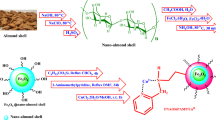
Abstract
The synthesis of 1,2,3-triazoles with immobilized Cu(I) in thiosemicarbazide-functionalized β-cyclodextrin (Cu@TSC-β‐CD) as a supramolecular catalyst was discussed. The catalyst was characterized by Fourier-transform infrared spectroscopy (FT-IR), thermogravimetric analysis (TGA), X-ray diffraction (XRD), scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDS), and Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) measurements. The catalyst showed high activity (up to 95% yields of triazole products under optimized reaction conditions), providing a one-pot, atom-economic, and highly regioselective green method for 1,2,3-triazoles synthesis in an azide-alkyne cycloaddition (AAC) protocol in water. High stability and no appreciable leaching of Cu(I) were observed, owing to its strong binding via the coordination with thiosemicarbazide functionality.
Similar content being viewed by others
Introduction
In recent years, supramolecular chemistry has found special attention in various fields of chemistry. Cram, Lehn, and Pedersen raised their study about host–guest chemistry for the first time, and in 1987 they won the Nobel Prize1,2,3,4,5. Among the various well-known supramolecules such as Calix[n]arenes6, cucurbits7, and rotaxanes8, cyclodextrins and their derivatives are very interesting to study, due to their hydrophobic cavities, and ability to encapsulate small molecules and generate inclusion complexes, which demonstrate host–guest relationships9. These compounds are very useful in drug delivery due to their non-toxicity in small amounts10,11, and in removing pollution from wastewater12,13,14. In recent years, cyclodextrins containing silver and gold nanoparticles have been used to detect heavy and polluting metals mercury, lead, magnesium, and cadmium, and as well as organic pollutants such as nitrobenzene15,16,17. Composites of β-Cyclodextrins with polymers or modified β-Cyclodextrins with the novel anionic or cationic acrylamide polymers have been recently used in the composite industr18 or enhancing oil recovery19. Cyclodextrins are cyclic oligosaccharides consisting of subunits with six (α-CD), seven (β-CD), eight (γ-CD), or more glucose subunits joined by α-1,4 glycosidic bonds20,21. Compared to other derivatives, β-cyclodextrin is the most accessible, most useful, with the lowest price, and is non-toxic one that has a hydrophilic exterior and a hydrophobic interior cavity22. There is much research currently being done in this area to show that β-CD and its functionalized derivatives are useful catalysts in synthetic organic reactions23. The formation of ethers and esters by the electrophilic attack at the OH-groups of cyclodextrins is the most frequently studied substitution reaction of cyclodextrins. The substitution of β-CD at the primary face with a ligand also demonstrates a similar conjoint of hydrophobicity and molecule reorganization properties as the CD. So, different synthetic modifications of β-CD were carried out for a better host–guest interaction24,25,26. On the other hand, metal complexes of β-CD have also been used for various transformations in aqueous media27. Mono tosylation of β-CD was a turning point in the derivation of β-CD, as it allowed derivation or coupling β-CD at the primary hydroxyl group position28. Rashidi Ranjbar and his research group did the green synthesis of triazolyl quinazolinone derivatives using a Cu@β-CD@SiO2@SPION catalyst29. In 2018 Jin and his co-workers used modified β-CD as a catalyst in Suzuki–Miyaura coupling30. In a similar study, Pd@aminopropanol-functionalized β-CD was used to catalyze the Suzuki reaction in the Jian research group in 201831.
Perhaps the most widely studied organic reaction of all copper-catalyzed reactions is Cu(I)-catalyzed azide-alkyne 1,3-dipolar cycloaddition (CuAAC) reaction32. The use of sodium ascorbate with copper (II) sulfate together, in aqueous tert-butanol, is a useful method in these reactions25. But in this type of reaction, because of their thermodynamic instability and initiation of undesired alkyne–alkyne coupling, the direct use of Cu(I) salts was restricted and the presence of heterogeneous catalysts can further contribute to the reaction process33,34. In recent years, various phosphorus-based or phosphine-free ligands such as diverse N-heterocyclic carbenes, diimines, diamines, and amides have attracted considerable attention as competent ligands for different organic reactions. Ligands have been employed to protect the metal center and enhance its catalytic activity35,36,37. Even in 2013, Runo and co-workers performed this reaction in solvent-free conditions with a ball-milling system38. Cu2O/reduced graphene oxide/TiO2 (Cu2O/rGO/TiO2) photocatalyst under ultrasonic irradiation was also used to synthesize these compounds with epoxide derivatives and alkynes39. Although many ways to synthesize alkyl azides have been reported in various research groups, methods for making aryl azides are limited40. Kaboudin et al. reported the one-pot synthesis of 1,2,3-triazoles of aryl boronic acids with sodium azide in the presence of Cu(II)-β-CD, in water41. Rostamnia's research group used a new ionic liquid system as a catalyst via aryl and alkyl halide substrates42.
