Abstract
Reactivating of fetal hemoglobin (HbF; α2γ2) can ameliorate the severity of β-thalassemia disease by compensating for adult hemoglobin deficiency in patients. Previously, microarray analysis revealed that zinc finger protein (ZNF)802 (also known as Juxta-posed with another zinc finger gene-1 (JAZF1)) was upregulated in human erythroblasts derived from adult peripheral blood compared with fetal liver-derived cells, implying a potential role as a HbF repressor. However, deficiency in ZNF802 induced by lentiviral shRNA in β0-thalassemia/hemoglobinE erythroblasts had no effect on erythroblast proliferation and differentiation. Remarkably, the induction of HBG expression was observed at the transcriptional and translational levels resulting in an increase of HbF to 35.0 ± 3.5%. Interestingly, the embryonic globin transcripts were also upregulated but the translation of embryonic globin was not detected. These results suggest ZNF802 might be a transcriptional repressor of the γ-globin gene in adult erythroid cells.
Similar content being viewed by others
Introduction
β-thalassemias and sickle cell disease are a group of inherited blood disorders caused by mutations in the β-globin gene cluster, which result in the reduced or absent production of β-globin chains of adult hemoglobin (HbA; α2β2). As a result, the relative excess of α-globin chains forms insoluble α-globin chain inclusions that cause intramedullary hemolysis and ineffective erythropoiesis1. Severely affected patients with β-thalassemia require lifelong blood transfusion and chelation therapy. Induction of fetal hemoglobin (HbF; α2γ2) synthesis can reduce the severity of β-thalassemias by improving the balance between α- and non-α-globin chains. The role of fetal hemoglobin in sickle cell disease was initially investigated more than 70 years ago when Janet Watson reported a few symptoms in infants with sickle cell disease who had high HbF levels in the blood2. Increased levels of HbF retard the polymerization of deoxy sickle hemoglobin and therefore reduce sickle hemoglobin concentrations3. The perinatal decline of HbF synthesis coupled with the increased synthesis of HbA occurs in the second wave of hemoglobin switching during human development, which requires the activation of several transcription factors, including those that bind to the globin gene promoters. However, the regulation of fetal to adult hemoglobin switching is not fully understood. Numerous transcription factors regulate HbF expression via silencing in definitive erythroid cells such as GATA1, KLFs, SOX6, MYB, LRF/ZBTB7A, and direct repeat erythroid-definitive (DRED) have been identified. Several studies suggested that γ-globin gene repression in adult cells may be regulated through the DRED complex4. The DRED complex is a tetrameric corepressor consisting of the orphan nuclear receptors TR2 (NR2C1), TR4 (NR2C2), and two co-repressor enzymes, namely DNA methyltransferase 1 (DMNT1) and lysine-specific demethylase 1 (LSD1 or KDM1a)5. The nuclear receptor TR4 is highly expressed in hematopoietic cells involved in the regulation of differentiation and proliferation of myeloid progenitor cells6. The orphan nuclear receptors have a strong binding affinity to direct repeat (DR1) elements located in the human embryonic ε-globin and fetal γ-globin promoters. Nonetheless, the human β-globin gene has no DR1-binding sites7,8. Inhibitory activity of the co-repressor enzymes, DNMT1 (by 5-azacytidine or decitabine) and LSD1 (by tranylcypromine or RN-1), led to the induction of γ-globin synthesis in adult definitive erythroid cells9,10,11. In addition, gene silencing of each of the DRED corepressors, TR2, TR4 or LSD1, induced embryonic and fetal globin expression in mice7 and human erythroid cells10. These findings suggested that γ-globin repression in adult cells may be regulated through the DRED complex. Recently, our dataset and meta-analysis revealed a significant upregulation of zinc finger protein (ZNF) 802, also known as Juxta-posed with another zinc finger gene-1 (JAZF1) or TAK1-interacting protein 27 (TIP27) located on chromosome 7, in adult basophilic erythroblasts (CD71high/CD235a+)12. ZNF802 was reported to interact with the nuclear receptor TR4 as demonstrated by pull-down analysis13. We hypothesized that ZNF802 functions as a transcriptional corepressor involved in erythropoiesis and hemoglobin synthesis. Therefore, the effects of ZNF802 knockdown were analyzed in healthy donor and β0-thalassemia/hemoglobin E (HbE) erythroblasts.
