Abstract
Epidemiological studies suggest that the Zinc-α-2-glycoprotein (ZAG) plays significant physiological roles. In this study we investigate whether ZAG could be considered as a clinical biomarker in the diagnosis and prognosis of metabolic syndrome (MetS) in Saudi population. As such insights urgently required for management of MetS. Thus, we have determined serum levels of ZAG in patients with MetS and normal individuals. We have also assessed the correlation between ZAG and different components of MetS. In this case–control study, clinical information of 200 Saudi male and female subjects (age range 30–65) with MetS (n = 100) and healthy controls (n = 100) were extracted from the database of the Chair of Biomarkers of Chronic Disease (CBCD) in King Saud University (KSU), Riyadh, Saudi Arabia. MetS was screened according to NCEP ATP III criteria (National Cholesterol Education Program Adult Treatment Panel III). Fasting glucose and lipid profile levels were measured using Konelab. Serum TNF-α, IL- 6, CRP and ZAG levels were measured using commercially available assays. There was an age-dependent significant increase in ZAG level among MetS subjects than controls (43.8 ± 19.5 vs 48.1 ± 14.8; P = 0.04). A significant inverse correlation between ZAG and serum HDL-cholesterol (r = − 0.20, P < 0.05) was observed. Whereas, triglycerides (r = 0.25, P < 0.01), waist circumference (WHR) (r = 0.17, P < 0.05) and CRP (r = 0.24, P < 0.01) were all significantly and positively associated with ZAG. Circulating ZAG is associated with MetS in an age-dependent manner. Serum ZAG is a potential biomarker for MetS.
Similar content being viewed by others
Introduction
Metabolic syndrome (MetS) is a complicated disorder. Progressions in proinflammatory and prothrombotic states are potential mechanisms contributing to the pathophysiology of MetS, due to adipocytokine production. There are also several established mechanisms in the etiology of MetS which involves the insulin resistance (IR) with fatty acid flux, low-level chronic inflammation and oxidative stress1. Obesity-mediated MetS and IR are highly prevalent in the Saudi society. In a study involving 17,293 Saudi patients, the prevalence of MetS was 39.3%2. Moreover, females have a higher MetS prevalence than males2,3. Low-level chronic inflammation combined with increased fat mass and damaging lipid profiles is associated with the pathophysiology of MetS and IR1. Increase in adipocyte has been shown to cause abundance of proinflammatory cytokines such as interleukin (IL)-6, resistin, tumor necrosis factor alpha (TNF-α) and C-reactive protein (CRP), and this could be due the presence of macrophages in adipose tissue which might be partially the source of the production of proinflammatory cytokines4. IR also was found to be associated with overproduction of proinflammatory cytokines and relative deficiency of adiponectin in muscle and liver4.
Growing evidence suggests that altered production of adipose-derived protein factors such as Zinc-α2-glycoprotein (ZAG) plays an important role in the pathophysiology of obesity and its associated complications such as MetS5,6. ZAG gene expression in adipocytes is primarily controlled by androgens and progestins, and Glucocorticoids7. Epidemiological studies showed that, the levels of serum ZAG were positively correlated with the levels of serum triglycerides (TAG) and adipocyte fatty acid-binding protein, whereas the levels of ZAG were inversely associated with HDL-cholesterol8. Overexpression of ZAG in cultured hepatocytes significantly inhibited lipogenesis by decrease metabolic nuclear receptors sterol regulatory element-binding protein (SREBP-1c), liver X receptor (LXR) and lipogenic enzymes in the liver, also lipolysis and fatty acid β-oxidation were stimulated. On the other hand, knocking down of ZAG resulted in inhibition of fatty acid β-oxidation, increased lipogenesis and lipid accumulation9. Findings of these studies suggest an important role of ZAG in lipolysis, and that the way ZAG is involved in lipid metabolism, is by enhancing the conversion of white adipose tissue into brown adipose tissue. The suggested mechanism of ZAG exerting its effect on white adipose tissue, is by increasing the expression of the peroxisome proliferator-activated receptor c (PPARc) and early B cell factor 2, that are considered in promoting cascade expression of genes involved in browning of the adipose tissue10.
We hypothesized that MetS components have different effects on the circulating levels of ZAG. Therefore, the aim of this study was to investigate the relationship between circulating ZAG level and MetS as well as its components, to provide an insight for predicting the occurrence of MetS in Saudi patients. Additional aim of the study was to assess circulating levels of ZAG and proinflammatory cytokines, CRP, IL- 6 and TNF-α in patients with or without MetS.