1,2,3-Triazole is a well-known substructure present in different biologically active compounds with bioactivities, such as antimicrobial43, antiviral44, antitumor45, and anti-inflammatory46 (Fig. 1). The structural features of 1,2,3-triazole enable it to be a good candidate for optical brighteners, light stabilizers, fluorescent whiteners, and corrosion retarding agents47,48. They can also be made from other starting materials such as nitroolefins with different catalysts49.
This work aims to develop a new catalyst (Cu@TSC-β‐CD) via functionalization of β-cyclodextrin by thiosemicarbazide and copper (I) chloride or iodide (Scheme 1) and investigate its use in performing Click reaction. We tried to use water as a solvent in most of our reactions, as a safe, non-toxic, environmentally friendly, and inexpensive solvent. In addition, the purpose of this study was to construct a homogeneous and green catalyst based on a natural compound. The stability of copper (I) iodide in an aqueous medium is very low and it turns gradually to Cu (0) and Cu (II) via disproportionation. Nitrogen-containing ligands protect the metal center from oxidation and disproportionation, by immobilizing the copper ion in the structure of the modified β-cyclodextrin. Also, the solubility of copper (I) ion as its iodide salt in water is extremely low and it can be increased by being in the structure of modified β-cyclodextrin. This also improves the recovery, reusability, and activity of the catalyst50.
Material and methods
Reagents and instrumentation
Except for copper (I) iodide salt (which was freshly prepared), the rest of the reagents like β‐cyclodextrin (98%) and thiosemicarbazide were purchased from commercial sources (Sigma-Aldrich and Merck). They were used without further purification. The melting points of the prepared derivatives were measured by an Electrothermal 9100 apparatus and were reported without any correction. The FT-IR spectra were recorded in the range of 400–4000 cm−1 using the AVATAR spectrometer from Thermo company by using KBr pellets. Elemental analysis was provided by EDX analysis, which was recorded by TESCAN4992. The morphology of the catalyst was studied by SEM using MIRA2 TESCAN instrument. The TGA of the prepared nanocomposite was obtained by an STD Q600. The XRD measurements were recorded with the Rigaku Ultima IV.
General experimental procedures
All click reactions were carried out under air or inert atmosphere (N2, Ar) in a single-neck, round bottom flasks fitted with a rubber septum. Thin-layer chromatography (TLC) was used for following the progress of the reactions. Visualization was done with a 254 nm UV light source.
Catalyst preparation
For simplicity, the detailed preparation for the Cu@TSC-β‐CD complex is illustrated in Scheme 1.
Synthesis of mono-6-(p-tosylsulfonyl)-6-deoxy-β-cyclodextrin (6-OTs-β-CD)51
β-Cyclodextrin (10.0 g, 8.8 mmol) was solved in 100 ml deionized water at 0–5 °C, and 2–3 ml NaOH (8 M) was added dropwise over 5 min until the solution was completely clear. An amount of 0.20 g (1.05 mmol) p-toluenesulfonyl chloride dissolved in 10 ml of acetonitrile was added dropwise over 10 min, forming a white precipitate. After stirring for 2 h at room temperature, the precipitate was acidified to about pH 6–7 with HCl (6 M) and kept in a refrigerator at 0–4˚C overnight. The resulting white precipitate was obtained by filtration. The white solid product was recrystallized from hot water. Finally, the product was dried for 6 h, at room temperature (Yield: 55%). IR: ν (cm−1) 3367(OH), 1641 (Ph-SO2-), 850 (Ph-SO2-O-R).