Results
ZNFs expression in human adult erythroblasts
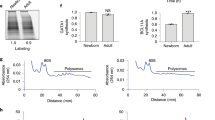
The global gene expression profiling of stage-specific erythroblasts derived from fetal liver and adult peripheral blood was previously analyzed by microarray12. The current study further explored the transcriptional levels of 570 ZNFs in erythroblasts. The three most upregulated ZNFs in adult erythroblasts were ZNF802, ZNF462, and ZNF563. We validated the expression of the three most upregulated ZNFs by RT-qPCR using the same RNA samples as those in the microarray study. We confirmed the high expressions of three ZNFs, ZNF802, ZNF462, and ZNF563 in adult erythroblasts compared with fetal erythroblasts (13.4-, 31.4-, and 9.9-folds change, respectively, Fig. 1A). In addition, three ZNF transcripts were upregulated during day 8 of adult erythroid cell differentiation when normalized to their expressions in proerythroblasts (day 6 of erythroid cell culture) derived from healthy donors (Fig. 1B). The expression of ZNF802 showed a significant 3–4fold increase from day 10 to day 12 of erythroid cell differentiation. The expression patterns of ZNF462 and ZNF563 were slightly increased during erythroid differentiation. Interestingly, the levels of ZNF802 expression in erythroblasts in the β0-thalassemia/HbE group (n = 9) cultured at day 8 and day 10 were significantly lower than in erythroblasts from healthy donors (n = 5; p-value = 0.04) (Fig. 1C) and had a strong negative correlation (r = -0.85; p-value = 0.0004) with the levels of HbF at day 14 (Fig. 1D). These results suggest that the decrease in ZNF802 transcripts in the β0-thalassemia/HbE group indicates ZNF802 as a transcriptional repressor responsible for HbF induction in β0-thalassemia/HbE patients. Next, we investigated the effect of ZNF802 on erythropoiesis and hemoglobin production in erythroblasts.
ZNF expression in human erythroid cells. (A) Validation of ZNF transcripts identified by microarray datasets by RT-qPCR. (B) ZNF802, ZNF462, and ZNF563 expressions during erythroid differentiation (day 8 of culture) and the fold change analysis normalized to its expression in proerythroblasts derived from healthy donors (day 6 of culture, n = 3). *p < 0.05, **p < 0.005. (C) ZNF802 expression at day 8 and day 10 in healthy donors (n = 5) and β0-thalassemia/HbE patients (n = 9). *p < 0.05. (D) Correlation of ZNF802 mRNA expression at day 10 and fetal hemoglobin in β0-thalassemia/HbE patients (n = 12). Error bars represent means and ± SD.
Knockdown of ZNF802 reactivates embryonic and fetal hemoglobins
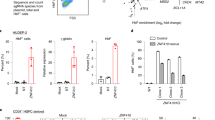
The expression of ZNF802 was knocked down in adult erythroid progenitor cells to investigate the potential role of ZNF802 as a transcriptional repressor of HbF synthesis in adult erythroid cells. The expression levels of six human globin genes, HBZ, HBE, HBG, HBA, HBD, and HBB were determined by RT-qPCR after ZNF802 knockdown in human erythroid progenitor cells from healthy donors and β0-thalassemia/HbE subjects. Lentiviral vectors carrying three specific ZNF802 shRNAs, including ZNF802sh-34, ZNF802sh-35, and ZNF802sh-71, reduced ZNF802 transcripts by more than 80% (Fig. 2A) and resulted in an almost undetectable protein level of ZNF802 in erythroblasts compared with the non-targeting control shRNA group (shNTC) (Fig. 2A). Knockdown of ZNF802 did not significantly affect the expressions of the major HbF repressors BCL11A and LRF, or DRED complex members including TR4, TR2, LSD1, and DMNT1 (Fig. 2B), which demonstrated the specificity of the ZNF802 shRNAs.
Effects of ZNF802 knockdown in erythroid progenitor cells from healthy donors (n = 2) and β0-thalassemia/HbE patients (n = 9). (A) RT-qPCR and western blotting depicting ZNF802 knockdown efficiency on day 10. RPS18 and β-actin were used as a housekeeping gene and loading control for RT-qPCR and western blot, respectively. (B) RT-qPCR of transcription factors, DRED complex repressors (TR2, TR4, LSD1, DMNT1), major repressor BCL11A, and LRF mRNA expression levels on day 10. *p < 0.05, **p < 0.005. (C) RT-qPCR analysis of the relative fold change of α-globin and β- globin cluster mRNA expression levels. Relative fold change represents the mRNA expression levels normalized to RPS18 of shRNAs targeting ZNF802 (ZNF802sh-34, ZNF802sh-35, and ZNF802sh-71) versus nontargeting control shRNA (shNTC) on day 10. Data are presented as the mean ± SD from healthy donors and β0-thalassemia/HbE patients, *p < 0.05. (D) Representative western blot analysis on day 10 showing each globin protein expression. β-actin was used as a loading control. (E) Illustrative hemoglobin typing showing HbF induction on day 14, *p < 0.05. Percentage increase of HbF compared with shNTC, ***p < 0.001. (F) Globin chain analysis to determine the relative ratio of γ-globin chain induction in healthy donors (n = 2) and β0-thalassemia/HbE patients (n = 3), *p < 0.05. (G). Illustrative flow cytometric analysis on day 10 and day 14 showing transferrin receptor (CD71) and glycophorin A (GPA) expression levels. R1 population, CD71high/GPAhigh; R2 population, CD71medium/GPAhigh; R3 population, CD71low/GPAhigh. (H) Representative cytospin preparations of modified Giemsa-stained cells on day 10 from healthy donors (n = 2) and β0-thalassemia/HbE patients (n = 5) visualized under a light microscope with 100 × magnification. Scale bar indicates 10 μm. Error bars represent means and ± SD.