Materials and method
Subjects
A total of 200 adult Saudi subjects (94 men; 106 women), aged 23–65 years were randomly selected from the database of Biomarker Screening in Riyadh Project (RIYADH COHORT) collected by Center for Biomarkers in Chronic Diseases (CBCD) in Riyadh, KSA11,12. All participants provided written and informed consent prior to inclusion. Ethical approval was granted by the Ethics Committee of the College of Science Research Center, King Saud University, Riyadh, Kingdom of Saudi Arabia (KSA). Participants completed a questionnaire their general health status, demographic information, and past medical history. Anthropometric and biochemical data from the database was utilized to assess the status of full MetS and its five components as present/absent (dichotomous data) according to the criteria set in the National Cholesterol Education Programme Adult Treatment Panel III (NCEP-ATP III) where MetS was present when at least three out of the five following components are present: Waist circumference of > 88 cm; fasting glucose > 5.6 mmol/L; HDL-cholesterol < 1.30 mmol/L; triglycerides > 1.7 mmol/L; systolic blood pressure > 130 mmHg and/or diastolic blood pressure > 85 mmHg13.
The sample size was calculated based on an earlier study by Yeung et al.8 where serum Zinc-alpha2-Glycoprotein in MetS patients was reported with an effect size of > 0.5. Thus, a total sample size of 176 subjects (88 per group) was required to detect the effect size of 0.5 with 95% power using 5% significance level. Based on this, 100 MetS and 100 non-MetS subjects were selected from the database by random selection based on the RAND function in the Microsoft excel. Prior to the final selection, the subjects with reported chronic conditions like liver, kidney, heart failures and pregnant women were removed from the selection and the process was repeated to get the final count of 100 in each group.
Inclusion and exclusion criteria
Subjects on anti-hyperglycemic treatment; pregnant or lactating women; with known renal, hepatic, pulmonary, cardiac, etc., complications were excluded from this study.
Anthropometry and blood collection
Anthropometry included height (cm), weight (kg), waist and hip circumference (cm), and mean systolic and diastolic blood pressure (mmHg, average of two reading). Body mass index (BMI) was calculated as weight in kilograms divided by height in square meters. Waist-hip ratio (WHR) was calculated as the quotient between waist and hip circumferences. They were asked to fast 10 h or overnight before blood withdrawal. Fasting blood samples (> 10 h) were collected and transferred immediately to a non-heparinized tube for centrifugation. The collected serum was transferred to pre-labeled new tubes, kept on ice, and delivered to the CBCD in King Saud University, Riyadh, KSA, for immediate storage at − 20 °C.
Blood chemistry
Fasting blood glucose and lipid profile (triglycerides, total and HDL-cholesterol) were assessed using routine chemical methods (Konelab analyzer, Espoo Finland). Hypertriglyceridemia was defined as circulating triglycerides ≥ 1.7 mmol/l was considered abnormal level13. Low HDL-cholesterol was defined as < 1.03 mmol/l and total cholesterol HDL ratio > 3.5. Low-HDL level for women was set at < 1.3 mmol/l14,15.
Inflammatory markers and ZAG
Serum TNF- α and IL-6 level were determined using MILLIPLEX MAP human adipokine magnetic bead panel 2 kit obtained from Millipore Corporation. Serum human CRP level were determined using ELISA kits obtained from R&D System, USA. Serum ZAG level were determined using ELISA kits using BioVendor-Laboratory Medicine.
Data analysis
Data were analyzed using SPSS (version 22 Chicago, IL, USA). Continuous data were presented as mean ± standard deviation (SD) for normal variables and non-Gaussian variables were presented in median (1st and 3rd) percentiles. Categorical data were presented as frequencies and percentages (%). All continuous variables were checked for normality using Kolmogorov–Smirnov test. Non-Gaussian variables were log-transformed prior to parametric analysis. Independent T-test and Mann Whitney U were used to compare mean differences in Gaussian and Non-Gaussian variables. Correlations between variables were done using Pearson’s and spearman correlation analysis. Stepwise regression analysis was performed for dependent predicators of ZAG (ug/ml) and area under the curve (AUC) was calculated using receiver operating characteristic (ROC) curve to determine viability of ZAG as a biomarker for MetS and its components. An AUC of 0.9 to 1 is considered excellent, 0.8 to 0.9 is considered good, 0.7 to 0.8 is considered fair, 0.6 to 0.7 is considered poor, and 0.5 to 0.6 is considered very poor. A p-value < 0.05 was considered statistically significant.