Synthesis of mono-6-(hydrazinylcarbothioamide)-6-deoxy-β-cyclodextrin (6-TSC-β-CD)52,53
At this step, 0.5 g of 6-OTs-β-CD with 0.02 g thiosemicarbazide was dissolved in 4 ml of DMF and a few drops (0.1 ml) of Et3N as a base was added to the above flask. The reaction mixture was stirred for 24 h in the reflux condition (a cream-yellow turbid solution was formed). Then, by adding 5–10 ml of acetone, a white precipitate appeared. The precipitate was filtered through a Buchner funnel under vacuum, washed with fresh acetone twice, and stored for the next step23 (Yield: 25%).
Modification of TSC-β‐CD with copper (I) chloride and copper (I) iodide (Cu@TSC-β‐CD)
Various methods for making fresh copper iodide salt have been reported54,55. By examining these methods, copper (I) iodide salt was freshly prepared with a slight change in the procedure in an easy, efficient, and cost-effective way56. Briefly, 0.5 g I2 (4 mmol) and 5 g NaI (33 mmol) were dissolved in 50 mL deionized water in a 100 mL round-bottom flask which was previously filled with a small amount of purified and polished Cu foil or granules. Then 2 drops of glacial acetic acid were added, and the reaction was carried out at 70–80 °C under vigorous stirring for 30 min. The change in color of the solution from brown to milky color indicated the formation of a product. The copper foil was completely removed, and the reaction mixture was poured into a container of deionized water and ice and stirred for 10 min. It was then filtered and washed with plenty of water and acetone and dried in a vacuum oven. This product can be stored fresh for two weeks under argon gas. Finally, the obtained ligand 6-TSC-β-CD was stirred with Cu (I) salt in dry toluene at reflux condition in an inert atmosphere (Ar or N2) for 24 h. The precipitate was filtered and washed with acetone, and dried at room temperature. In addition to copper (I) iodide, we also used copper (I) chloride salt to modification of the TSC-β-CD ligand. Comparisons of two modified catalysts showed that copper (I) iodide had better performance.
General procedure for azidation of arylamines
Typically, aryl azide compounds are prepared through nucleophilic substitution of halides in activated arenes by the azide anion, or by azido-demetalation of aryl magnesium halides and aryl lithium reagents (alkali azides, trimethylsilyl azide, or p-toluenesulfonyl azide are the most frequent azide sources)57. We found an efficient and reliable procedure for the synthesis of aryl azides through the reaction of arene diazonium tosylates with sodium azide that was developed in the D. Filimonov research group in 201358. In brief, to a solution of p-TSA in deionized water (9 mmol in 9 mL H2O), 1 mmol Ar-NH2 was added and stirred for 1 min. NaNO2 (9 mmol) was dissolved in 1 ml H2O and added dropwise to the reaction (in 5 min) in an ice bath. The mixture of the reaction was stirred at 5–25 °C for 20 min-1 h. An Amount of 1.6 mmol anhydrous NaN3 is added slowly to the reaction flask. Observation of immediate emission of N2 gas was a sign of product formation. After 15–30 min, solid products were filtered off and washed with cold water, and oil products were extracted with EtOAc (3 × 10 mL) and dried with Na2SO4 and the solvent was removed in a rotary evaporator under reduced pressure (Scheme 2). The products were used in the next step without any purification.
General procedure for Click reaction with organic halides or aryl azides
NaN3 (72 mg, 1.1 mmol), benzyl bromide (119 mg, 1 mmol) or other aryl halides, and terminal alkyne (e.g., phenylacetylene (110 µl, 1 mmol)) were added to a suspension of Cu(I)@TSC-β‐CD in an appropriate solvent (mostly water) under an atmosphere of air or inert atmosphere (see Tables 3 and 4). The reaction progress was being monitored by TLC. The reaction mixture was extracted with EtOAc (3 × 10 ml). The organic phase was dried with anhydrous MgSO4, and the solvent was removed in vacuo to give the crude material. Most products did not require further purification and were only recrystallized from ethanol. The catalyst was dissolved in large amounts of water. To recycle the catalyst from the water, acetone was added and the resulting precipitate was filtered off and dried.