We observed a significant upregulation of the embryonic transcripts; HBZ 7-fold change and 10-fold change, HBE 8- fold change and 12-fold change, and fetal transcripts; HBG 8-fold change and 4-fold change of mRNA expression in ZNF802 knockdown cells compared with shNTC cells at day 10 in healthy donors and β0-thalassemia/HbE cases, respectively (Fig. 2C). The expression of globin gene transcripts in ZNF802sh-34, ZNF802sh-35, and ZNF802sh-71 knockdown erythroblast was reported as fold change compared with its expression derived from shNTC. When ZNF802 expression was diminished in erythroblasts derived from a healthy donor, the HBA, HBB and HBD transcript were expressed in the range of 1.2–1.9 fold change, 1.2–1.6 fold change and 0.9–2.1 fold change, respectively. In β0-thalassemia/HbE, the HBA, HBB and HBD transcripts were expressed in the range of 1.2–1.3 fold change, 0.2–1.1 fold change and 0.8–1.3 fold change, respectively. The HBA, HBD, and HBB transcripts were consistently expressed after ZNF802 shRNA transduction by lentiviral vector. Western blot analysis demonstrated that only the levels of γ-globin protein post ZNF802-sh35 knockdown were increased (1.7- and 1.2-fold changes in healthy donors and β0-thalassemia/HbE, respectively), whereas the embryonic globin (HBZ, HBE) proteins were undetectable in erythroblasts transduced with ZNF802-shRNA (Fig. 2D). Knocking down the expression of ZNF802 by ZNF802sh-35 and ZNF802sh-71 in β0-thalassemia/HbE erythroid cells reactivated HbF to 26.9 ± 7.4% and 26.4 ± 8.8%, respectively, whereas the baseline HbF levels of shNTC varied between 12–23% with a mean of 18.5 ± 6.8%, (n = 9, Fig. 2E). Therefore, the increase in the percentage of fetal hemoglobin (% increase of HbF) was analyzed by the ratio of HbF in the ZNF802 knockdown compared with the shNTC in individual β0-thalassemia/HbE samples. The percentage of HbF was significantly increased to 26.3 ± 4.2% in the ZNF802sh-34 group, 35.0 ± 3.5% in the ZNF802sh-35 group, and 30.2 ± 4.8% in the ZNF802sh-71 group compared with the shNTC of β0-thalassemia/HbE (p-value < 0.0001, Fig. 2E). The increase in γ-globin chain levels in ZNF802 knockdown erythroblasts was demonstrated by the relative ratio γ to α-globin chain (γA + γG/α-globin chain) compared with shNTC treatment in the same donor (Fig. 2F). These changes in HbF levels were concomitant with a significant increase in γ-globin transcripts and γ-globin chain analysis by HPLC (Figs. S1, 2F). Erythroid differentiation and maturation were analyzed based on the expressions of CD71 and GPA, including the CD71-high/GPA-high population (R1), the CD71-medium/GPA-high population (R2), and the CD71-low/GPA-high population (R3). In this culture system, β0-thalassemia/HbE-derived erythroblasts demonstrated delayed erythroid differentiation (greater R1 portion) compared with those from healthy donors (Fig. 2G). The downregulation of ZNF802 in erythroblasts derived from healthy donors and the β0-thalassemia/HbE group did not affect erythroid differentiation or maturation as visualized by flow cytometry and Giemsa staining (Figs. 2G, H).