Institutional review board statement
Ethical approval was obtained from the Ethics Committee of the College of Science Research Center, King Saud University, Riyadh, Saudi Arabia (approval No. E-20-5369). All methods were carried out in accordance with relevant guidelines and regulations.
Informed consent statement
Written informed consent was obtained from all subjects involved in the study.
Results
General characteristics of the study subjects
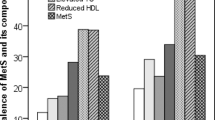
A total of 200 subjects (94 males and 106 females), age range 30–65; mean BMI 29.8 ± 5.5 kg/m2) participated in this study. Half of all subjects had MetS, with significant increase in blood pressure, fasting glucose and triglycerides. Also, there was a decrease in HDL-cholesterol among the MetS subjects. TNF- α and CRP were significantly elevated in MetS subjects than normal subjects (Table 1).
Correlation of ZAG level with proinflammatory cytokines in different metabolic component
A significant inverse correlation between ZAG and serum HDL-cholesterol (R = − 0.20, P < 0.05) was observed. Whereas, triglycerides (R = 0.25, P < 0.01), waist circumference (WHR) (R = 0.17, P < 0.05) and CRP (R = 0.24, P < 0.01) were all significantly and positively associated with ZAG (Fig. 1). We also observed a positive correlation between ZAG and CRP in non-central obesity group (R = 0.31, P < 0.01), as well in female group (R = 0.42, P < 0.01). Also, CRP and ZAG have positive correlations with the hypertensive group (R = 0.28, P < 0.05), and in hypertensive female group (R = 0.40, P < 0.01). While TNF-α and ZAG have an inverse correlation in non-hypertensive males (R = − 0.47, P < 0.05). Furthermore, there was an inverse correlation between IL-6 and ZAG level in the hyperglycemic group in all subjects (R = − 0.26, P < 0.05). In the female group with normal HDL-cholesterol and triglycerides level, there were significant positive correlations between CRP and ZAG (R = 0.44, P < 0.01), (R = 0.35, P < 0.05) respectively (Table 2).
Logistic regression analysis was carried out to identify the relationship between MetS components and ZAG tertiles. The odds-ratios for hypertriglyceridemia were significantly higher for 2nd [OR: 3.7 (1.6–8.3); P = 0.001) and 3rd [OR: 2.9 (1.3–6.4); P = 0.009] tertiles than the first tertile in the multivariate model. Furthermore, the odds ratios of MetS were also significantly higher for 2nd [OR: 3.0 (1.3–6.6); P = 0.006] and 3rd [OR: 2.3 (1.1–5.1); P = 0.04] tertiles than the first tertile in the multivariate model. The rest of the odds ratios were not significant (Table 3).
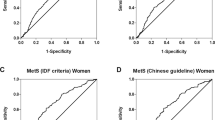
Lastly, AUC was done to determine whether ZAG can be used as a clinical biomarker for the diagnosis of MetS and its components (Table 4). In all subjects, ZAG was not a sensitive biomarker for MetS [AUC 0.57 (95% CI 0.5–0.6; P = 0.10] and a significant but poor biomarker of hypertriglyeridemia [0.61 (0.5–0.7; P = 0.01)] with sensitivity and specificity of 59.8% and 58.0% respectively (ZAG cutoff: 45.5 ug/ml). When stratified according to sex, ZAG is a fair biomarker for hypertriglyceridemia in males [0.7 (95% CI 0.6–0.8; P = 0.003] with sensitivity and specificity of 68.8% and 62.2% respectively (ZAG cutoff: 44.9 ug/ml). Furthermore, ZAG is a poor but significant biomarker for low-HDL cholesterol in females [0.6 (95% CI 0.5–0.8; P = 0.02] with sensitivity and specificity of 60.9% and 61.0% respectively (ZAG cutoff: 44.3 ug/ml) (Fig. 2).