Result and discussion
Characterization of catalysts
FT-IR spectroscopy
Figure 2 shows the FT-IR spectra of (a) β-Cyclodextrin, (b) β-CD-OTs, (c) 6-TSC-β-CD, and (d) Cu@TSC-β‐CD. In Fig. 2a, the strong absorption bands at 3380 cm−1 and 1640 cm−1 correspond to the stretching vibration and bending vibrations of OH groups, respectively. The aliphatic CH absorption bands of cyclodextrin can be seen at 2925 cm−1. The peak in the curve (b) at 1370 cm−1 corresponds to the characteristic bands of the S =O tosyl group. The IR-absorption bands 3349, and 3448 cm−1 in Fig. 2d were due to the free –OH and –NH2 groups of β-CD and thiosemicarbazide of 6-TSC-β-CD. In Fig. 2c, the band around 1658 cm−1 corresponds to the ν(C= S) of TSC, which moved to ca. 1633 cm−1 in Cu@TSC-β‐CD upon complexation with copper (Fig. 2d). It is noteworthy to mention that the amount of TSC was not enough high to change the spectra considerably. The presence of TSC and copper were approved by further data as EDAX and ICP-OES as follows.
EDAX and ICP analyses
Energy Dispersive X-Ray Analysis (EDAX) was used to identify the elemental composition of (a) β-CD-TSC and (b) Cu@TSC-β‐CD (Fig. 3). As expected, the presence of sulfur and nitrogen in the β-CD-TSC and copper in the structure of the final catalyst is proved. The presence of the copper on the catalyst was confirmed with the bands of 8.04, 8.90 keV (K lines), and 0.92 keV (L line).
The exact amount of copper in the catalyst was measured by ICP analysis. This showed that the copper loading was about 0.05 mmol per gram of the Cu@TSC-β‐CD.
Microscopic properties
The surface morphology and the size of the Cu@TSC-β‐CD particles were studied by scanning electron microscopy. The SEM images of the catalyst on 3 scales are shown in Fig. 4. It is observed that most parts of the sample display monodispersed spherical particles. The diameter of the nanospheres is mostly in the range of < 100 nm.
UV–Vis analysis
Figure 5A shows step-by-step UV–Vis spectra of all components. In A1, no absorption band was noted in the UV absorption spectrum of β-CD. The significant increase in Amax = 220–250 may be due to the interaction of the chromophoric group of tosylate in A2 with auxochromic groups in β-CD. In addition to the covalent bond between TSC and β-CD, different kinds of interaction forces are present, such as hydrogen bonding, and dipole–dipole interaction, which will make different contributions to the conformation variation, and immobilization of the TSC. Due to the different interaction forces, the resonant system will be affected for concerning bond angles and bond lengths, to decrease the overlapping of the adjacent π orbital, which will affect the UV–Vis absorption spectra. Since there is no UV–Vis absorption from β-CD itself, the absorption peaks of β-CD-TSC in A3 are assigned to the corresponding TSC molecules. Blue shifts reveal the higher electronic energy gaps (π–π*) of the TSC included in β-CD.
XRD analysis
The XRD patterns of (1) β-Cyclodextrin, (2) β-CD-OTs, (3) β-CD-TSC, and (4) Cu@TSC-β‐CD are shown in Fig. 5B. A scan efficiency of 0.1°·S−1 was applied to record the powder patterns in the range of 3° ≤ 2θ ≤ 80°. The XRD pattern of β-CD showed its characteristic peaks with crystalline nature. Here is no obvious change in the structure of β-Cyclodextrin after functionalization with TSC. The diffraction peaks at 2θ = 35.76°, 39.60° in @Cu β-CD-TSC could be indexed to the (111), and (200) planes of Cu, which is very close to the values in JCPDS– International Center for Diffraction Data.
TGA analysis
The thermogravimetric analysis (TGA) curves for (1) β-CD-TSC, and (2) Cu@TSC-β‐CD are shown in Fig. 5C. Weight loss at temperatures less than 200 °C can be attributed to the elimination of adsorbed water and other solvents. When heated to 600 °C, the weight loss can be attributed to the decomposition of the organic moiety. In the case of β-CD-TSC, the whole structure is decomposed up to 500 °C. In β-CD-TSC@Cu, a weight loss of 77.78% occurred at temperatures of 200–350 °C.