Discussion
The phenomenon of hemoglobin switching is mediated by transcriptional changes in hemoglobin composition (embryonic-to fetal-to adult-globin) at different developmental stages14. This process has been studied intensively because the reactivation of HbF can ameliorate the clinical symptoms of β-hemoglobinopathies. However, the mechanisms underlying the switching remain unclear. The critical switching regulators of fetal to adult globin gene expression potentially involve DNA-binding TFs BCL11a and LRF (ZBTB7A) and the nucleosome remodeling and deacetylase (NuRD) chromatin complex15,16,17. Zinc-finger TFs, BCL11a and LRF bind to unique site at the proximal promoters of the duplicated gene HBG1 and HBG2 and interact with NuRD to silence γ-globin transcription16,18. The general mechanism allowing the reactivation of HbF levels is through the inactivation by any silencer or repressor (s) of fetal globin expression in adult erythroid cells. However, the reactivation HbF by inactivated HbF silencers such as BCL11a and LRF may negatively contribute to various hematopoietic lineages19,20 and terminal erythropoiesis21, respectively. Therefore, new target genes or drugs that can induce HbF with less or no adverse effects are highly needed. In this study, we took advantage of our previous microarray analysis of the comparative gene expression profiling of human erythroid cells derived from the fetal liver and adult peripheral blood. We explored the expression of 570 ZNFs in erythroblasts12. ZNFs contain a zinc-finger domain that typically binds to DNA, RNA, and proteins involved in several cellular processes, which mediate their effects through different molecular mechanisms in eukaryotes22. Among the significantly differentially expressed ZNF transcripts, the upregulation of ZNF802 in adult erythroid cells was confirmed by RT-qPCR. In addition, the level of ZNF802 expression in erythroblasts (day 8) revealed a negative correlation with HbF levels in β0-thalassemia/HbE erythroid cells, which support a repressive role for ZNF802 in HbF production (day 14). Recently, a genome-wide association study of an African American cohort with sickle cell disease reported a single nucleotide polymorphism (SNP) of ZNF802/JAZF1 (SNP ID = rs740127, base position 28,004,900) associated with high HbF levels with a low genome-wide significance23. This evidence supports our assumption that ZNF802 may have an important role of hemoglobin production during adult erythropoiesis.
To investigate whether ZNF802 has a role in globin regulation related to diverse HbF baseline levels, we performed lentiviral shRNA-mediated ZNF802 knockdown in erythroid progenitor cells from healthy donor adults and β0-thalassemia/HbE patients. We successfully knocked down ZNF802 expression, which resulted in the induction of embryonic globin (HBZ and HBE) and fetal globin (HBG) gene expressions in erythroid progenitor cells from healthy donors and β0-thalassemia/HbE subjects, similar to the increase in embryonic εy and βh1 globins when the DRED complex partnership, TR2 and TR4, was knocked out in mouse erythroid cells24. The upregulation of embryonic and fetal globin gene expression in ZNF802 knockdown erythroid cells supported our assumption that ZNF802 might function as a corepressor related to TR4 binding at the DR-1 binding site of these globin promoters13. The diminished ZNF802 may perturb the binding affinity of TR4 on the DR1 element, resulted in γ-globin and ε-globin mRNA induction. The reactivation of γ-globin expression could bind to excess α-globin in β0-thalassemia/HbE cells, which do not have adult hemoglobin, then formed tetrameric of HbF. In comparison, the assumption may not happen in healthy donors who have balance of β/α- globin ratio. Interestingly, the downregulation of ZNF802 did not significantly affect the expression levels of major repressors of HBG including BCL11A and LRF, or other transcription factors of the DRED complex, suggesting an independent mechanism of globin regulation. We did not detect the protein levels of embryonic globins by western blotting or globin chain analysis via HPLC. This might be because the transcripts of embryonic globin were not translated into proteins or the levels of embryonic globin chains were lower than the limit of detection. Commonly, the basal level of HbF in peripheral blood derived from healthy donors is very low (1–3% HbF). The γ-globin protein was increased 1.7-fold by diminishing ZNF802 compared with shNTC in healthy donors, which might have been related to the slight increase in HbF. Conversely, γ-globin expression was upregulated 1.2-fold when ZNF802 was knocked down in erythroblasts from β0-thalassemia/HbE patients, as reflected by the transcriptional and translational levels of HbF compared with those in the shNTC group in terms of % increased HbF. In this study, we focus on the β0-thalassemia/HbE group with a wide range of HbF levels (9.6–31.1%) to study the diminishing effect of ZNF802 on fetal hemoglobin regulation. The diminished ZNF802 expression in sickle erythroblasts or other β-hemoglobinopathies may reactivate the γ-globin expression via the DRED complex and increase HbF levels, but this needs to be verified further study. However, the diminishing effect of ZNF802 in some groups of β-thalassemia like homozygous β+-thalassemia and homozygous β0-thalassemia who a high levels of fetal hemoglobin (70–90%)25, may pose some difficulties in distinguishing the upregulation of γ-globin expression and HbF production.
Recently, ZNF410 (pentadeacytl ZF protein) has emerged as a DNA-binding protein that directly interacts with CHD4, as shown by CRISPR-Cas9 screening26,27. Loss of ZNF410 in the adult stage of erythroid cell culture systems and xenotransplantation diminishes CHD4 levels and derepresses the fetal hemoglobin genes26. In addition, knocking down of ZNF410 demonstrated no defects on erythroid maturation or hematopoietic reconstitution27. ZNF410 is a novel HbF repressor that does not directly bind to HBG promoter regions but it acts specifically to enhance the expression of the CHD4 component of the NuRD complex. The discovery of ZNF410 demonstrated a new repressor that regulated γ-globin via the CHD4 component of the NuRD complex. Conversely, the DRED complex corepressor is one of the critical factors for γ-globin repressors in adult erythropoiesis4. We speculate that ZNF802 may be a member of the DRED complex that interacts with TR4 and binds on the ε- and γ- promoter regions of DR1. The transcriptomic profile derived from knocking down ZNF802 in erythroid cells may involve the responsive target gene cluster that reactivates γ-globin and ε-globin, but the RNA derived from ZNF802 knockdown erythroid cells was not qualified for RNAseq. The lacking information on DNA-binding of ZNF802 hampered the attempt to determine the mechanism of how ZNF802 regulates HBG gene expression, while chromatin immunoprecipitation (ChIP) revealed no suitable candidate antibody28.