Discussion
In this study we investigated the relationship between circulating ZAG concentrations and MetS components, and analyze the effects of the circulating levels of ZAG on proinflammatory cytokines, CRP and TNF-α in Saudi adults with and without MetS. To the best of our knowledge, there are a few studies on ZAG level in MetS patient worldwide, and this study is the first to be conducted in Saudi Arabia. The current study showed that circulating ZAG levels in MetS patients were significantly higher than in those without MetS in age dependent manner. In contrast, the results of a study by Lei et al.16 and Wang et al.17 revealed that circulating ZAG levels were lower in MetS subjects than in those without MetS. This controversy between the findings could be due to differences in the study design, including sample size, age, and more importantly subjects involved in the two studies were from different population. Age effect on ZAG levels was confirmed in a recent study by Tan et al.18 were they reported significantly lower circulating ZAG level in young women with MetS components. Whereas, in agreement with findings of our study, the results of Yeung et al.8 study on Chinese subjects have shown that ZAG levels were significantly elevated in MetS subjects and it was also elevated progressively with an increasing number of components of the MetS. Our results revealed that ZAG also correlate positively with some of MetS component and significantly with triglycerides, waist and inversely correlated with HDL-cholesterol. Similar findings were shown by previous study8. Animal studies have shown that ZAG act as a regulator of lipid metabolism, and our results multiplied these finding and we showed significantly positive correlation between ZAG and triglyceride, and inverse correlation with HDL-cholesterol18,19. These clinical finding suggest that ZAG may play an important regulatory role in lipid metabolism in humans as well. As ZAG have been shown to possess beneficial effect on mice obesity reduction, decreasing body fat content, and stimulating lipolysis in differentiated adipocytes in vitro18. Based on research finding we speculate that, the observed elevation of serum level of ZAG in MetS patient could be a compensatory process for the human body to overcome the metabolic stress induced by obesity. Alternatively, obesity, could be contributing to ZAG resistance which leads to its upregulation. SBP, DBP and Glucose level were significantly higher in MetS patient than the healthy control, but there was no significant correlation between ZAG and hypertension or fasting glucose. However, in previous studies, it was found that there is an inverse correlation between ZAG and hypertension16. Elevation of visceral adipocytes in MetS disturbs the adipocytokine secretion and leads to a low-grade chronic inflammatory state by the macrophages of adipocytes. This inflammatory state is found to be associated with IR and MetS20. CRP is well known marker for obesity-related inflammation and the Mets, thus we measured its levels. Subjects with MetS had markedly higher CRP level compared to patients without MetS, this finding came in agreement with other study21. CRP level also was significantly higher in obese patient than normal subjects. Furthermore, both CRP and ZAG have been found significantly and positively associated with each other8. Apparently, inflammatory cytokines are able to induce IR in both adipose tissue and muscle.in case of obesity, adipocytes produce plenty of cytokines such as TNF-α and IL-6, the primary stimulator of the production of CRP in the liver. These responses seem to link MetS and inflammation, and explain the increase of CRP level in MetS patients, and the strong relationship between BMI and CRP levels8,22.
Proinflammatory TNF-α plays a crucial role in the inflammatory cytokine cascade that is involved in multiple disease pathogenesis. TNF-α has been studied by Gormez et al.21 and it was found significantly higher in MetS patient than normal subjects. The significant increase in TNF-α level was due to the increased its gene expression in Mets patient compare to control21. In addition, TNF-α level was found to be higher in obese patients than their non-obese counterparts23. We found that TNF-α was higher in female than male. Interestingly, our data reveals a positive correlation between TNF-α and ZAG in normal subjects but inverse correlation in MetS subjects. This inverse correlation was reported in cultured human adipocytes upon treatment with TNF-α, which lowered ZAG mRNA levels. Among inflammatory markers, TNF-α is one of the most important inflammatory cytokines that is critically involved in the development of insulin resistance and pathogenesis of T2DM. Additionally, neutralization of TNF-α also improved the hepatic IR in animal model, also TNF-α-deficient mice have shown improved insulin sensitivity24,25. With regards to the proinflammatory cytokine IL-6 levels, there was no significant change in IL-6 found in both groups, but there was a significant decrease in male subjects with MetS as compared to normal male subjects. Also, there was an inverse correlation between IL-6 and ZAG in normal and MetS subjects. We have no knowledge of any previous study that studied the relationship between ZAG and IL-6. IL-6 was found to be elevated in obesity and type 2 diabetes mellitus patients, while Circulating ZAG levels in the serum and adipose tissue of obese patients and obese mice are notably lower in compare to normal subjects and ZAG were found to be oppositely associated with insulin resistance in type 2 diabetes mellites patients26,27,28,29.