Application of the catalyst in the click reaction
The catalytic activity of Cu@TSC-β‐CD was investigated for the synthesis of 1-benzyl-4-phenyl-1H-1,2,3-triazole as a model reaction at ambient temperature (Table 1, Entry 1). In the absence of any catalyst, the reaction needs temperatures of 100 °C and higher, and a long reaction time. In the presence of catalytic amounts of triethylamine (Et3N) under different solvent conditions such as dichloromethane (DCM), toluene, ethanol, methanol, and water, there was no sign of the formation of the corresponding product at room temperature (Table 1, Entry 2). The addition of copper salts CuI or Cu(OAc)2 on β-CD did not result in good yields (Table 1, Entries 4, 5). The model reaction proceeded smoothly using Cu@TSC-β‐CD (Table 1, Entries 6–11). The catalyst was prepared using two copper salts (CuCl and CuI) and the catalytic activity of both of them was investigated. Because copper iodide salt was freshly prepared, its activity with the same reaction conditions as with copper chloride was higher (Table 1, entry 7,8). Therefore , it was possible to use this catalyst without sodium ascorbate in an inert gas atmosphere (N2 or Ar). After testing several conditions, it was found that 5 mol % of the catalyst Cu@TSC-β‐CD in water as solvent was sufficient for the efficient synthesis of triazole compounds (Table 1, entry 7). By increasing the amount of catalyst concentration to 10 mol%, no further improvement in the product yield was recorded, but decreasing of its concentration to less than 5 mol% resulted in a significant reduction in product yield (Table 1, entries 6 and 9). The reaction was then performed with the solvent’s ethanol, ethanol/water, acetonitrile, and PEG/water (Table 1, entries 8–11). No better result than in pure water was observed.
The obtained optimized conditions were applied for the reaction of different alkynes with various benzyl halides and sodium azide as summarized in Tables 2 and 4. All the products were produced with very good to excellent yields and selectivity. The use of 2-bromoacetophenone derivatives slightly reduced the product yields and increased the reaction time (Tables 2, 3f–i). The presence of electron-withdrawing groups on the benzene ring of 2-bromoacetophenone derivatives increased the product yield (Tables 2, 3f). The products made with propargyl alcohol instead of phenylacetylene had less yield (Tables 2, 3c,g).
Dipolar cycloaddition reactions of aryl azides with alkynes in the presence of copper catalysts and copper salts may cause the formation of 1,4-disubstituted and 1,5-disubstituted 1,2,3-triazoles products. We decided to study also this reaction in the presence of our catalyst. The derivatives 5a-5n were first prepared from primary aromatic amines in high yield (Table 3). No side-products were detected (e.g., phenols and triazenes), which are often formed in the reactions with diazonium salts. So, by a one-pot reaction of p-TsOH with sodium nitrite in water, followed by the reaction with sodium azide without isolation of the intermediate, a variety of aromatic amines can be directly converted into the corresponding aromatic azides (Table 3, 5g, 5h, 5i, 5j).
The reaction between synthesized aryl azides and alkyne described in Table 4 can afford 1,2,3-triazoles in high yields with excellent regioselectivity. In derivatives with an electron-withdrawing group (EWG) on the benzene ring, the product yields were higher than in those with electron-donating groups (7h, 7g). The amount of the catalyst used to prepare the products 7a-h was more than 3a-i products. The final products obtained from 5 h, 5i, 5j azides had very low yields.
The plausible reaction pathway is as in Scheme 2. The Cu(I) species reacts with the alkyne moiety to give a copper acetylide. The subsequent 1,3-dipolar cyclization of the resulting Cu acetylide with an organic azide and following protonation provided the formation of the triazole and the regeneration of the Cu(I) catalyst. It seems that the hydrophobic interior cavity of β-cyclodextrin provides a suitable place for the organic ingredients, as well as copper ions, and they can interact more effectively with each other.
The recyclability of the catalyst
The recyclability of the β-CD-TSC@Cu as a catalyst was tested several times (Fig. 6) in the Click reaction of benzyl azide and phenylacetylene. After each run, the product was extracted from aqueous solution with ethyl acetate. After addition of acetone, the catalyst was precipitated from the solution, filtered out, and dried, and reused under the same condition. The ICP-OES analysis of the filtrate did not detect a significant amount for the leaching of copper species at the 3rd stage of the recyclability study of the catalyst (≤ 2 ppm). The results indicated that the recovered catalyst was still enough active without a significant loss of its performance. At the end of the seventh cycle, a yield of 82% of the product has been achieved.