Epigenetic factors might contribute to the modifying of the chromatin structure of globin genes (and eventually other genes) and be responsible for regulating globin gene expression and disease severity. Recently, ZNF802 was suggested to be a chromatin modulator that recruits components of the histone H2A.Z chaperone complex in regulatory regions to control gene expression29. Incorporating of histone H2A.Z into chromatin might have allowed enhancers more access by transcription factors or coactivators before transcription initiation. H2A.Z was highly enriched on active enhancers or locus control regions (LCR HSs; HS4, HS2, HS1) of the β-globin locus, either the active γ-globin promoter of the erythroleukemic cell line, K562, or the active β-globin promoter of murine erythroleukemia cells30, suggesting its role in chromatin reorganization during erythropoiesis and hemoglobin production. H2A.Z incorporation facilities RNA pol II elongation31. Disrupting the expression of ZNF802 in erythroid cells by shRNA may be associated with different epigenetic regulatory mechanisms, including the deacetylation of H2A.Z on LCR or adult β-globin gene promoters. ZNF802 may function in combination with other repressors of the DRED complex by binding at the γ-globin promoter or serve as a chaperone partner with histone H2A.Z at LCR or γ-globin promoters. In this context, we speculate that ZNF802 represses the ɛ- and γ-globin genes via the interaction with TR4 component of the DRED complex binding to the DR1 region of ε- globin and γ-globin promoter and the potentially modulate the histone modification of H2A.Z at specific LCR hypersensitive sites and promoters of active globin genes. Further investigation should elucidate the role of ZNF802 in chromatin remodeling at LCR looping to the γ-globin or β-globin promoters on β-clusters.
Methods
Subjects and sample collection
This study was performed after obtaining institutional ethical approval (MURA2017/375) from the Ethics Committee on Human Rights Related to Research Involving Human Subjects, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Thailand. Mobilized peripheral blood progenitor cells were collected by leukapheresis from healthy donors. Bone marrow samples were obtained as part of pre-stem cell transplantation back up from β0-thalassemia/HbE patients at the Department of Pediatrics, Faculty of Medicine Ramathibodi Hospital. Each participating subject provided written informed consent and all experiments were performed in following relevant guidelines and regulations.
Isolation of hematopoietic stem cells
Mononuclear cells were separated from the mobilized peripheral blood of healthy donors and bone marrow from β0-thalassemia/HbE patients by the gradient density centrifugation (1.077 g/mL Lymphoprep®, Axis-Shield PoC AS, Oslo, Norway) and subsequently selected for CD34+ cells using positive immunomagnetic selection (CD34 MicroBead Kit) (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s recommendations.
Erythroid differentiation of CD34+ cells
Purified CD34+ cells were cultured and differentiated ex vivo into the erythroid lineage using the two-phase culture method. Cells were cultured for 4 days in phase I medium consisting of Iscove’s Modified Dulbecco’s Medium (IMDM; Gibco, Grand Island, NY, USA) supplemented with 20% of fetal bovine serum (FBS; Sigma-Aldrich, St-Louis, MO, USA), 300 μg/mL of holo-transferrin (holo-TF; PromoCell, Heidelberg, Germany), 10 ng/mL of interleukin-3 (IL-3; Cell Signaling Technologies, Beverly, MA, USA), 50 ng/mL of human stem cell factor (SCF; Cell Signaling Technologies, Beverly, MA, USA), and 2 units/mL of human recombinant erythropoietin (EPO; CILAG GmbH, Zug, Switzerland). After 4 days, suspended cells were collected and re-seeded in phase II medium consisting of IMDM supplemented with 20% of FBS, 300 μg/mL of holo-TF, and 5 units/mL of EPO. The culture was maintained under an atmosphere of 5% CO2 at 37 °C for 10 days in phase II medium. Erythroblast differentiation was monitored by cell surface marker analysis using flow cytometry on a FACSVerse flow cytometer (BD Biosciences, San Jose, CA, USA), in which cells were immunostained with allophycocyanin-conjugated anti-transferrin receptor (CD71-APC) (BD Biosciences Pharmingen, San Diego, CA, USA) and fluorescein isothiocyanate-conjugated anti-glycophorin A (GPA-FITC) (BioLegend, San Diego, CA, USA) antibodies. In addition, cell maturation was monitored by Giemsa-stained cytospin preparations. Cell morphology was observed under a light microscope.
