alvaro, C. & Lorenzo, M. Dual role of interleukin-6 in regulating insulin sensitivity in murine skeletal muscle. Diabetes 57, 3211–3221. https://doi.org/10.2337/db07-1062 (2008).
Whitham, M. et al. Contraction-induced interleukin-6 gene transcription in skeletal muscle is regulated by c-Jun terminal kinase/activator protein-1. J. Biol. Chem. 287, 10771–10779. https://doi.org/10.1074/jbc.M111.310581 (2012).
Pedersen, B. K. & Febbraio, M. Exercise and interleukin-6 action. Expert Rev. Endocrinol. Metab. 1, 319–321. https://doi.org/10.1586/17446651.1.3.319 (2006).
Belizário, J. E., Fontes-Oliveira, C. C., Borges, J. P., Kashiabara, J. A. & Vannier, E. Skeletal muscle wasting and renewal: A pivotal role of myokine IL-6. Springerplus 5, 619. https://doi.org/10.1186/s40064-016-2197-2 (2016).
Buck, M. & Chojkier, M. Muscle wasting and dedifferentiation induced by oxidative stress in a murine model of cachexia is prevented by inhibitors of nitric oxide synthesis and antioxidants. EMBO J. 15, 1753–1765 (1996).
Anker, S. D. & Rauchhaus, M. Insights into the pathogenesis of chronic heart failure: Immune activation and cachexia. Curr. Opin. Cardiol. 14, 211–216. https://doi.org/10.1097/00001573-199905000-00004 (1999).
Tisdale, M. J. Wasting in cancer. J. Nutr. 129, 243s–246s. https://doi.org/10.1093/jn/129.1.243S (1999).
Farber, M. O. & Mannix, E. T. Tissue wasting in patients with chronic obstructive pulmonary disease. Neurol. Clin. 18, 245–262. https://doi.org/10.1016/s0733-8619(05)70188-2 (2000).
Reid, M. B. & Li, Y. P. Tumor necrosis factor-alpha and muscle wasting: A cellular perspective. Respir. Res. 2, 269–272. https://doi.org/10.1186/rr67 (2001).
Verghese, J. et al. Inflammatory markers and gait speed decline in older adults. J. Gerontol. Ser. A 66A, 1083–1089. https://doi.org/10.1093/gerona/glr099 (2011).
Renner, S. W. et al. Association of fatigue, inflammation, and physical activity on gait speed: The Long Life Family Study. Aging Clin. Exp. Res. https://doi.org/10.1007/s40520-021-01923-x (2021).
Gorelick, P. B. Role of inflammation in cognitive impairment: Results of observational epidemiological studies and clinical trials. Ann. N. Y. Acad. Sci. 1207, 155–162 (2010).
Nardone, O. M. et al. Inflammatory bowel diseases and sarcopenia: The role of inflammation and gut microbiota in the development of muscle failure. Front. Immunol. https://doi.org/10.3389/fimmu.2021.694217 (2021).
Peake, J., Della Gatta, P., Suzuki, K. & Nieman, D. Cytokine expression and secretion by skeletal muscle cells: Regulatory mechanisms and exercise effects. Exerc. Immunol. Rev. 21, 8–25 (2015).
Supinski, G. S. & Callahan, L. A. Free radical-mediated skeletal muscle dysfunction in inflammatory conditions. J. Appl. Physiol. 102, 2056–2063 (2007).
Thomas, D. R. Loss of skeletal muscle mass in aging: Examining the relationship of starvation, sarcopenia and cachexia. Clin. Nutr. 26, 389–399 (2007).
Tomioka, K., Iwamoto, J., Saeki, K. & Okamoto, N. Reliability and validity of the international physical activity questionnaire (IPAQ) in elderly adults: The Fujiwara-kyo study. J. Epidemiol. Jpn. Epidemiol. Assoc. 21, 459–465. https://doi.org/10.2188/jea.JE20110003 (2011).
Acknowledgements
We are grateful to everyone who kindly participated in our study.
Funding
The financial support for this study comes from the Tehran Endocrine and Metabolism Research Center and the Tehran University of Medical Science.
Author information
Authors and Affiliations
Contributions
F.A., F.D., S.R., R.He., M.B., Z.J., and R.Ha. contributed to the design, conception, data acquisition, analysis, and interpretation of the data, manuscript writing, and review of the final version of the manuscript. All authors approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Asoudeh, F., Dashti, F., Raeesi, S. et al. Inflammatory cytokines and sarcopenia in Iranian adults-results from SARIR study. Sci Rep 12, 5471 (2022). https://doi.org/10.1038/s41598-022-09139-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-09139-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.