Comparison with other catalysts
The comparison of some previously reported catalysts in the synthesis of cyclic triazole compounds showed that the catalyst synthesized by our group has the necessary efficiency for this reaction (Table 5).
Conclusion
In summary, we designed and synthesized a water-soluble, homogeneous, and stable new catalyst consisting of copper (I) ions supported on functionalized β-cyclodextrin as a supramolecular substrate. The catalyst was fully characterized by FT-IR, SEM, TGA, EDAX, ICP-OES, and XRD analyses. The effectiveness and its application were tested in the synthesis of 1,4-disubstituted-1,2,3-triazoles through a multicomponent alkyne–azide 1,3-dipolar cycloaddition. Moreover, this catalyst was successfully used in the synthesis of a wide range of triazoles from different terminal alkynes and benzyl chlorides and bromides, as well as aryl azides with high to excellent yields (up to 98%). The reactions proceeded well in an environmentally benign and mild conditions. The catalyst can be easily recovered by simple anti-solvent precipitation, and filtration, and reused at least 7 times without significant loss in its activity.
References
Cram, D. J. & Cram, J. M. Host-guest chemistry. Science 183(4127), 803–809 (1974).
Dietrich, B., Lehn, J. M. & JP, S. Tetrahedron Lett. 10, 2889 (1969).
Dietrich, B., Lehn, J. M., Sauvage, J. P. & Blanzat, J. Cryptates—X: Syntheses et proprietes physiques de systemes diaza-polyoxa-macrobicycliques. Tetrahedron 29(11), 1629–1645 (1973).
Pedersen, C. J. Am. Chem. Soc. 89, 7017–7036 (1967).
Dindulkar, S. D., Jeong, D., Kim, H. & Jung, S. Functionalized β-cyclodextrin as supramolecular ligand and their Pd (OAc)2 complex: highly efficient and reusable catalyst for Mizoroki-Heck cross-coupling reactions in aqueous medium. Carbohydrate 430, 85–94 (2016).
Zhou, Y. et al. Modification of collagen with three novel tannages, sulfonated calix [4] arenes. Int. J. Biol. Macromol. 116, 1004–1010 (2018).
Gao, R.-H., Huang, Y., Chen, K. & Tao, Z. Cucurbit [n] uril/metal ion complex-based frameworks and their potential applications. Coord. Chem. Rev. 437, 213741 (2021).
Aoki, D. & Takata, T. Mechanically linked supramolecular polymer architectures derived from macromolecular [2] rotaxanes: Synthesis and topology transformation. Polymer 128, 276–296 (2017).
Ying-Hui, D. et al. Preparation, characterization and water solubility of inclusion complexes of daidzein with amino-modified β-cyclodextrins. Chin. J. Anal. Chem. 45(5), 648–653 (2017).
Kim, C., Jeong, D., Kim, S., Kim, Y. & Jung, S. Cyclodextrin functionalized agarose gel with low gelling temperature for controlled drug delivery systems. Carbohydrate 222, 115011 (2019).
Zhou, H.-Y., Jiang, L.-J., Zhang, Y.-P. & Li, J.-B. β-Cyclodextrin inclusion complex: preparation, characterization, and its aspirin release in vitro. Front. Mater. Sci. 6(3), 259–267 (2012).
Herrera, B. A. et al. A surface functionalized with per-(6-amino-6-deoxy)-β-cyclodextrin for potential organic pollutant removal from water. Carbohydrate 233, 115865 (2020).
Abou-Elanwar, A. M. et al. Nanocomposite hollow fiber membranes with recyclable β-cyclodextrin encapsulated magnetite nanoparticles for water vapor separation. J. Mater. Chem. A 6(47), 24569–24579 (2018).
Xu, L. et al. Amino-Functionalized β-Cyclodextrin to Construct Green Metal-Organic Framework Materials for CO2 Capture. ACS Appl. Mater. Interfaces 12(2), 3032–3041 (2019).
Manivannan, S. & Ramaraj, R. Silver nanoparticles embedded in cyclodextrin–silicate composite and their applications in Hg (II) ion and nitrobenzene sensing. Analyst 138(6), 1733–1739 (2013).
Aswathy, B., Avadhani, G., Suji, S. & Sony, G. Synthesis of β-cyclodextrin functionalized gold nanoparticles for the selective detection of Pb 2+ ions from aqueous solution. Front. Mater. Sci. 6(2), 168–175 (2012).