Lentiviral shRNA-ZNF802 production and titer determination, and ZNF802 knockdown
shRNAs targeting human ZNF802 mRNA were obtained from the Broad Institute Genetic Perturbation Platform Web Portal and Sigma Aldrich. Three selected target shRNA sequences (shRNA-TRCN0000078534, shRNA-TRCN0000078535, and shRNA-TRCN0000256871) were cloned into the third-generation lentiviral vector, pLL3.7-puro, which was an in-house modified form of pLL3.7 (Addgene plasmid #11795) that replaced the EGFP gene with a puromycin resistance gene as a selectable marker and containing the mouse U6 promoter to drive shRNA expression (a gift from Dr. Khamphikham)32. Lentiviruses expressing different shRNAs targeting ZNF802 mRNA were produced by co-transfecting 10 μg of the expression constructs with packaging plasmids including 2.5 μg of pMD2.G (Addgene plasmid #12259), 3.75 μg of pMDLg/pRRE (Addgene plasmid #12,251), and 3.75 μg of pRSV-Rev (Addgene plasmid #12253) into HEK293T cells using X-tremeGENE HP Transfection reagent (Roche Molecular Systems, CA, USA). Supernatants were collected at 48 and 72 h after transfection and were filtered through a 0.45-μm membrane. The filtrates were concentrated using a Lenti-X concentrator (Clontech, Mountain View, CA, USA) and centrifuged at 1500 × g at 4 °C for 1 h. Lentiviral titers were measured by crystal violet staining (https://horizondiscovery.com/-/media/Files/Horizon/resources/Protocols/titer-crystal-violet-protocol.pdf). Briefly, HEK293T cells were transduced with serial dilutions of lentiviral carrying shRNA in the presence of 4.0 μg/mL polybrene (Sigma-Aldrich), and subsequently challenged with 2.0 μg/mL puromycin (Invitrogen, Carlsbad, CA, USA) at 48 h post-transduction then stained with 1% crystal violet in 10% ethanol. Transducing units per ml (TU/mL) were calculated by colony counts per volume (mL) multiplied by the dilution rate. A non-targeting control shRNA sequence (SHC016V; Sigma-Aldrich) was used as a negative control (shNTC). Day 4 erythroblast cells in culture were transduced with the lentiviruses at a multiplicity of infection (MOI) of 20 in phase II medium supplemented with 8.0 μg/mL polybrene overnight. The transduced cells were treated with 1.0 μg/mL puromycin for 48 h post-transduction, and then cultured in phase II medium (without puromycin) until day 14.
RNA isolation and reverse-transcription quantitative PCR (RT-qPCR)
Total RNA was extracted from erythroid cultures (2 × 106 cells) using TRIzol Reagent (Thermo Fisher Scientific, MA, USA) according to the manufacturer’s instructions. cDNA was synthesized by reverse transcriptase reaction using the RevertAid First Strand cDNA synthesis kit (Thermo Fisher Scientific), following the manufacturer’s protocol. RT-qPCR was performed in duplicate with the specific primers listed in Table S1 using FastStart Essential DNA Green Master Mix (Roche Diagnostics, CA, USA) and analyzed by a LightCycler® 96 System (Roche Molecular Systems). All target gene expression levels were normalized to ribosomal RNA S18 (RPS18). The relative fold change was analyzed using the 2-∆∆Ct method33.
Western blotting
Nuclear and cytoplasmic proteins were extracted from a pellet of at least 5 × 106 cultured cells on day 10 using NE-PER, Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific) following the manufacturer’s instructions. Protein concentration was determined by the Bradford Protein Assay (BioRad, CA, USA). Ten micrograms of nuclear extract protein was run on a 12% SDS polyacrylamide gel, transferred to a polyvinylidene fluoride membrane, and blocked with 5% skimmed milk in phosphate buffer saline supplemented with 0.05% Tween 20 (PBST) (Sigma-Aldrich) for 1 h. Immunoblotting was performed using specific antibodies against their target proteins (Table S1) overnight at 4 °C. The membrane was washed three times for 10 min each with PBST. HRP-conjugated secondary antibodies were used to probe at room temperature for 1 h, followed by three times wash before signal development. Chemiluminescent detection was carried out using ECL™ western blotting detection reagents (Thermo Fisher Scientific) and detected by exposure to an X-ray film.
Hemoglobin typing
Hemolysates were prepared from at least 1 × 106 cultured cells on day 14 in VAR-β-THAL Elution buffer 1 (BioRad) and used for high-performance liquid chromatography (HPLC) for hemoglobin type analysis using a Bio-Rad VARIANT II Hemoglobin Testing System with β-Thalassemia Short Program (BioRad) according to the manufacturer’s recommendations.