Conclusions
This study shows that circulating ZAG levels in patients with MetS are elevated compared to healthy controls. ZAG was positively associated with MetS component like triglycerides, waist as well with inflammatory CRP, while inversely correlated with HDL-cholesterol. These findings support the pathological role of ZAG in human obesity and its related metabolic disorders. Further studies involving bigger size populations are needed to validate the use of ZAG as a biomarker for MetS and other cardiometabolic disorders.
Data availability
All data are available within the article.
References
McCracken, E., Monaghan, M. & Sreenivasan, S. Pathophysiology of the metabolic syndrome. Clin Dermatol 36, 14–20. https://doi.org/10.1016/j.clindermatol.2017.09.004 (2018).
Al-Nozha, M. et al. Metabolic syndrome in Saudi Arabia. Saudi Med J 26, 1918–1925 (2005).
Al-Daghri, N. M. et al. Decreasing prevalence of the full metabolic syndrome but a persistently high prevalence of dyslipidemia among adult Arabs. PLoS ONE 5(8), e12159. https://doi.org/10.1371/journal.pone.0012159 (2010).
Eckel, R. H., Grundy, S. M. & Zimmet, P. Z. The metabolic syndrome. Lancet 365, 1415–1428. https://doi.org/10.1016/S0140-6736(05)66378-7 (2005).
Gohda, T. et al. Identification of epistatic interaction involved in obesity using the KK/Ta mouse as a Type 2 diabetes model: is Zn-alpha2 glycoprotein-1 a candidate gene for obesity?. Diabetes 52, 2175–2181. https://doi.org/10.2337/diabetes.52.8.2175 (2003).
Tedeschi, S. et al. Serum adipokine zinc alpha2-glycoprotein and lipolysis in cachectic and noncachectic heart failure patients: relationship with neurohormonal and inflammatory biomarkers. Metabolism 61, 37–42. https://doi.org/10.1016/j.metabol.2011.05.011 (2012).
Hassan, M. I., Waheed, A., Yadav, S., Singh, T. P. & Ahmad, F. Zinc alpha 2-glycoprotein: a multidisciplinary protein. Mol Cancer Res 6, 892–906. https://doi.org/10.1158/1541-7786.MCR-07-2195 (2008).
Yeung, D. C., Lam, K. S., Wang, Y., Tso, A. W. & Xu, A. Serum zinc-alpha2-glycoprotein correlates with adiposity, triglycerides, and the key components of the metabolic syndrome in Chinese subjects. J Clin Endocrinol Metab 94, 2531–2536. https://doi.org/10.1210/jc.2009-0058 (2009).
Xiao, X. et al. Zinc alpha2 glycoprotein alleviates palmitic acid-induced intracellular lipid accumulation in hepatocytes. Mol Cell Endocrinol 439, 155–164. https://doi.org/10.1016/j.mce.2016.06.003 (2017).
Elattar, S., Dimri, M. & Satyanarayana, A. The tumor secretory factor ZAG promotes white adipose tissue browning and energy wasting. FASEB J 32, 4727–4743. https://doi.org/10.1096/fj.201701465RR (2018).
Al-Daghri, N. M. et al. Habitual physical activity is associated with circulating irisin in healthy controls but not in subjects with diabetes mellitus type 2. Eur J Clin Invest. 45(8), 775–781. https://doi.org/10.1111/eci.12468 (2015).
Al-Daghri, N. M. et al. Sensitivity of various adiposity indices in identifying cardiometabolic diseases in Arab adults. Cardiovasc Diabetol. 14, 101. https://doi.org/10.1186/s12933-015-0265-5 (2015).
Al-Daghri, N. M., Al-Attas, O. S., Wani, K., Sabico, S. & Alokail, M. S. Serum Uric Acid to Creatinine Ratio and Risk of Metabolic Syndrome in Saudi Type 2 Diabetic Patients. Sci Rep 7, 12104. https://doi.org/10.1038/s41598-017-12085-0 (2017).
Grundy, S. M. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab 89, 2595–2600. https://doi.org/10.1210/jc.2004-0372 (2004).
Lv, J. et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: a systematic review and meta-analysis. PLoS Med 9, e1001293. https://doi.org/10.1371/journal.pmed.1001293 (2012).