Jullian, C. et al. Supramolecular assemblies of phenyl-pyridyl-triazolopyridine and β-cyclodextrin as sensor of divalent cations in aqueous solution. Carbohydr. 121, 295–301 (2015).
Tang, W., Zou, C., Da, C., Cao, Y. & Peng, H. A review on the recent development of cyclodextrin-based materials used in oilfield applications. Carbohydr. Polym. 240, 116321 (2020).
Zou, C., Zhao, P., Ge, J., Lei, Y. & Luo, P. β-Cyclodextrin modified anionic and cationic acrylamide polymers for enhancing oil recovery. Carbohyd. Polym. 87(1), 607–613 (2012).
Dass, C. R. & Jessup, W. Apolipoprotein A-I, cyclodextrins and liposomes as potential drugs for the reversal of atherosclerosis. A review. J. Pharm. Pharmacol. 52(7), 731–761 (2000).
Del Valle, E. M. Cyclodextrins and their uses: A review. Process Biochem. 39(9), 1033–1046 (2004).
Tang, W., Zou, C., Da, C., Cao, Y. & Peng, H. A review on the recent development of cyclodextrin-based materials used in oilfield applications. Carbohydrate 2, 116321 (2020).
Tahir, M. N., Nielsen, T. T. & Larsen, K. L. β-cyclodextrin functionalized on glass micro-particles: A green catalyst for selective oxidation of toluene to benzaldehyde. Appl. Surf. Sci. 389, 1108–1112 (2016).
Deng, Z., Guo, Y., Zhao, X., Ma, P. X. & Guo, B. Multifunctional stimuli-responsive hydrogels with self-healing, high conductivity, and rapid recovery through host–guest interactions. Chem. Mater. 30(5), 1729–1742 (2018).
Tornøe, C. W.; Meldal, M., Peptidotriazoles: Copper (I)-catalyzed 1, 3-dipolar cycloadditions on solid-phase. In Peptides: The Wave of the Future, Springer 263–264 (2001).
Ren, Y., Yang, B. & Liao, X. The amino side chains do matter: chemoselectivity in the one-pot three-component synthesis of 2-amino-4 H-chromenes by supramolecular catalysis with amino-appended β-cyclodextrins (ACDs) in water. Catal. Sci. Technol. 6(12), 4283–4293 (2016).
Dindulkar, S. D., Jeong, D., Kim, H. & Jung, S. Functionalized β-cyclodextrin as supramolecular ligand and their Pd (OAc) 2 complex: highly efficient and reusable catalyst for Mizoroki-Heck cross-coupling reactions in aqueous medium. Carbohydr. Res. 430, 85–94 (2016).
Tripodo, G., Wischke, C., Neffe, A. T. & Lendlein, A. Efficient synthesis of pure monotosylated beta-cyclodextrin and its dimers. Carbohyd. Res. 381, 59–63 (2013).
Bahadorikhalili, S., Ashtari, A., Ma’mani, L., Ranjbar, P. R. & Mahdavi, M. Copper-supported β-cyclodextrin-functionalized magnetic nanoparticles: Efficient multifunctional catalyst for one-pot ‘green’synthesis of 1, 2, 3-triazolylquinazolinone derivatives. Appl. Organomet. Chem. 32(4), e4212 (2018).
Luo, K., Zhang, L., Yang, R., Jin, Y. & Lin, J. Picolinamide modified β-cyclodextrin/Pd (II) complex: Asupramolecular catalyst for Suzuki-Miyaura coupling of aryl, benzyl and allyl halides with arylboronic acids in water. Carbohydrate 200, 200–210 (2018).
Zhou, X., Guo, X., Jian, F. & Wei, G. Highly efficient method for Suzuki reactions in aqueous media. ACS Omega 3(4), 4418–4422 (2018).
Buckley, B., Figueres, M. M., Khan, A. N. & Heaney, H. A new simplified protocol for copper (I) alkyne–azide cycloaddition reactions using low substoichiometric amounts of copper (II) precatalysts in methanol. Synlett 27, 51–56 (2016).
Aucagne, V. & Leigh, D. A. Chemoselective formation of successive triazole linkages in One Pot:“Click− Click” chemistry. Org. Lett. 8(20), 4505–4507 (2006).