Globin chain analysis by high-performance liquid chromatography
Cells were lysed in HPLC-quality distilled water and then underwent two freeze–thaw cycles. A clear cell lysate was separated by centrifugation at 14,000 × g for 10 min at 4 °C and the supernatant was transferred into an HPLC micro vial. Analyzes were performed on a Waters HPLC alliance e2695 (Waters Corporation, MA, USA) separations module and detector. The stationary phase was collected on an Aeris 3.6-µm WIDEPORE-C4 200 Å column behind a SecurityGuard UHPLC Wide-pore C18; 4.6 mm guard column (Phenomenex, CA, USA). The mobile phase was composed of buffer A, 0.1% trifluoroacetic acid (Sigma-Aldrich), in deionized water and buffer B, 0.1% trifluoroacetic acid in 95% acetonitrile (E-CHROMASOLV for HPLC; Sigma Aldrich). At the start of each sample injection, the ratio of mobile phase Buffer A and Buffer B was 60:40%. Buffer B was gradually increased to 53% after 55 min with a constant flow rate of 1 mL/min. The eluted globin proteins were measured at 220 nm with a UV detector (photodiode array detector). Empower 3 chromatography software was used for data acquisition and data analysis.
Statistical analysis
All statistical analyses were performed using an unpaired Student’s t-test and Prism 8 version 8.4.3 (GraphPad Software, San Diego, CA). Results are presented as the mean ± SD and p-values < 0.05 were considered significant.
Ethics declarations
This study was performed after obtaining institutional ethical approval (MURA2017/375) from the Ethics Committee on Human Rights Related to Research Involving Human Subjects, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Thailand.
Change history
13 April 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41598-022-10541-0
References
Mettananda, S., Gibbons, R. J. & Higgs, D. R. α-Globin as a molecular target in the treatment of β-thalassemia. Blood 125, 3694–3701. https://doi.org/10.1182/blood-2015-03-633594 (2015).
Watson, J. The significance of the paucity of sickle cells in newborn Negro infants. Am. J. Med. Sci. 215, 419–423. https://doi.org/10.1097/00000441-194804000-00008 (1948).
Somervaille, T. Disorders of hemoglobin: genetics, pathophysiology, and clinical management. J. R. Soc. Med. 94, 602–603 (2001).
Suzuki, M., Yamamoto, M. & Engel, J. D. Fetal globin gene repressors as drug targets for molecular therapies to treat the β-globinopathies. Mol. Cell. Biol. 34, 3560–3569. https://doi.org/10.1128/MCB.00714-14 (2014).
Cui, S. et al. Nuclear receptors TR2 and TR4 recruit multiple epigenetic transcriptional corepressors that associate specifically with the embryonic β-type globin promoters in differentiated adult erythroid cells. Mol. Cell. Biol. 31, 3298–3311. https://doi.org/10.1128/MCB.05310-11 (2011).
Koritschoner, N. P. et al. The nuclear orphan receptor TR4 promotes proliferation of myeloid progenitor cells. Cell Growth Differ. Mol. Biol. J. Am. Assoc. Cancer Res. 12, 563–572 (2001).
Tanabe, O. et al. Embryonic and fetal beta-globin gene repression by the orphan nuclear receptors, TR2 and TR4. EMBO J. 26, 2295–2306. https://doi.org/10.1038/sj.emboj.7601676 (2007).
Tanabe, O. et al. The TR2 and TR4 orphan nuclear receptors repress Gata1 transcription. Genes Dev. 21, 2832–2844. https://doi.org/10.1101/gad.1593307 (2007).
McCaffrey, P. G., Newsome, D. A., Fibach, E., Yoshida, M. & Su, M. S. Induction of gamma-globin by histone deacetylase inhibitors. Blood 90, 2075–2083 (1997).
Shi, L., Cui, S., Engel, J. D. & Tanabe, O. Lysine-specific demethylase 1 is a therapeutic target for fetal hemoglobin induction. Nat. Med. 19, 291–294. https://doi.org/10.1038/nm.3101 (2013).
Molokie, R. et al. Oral tetrahydrouridine and decitabine for non-cytotoxic epigenetic gene regulation in sickle cell disease: a randomized phase 1 study. PLoS Med. 14, e1002382. https://doi.org/10.1371/journal.pmed.1002382 (2017).
Tangprasittipap, A. et al. Comparison of gene expression profiles between human erythroid cells derived from fetal liver and adult peripheral blood. PeerJ 6, e5527. https://doi.org/10.7717/peerj.5527 (2018).
Nakajima, T., Fujino, S., Nakanishi, G., Kim, Y. S. & Jetten, A. M. TIP27: a novel repressor of the nuclear orphan receptor TAK1/TR4. Nucl. Acids Res. 32, 4194–4204. https://doi.org/10.1093/nar/gkh74 (2004).
Vinjamur, D. S., Bauer, D. E. & Orkin, S. H. Recent progress in understanding and manipulating haemoglobin switching for the haemoglobinopathies. Br. J. Haematol. 180, 630–643. https://doi.org/10.1111/bjh.15038 (2018).
Sankaran, V. G. et al. Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science 322, 1839–1842. https://doi.org/10.1126/science.1165409 (2008).