Lei, L. et al. Circulating zinc-alpha2-glycoprotein levels are low in newly diagnosed patients with metabolic syndrome and correlate with adiponectin. Nutr Metab (Lond) 14, 53, doi:https://doi.org/10.1186/s12986-017-0210-6 (2017).
Wang, L. et al. Low Serum ZAG Levels Correlate With Determinants of the Metabolic Syndrome in Chinese Subjects. Front Endocrinol (Lausanne) 11, 154, doi:https://doi.org/10.3389/fendo.2020.00154 (2020).
Tan, X. et al. Association of metabolic syndrome components with circulating levels of cytokine clusters in young women. Endocr Connect 10, 66–75. https://doi.org/10.1530/EC-20-0569 (2021).
Russell, S. T. & Tisdale, M. J. Studies on the anti-obesity activity of zinc-alpha2-glycoprotein in the rat. Int J Obes (Lond) 35, 658–665. https://doi.org/10.1038/ijo.2010.193 (2011).
Russell, S. T., Zimmerman, T. P., Domin, B. A. & Tisdale, M. J. Induction of lipolysis in vitro and loss of body fat in vivo by zinc-alpha2-glycoprotein. Biochim Biophys Acta 1636, 59–68. https://doi.org/10.1016/j.bbalip.2003.12.004 (2004).
Gormez, S. et al. Adipose tissue gene expression of adiponectin, tumor necrosis factor-alpha and leptin in metabolic syndrome patients with coronary artery disease. Intern Med 50, 805–810. https://doi.org/10.2169/internalmedicine.50.4753 (2011).
Stejskal, D. et al. Determination of serum zinc-alpha-2-glycoprotein in patients with metabolic syndrome by a new ELISA. Clin Biochem 41, 313–316. https://doi.org/10.1016/j.clinbiochem.2007.11.010 (2008).
Grundy, S. M. et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112, 2735–2752. https://doi.org/10.1161/CIRCULATIONAHA.105.169404 (2005).
Park, H. S., Park, J. Y. & Yu, R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract 69, 29–35. https://doi.org/10.1016/j.diabres.2004.11.007 (2005).
Popa, C., Netea, M. G., van Riel, P. L., van der Meer, J. W. & Stalenhoef, A. F. The role of TNF-alpha in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J Lipid Res 48, 751–762. https://doi.org/10.1194/jlr.R600021-JLR200 (2007).
Akash, M. S. H., Rehman, K. & Liaqat, A. Tumor Necrosis Factor-Alpha: Role in Development of Insulin Resistance and Pathogenesis of Type 2 Diabetes Mellitus. J Cell Biochem 119, 105–110. https://doi.org/10.1002/jcb.26174 (2018).
Gong, F. Y. et al. Zinc-alpha2-glycoprotein is involved in regulation of body weight through inhibition of lipogenic enzymes in adipose tissue. Int J Obes (Lond) 33, 1023–1030. https://doi.org/10.1038/ijo.2009.141 (2009).
Esser, N., Legrand-Poels, S., Piette, J., Scheen, A. J. & Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract 105, 141–150. https://doi.org/10.1016/j.diabres.2014.04.006 (2014).
Barraco, G. M., Luciano, R. & Manco, M. Zinc-alpha2 -glycoprotein is associated with insulin resistance in children. Obesity (Silver Spring) 23, 5–6. https://doi.org/10.1002/oby.20948 (2015).
Acknowledgements
The authors would like to thank the research team from different primary care centers for the recruitment of subjects and Mr. Syed D. Hussain for statistical assistance.
Funding
The authors are grateful to the Deanship of Scientific Research, King Saud University for funding this research project through Vice Deanship of Scientific Research Chairs.
Author information
Authors and Affiliations
Contributions
Conceptualization, N.M.A., K.W.; methodology, S.S., A.M.A.; formal analysis, L.F.A., A.M.A., O.E.A., data curation and statistical analysis, M.N.K.K.; writing—original draft preparation, L.F.A.; writing—review and editing, S.S., A.M.A., K.W., N.M.A. M.S.A.; supervision, N.M.A., A.M.A.; funding acquisition, N.M.A. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alenad, A.M., Alkaltham, L.F., Sabico, S. et al. Associations of zinc-α-2-glycoprotein with metabolic syndrome and its components among adult Arabs. Sci Rep 12, 4908 (2022). https://doi.org/10.1038/s41598-022-09022-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-09022-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.