Masuda, T. et al. Transcription factors LRF and BCL11A independently repress expression of fetal hemoglobin. Science 351, 285–289. https://doi.org/10.1126/science.aad3312 (2016).
Sher, F. et al. Rational targeting of a NuRD subcomplex guided by comprehensive in situ mutagenesis. Nat. Genet. 51, 1149–1159. https://doi.org/10.1038/s41588-019-0453-4 (2019).
Liu, N. et al. Direct promoter repression by BCL11A controls the fetal to adult hemoglobin switch. Cell 173, 430-442.e17. https://doi.org/10.1016/j.cell.2018.03.016 (2018).
Liu, P. et al. Bcl11a is essential for normal lymphoid development. Nat. Immunol. 4, 525–532. https://doi.org/10.1038/ni925 (2003).
Tsang, J. C. et al. Single-cell transcriptomic reconstruction reveals cell cycle and multi-lineage differentiation defects in Bcl11a-deficient hematopoietic stem cells. Genome Biol. 16, 178. https://doi.org/10.1186/s13059-015-0739-5 (2015).
Maeda, T. Regulation of hematopoietic development by ZBTB transcription factors. Int. J. Hematol. 104, 310–323. https://doi.org/10.1007/s12185-016-2035-x (2016).
Cassandri, M. et al. Zinc-finger proteins in health and disease. Cell Death Discov. 3, 17071. https://doi.org/10.1038/cddiscovery.2017.71 (2017).
Liu, L. et al. Original research: a case-control genome-wide association study identifies genetic modifiers of fetal hemoglobin in sickle cell disease. Exp. Biol. Med. 241, 706–718. https://doi.org/10.1177/1535370216642047 (2016).
Cui, S. et al. Compound loss of function of nuclear receptors Tr2 and Tr4 leads to induction of murine embryonic β-type globin genes. Blood 125, 1477–1487. https://doi.org/10.1182/blood-2014-10-605022 (2015).
Sripichai, O. et al. A scoring system for the classification of beta-thalassemia/Hb E disease severity. Am. J. Hematol. 83, 482–484. https://doi.org/10.1002/ajh.21130 (2008).
Lan, X. et al. ZNF410 uniquely activates the NuRD component CHD4 to silence fetal hemoglobin expression. Mol. Cell 81, 239-254.e8. https://doi.org/10.1016/j.molcel.2020.11.006 (2021).
Vinjamur, D. S. et al. ZNF410 represses fetal globin by singular control of CHD4. Nat. Genet. 53, 719–728. https://doi.org/10.1038/s41588-021-00843-w (2021).
Kobiita, A. et al. The diabetes gene JAZF1 is essential for the homeostatic control of ribosome biogenesis and function in metabolic stress. Cell Rep. 32, 107846. https://doi.org/10.1016/j.celrep.2020.107846 (2020).
Procida, T. et al. JAZF1, a novel p400/TIP60/NuA4 complex member, regulates H2A.Z acetylation at regulatory regions. Int. J. Mol. Sci. 22, 678. https://doi.org/10.3390/ijms22020678 (2021).
Kang, J., Kim, Y. W. & Kim, A. Histone variants H3.3 and H2A.Z are incorporated into the β-globin locus during transcription activation via different mechanisms. Biochim. Biophys. Acta Gene Regul. Mech. S1874-9399, 30082–30088. https://doi.org/10.1016/j.bbagrm.2018.05.005 (2018).
Weber, C. M., Ramachandran, S. & Henikoff, S. Nucleosomes are context-specific, H2A.Z-modulated barriers to RNA polymerase. Mol. Cell. 53, 819–830. https://doi.org/10.1016/j.molcel.2014.02.014 (2014).
Khamphikham, P., Jearawiriyapaisarn, N., Tangprasittipap, A. & Hongeng, S. Downregulation of KLF4 activates embryonic and fetal globin mRNA expression in human erythroid progenitor cells. Exp. Ther. Med. 22, 1105. https://doi.org/10.3892/etm.2021.10539 (2021).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. https://doi.org/10.1006/meth.2001.1262 (2001).
Acknowledgements
This research project was supported by Mahidol University and the Ramathibodi Foundation.
Author information
Authors and Affiliations
Contributions
O.S. and A.T. conceived and designed the study. D.S, U.A., and S.H. provided specimens and supervised the study. C.W. and S.C. conducted the experiments and collected the data. O.S., N.S., and A.T. interpreted the data. C.W. and A.T. wrote the original draft of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained an error in the spelling of the author Usanarat Anurathaphan which was incorrectly given as Anurathaphan Usanarat.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wongborisuth, C., Chumchuen, S., Sripichai, O. et al. Down-regulation of the transcriptional repressor ZNF802 (JAZF1) reactivates fetal hemoglobin in β0-thalassemia/HbE. Sci Rep 12, 4952 (2022). https://doi.org/10.1038/s41598-022-08920-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-08920-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.