Siemsen, P., Livingston, R. C. & Diederich, F. Acetylenic coupling: A powerful tool in molecular construction. Angew. Chem. Int. Ed. 39(15), 2632–2657 (2000).
Tornøe, C. W., Christensen, C. & Meldal, M. Peptidotriazoles on solid phase:[1, 2, 3]-triazoles by regiospecific copper (I)-catalyzed 1, 3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 67(9), 3057–3064 (2002).
Rostovtsev, V. V., Green, L. G., Fokin, V. V. & Sharpless, K. B. A stepwise huisgen cycloaddition process: copper (I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 114(14), 2708–2711 (2002).
Lal, S., McNally, J., White, A. J. & Díez-González, S. Novel phosphinite and phosphonite copper (I) complexes: Efficient catalysts for click azide–alkyne cycloaddition reactions. Organometallics 30(22), 6225–6232 (2011).
Mukherjee, N., Ahammed, S., Bhadra, S. & Ranu, B. C. Solvent-free one-pot synthesis of 1, 2, 3-triazole derivatives by the ‘Click’reaction of alkyl halides or aryl boronic acids, sodium azide and terminal alkynes over a Cu/Al 2 O 3 surface under ball-milling. Green Chem. 15(2), 389–397 (2013).
Mirza-Aghayan, M., Saeedi, M. & Boukherroub, R. Cu2O/reduced graphene oxide/TiO2 nanomaterial: An effective photocatalyst for azide-alkyne cycloaddition with benzyl halides or epoxide derivatives under visible light irradiation. Appl. Organomet. Chem. 34(11), e5928 (2020).
Yang, H., Li, Y., Jiang, M., Wang, J. & Fu, H. General copper-catalyzed transformations of functional groups from arylboronic acids in water. Chem. A Eur. J. 17(20), 5652–5660 (2011).
Kaboudin, B., Abedi, Y. & Yokomatsu, T. One-pot synthesis of 1, 2, 3-triazoles from boronic acids in water using Cu (II)–β-cyclodextrin complex as a nanocatalyst. Org. Biomol. Chem. 10(23), 4543–4548 (2012).
Hosseini, H. G. et al. Combining ethylenediamine and ionic liquid functionalities within SBA-15: A promising catalytic pair for tandem Cu–AAC reaction. Appl. Catal. A 548, 96–102 (2017).
Gondru, R. et al. 1, 2, 3-triazole-thiazole hybrids: Synthesis, in vitro antimicrobial activity and antibiofilm studies. Bioorg. Med. Chem. Lett. 33, 127746 (2021).
Krajczyk, A. et al. Antivirally active ribavirin analogues–4, 5-disubstituted 1, 2, 3-triazole nucleosides: Biological evaluation against certain respiratory viruses and computational modelling. Antivir. Chem. Chemother. 23(4), 161–171 (2014).
Ashwini, N. et al. Synthesis of 1, 2-benzisoxazole tethered 1, 2, 3-triazoles that exhibit anticancer activity in acute myeloid leukemia cell lines by inhibiting histone deacetylases, and inducing p21 and tubulin acetylation. Bioorg. Med. Chem 23(18), 6157–6165 (2015).
Kim, T. W. et al. Synthesis and biological evaluation of phenyl-1H-1, 2, 3-triazole derivatives as anti-inflammatory agents. Bioorg. chem. 59, 1–11 (2015).
Gouault, N., Cupif, J.-F., Sauleau, A. & David, M. γ-Methyl-substituted-γ-butyrolactones: Solid-phase synthesis employing a cyclisation–cleavage strategy. Tetrahedron Lett. 41(38), 7293–7297 (2000).
Huo, J. et al. Preparation and characterization of poly-1, 2, 3-triazole with chiral 2 (5 H)-furanone moiety as potential optical brightening agents. ACS Omega 2(9), 5557–5564 (2017).
Zhang, H., Dong, D.-Q. & Wang, Z.-L. Direct synthesis of N-unsubstituted 4-aryl-1, 2, 3-triazoles mediated by Amberlyst-15. Synthesis 48(01), 131–135 (2016).
Marra, A., Vecchi, A., Chiappe, C., Melai, B. & Dondoni, A. Validation of the copper (I)-catalyzed azide− alkyne coupling in ionic liquids. Synthesis of a triazole-linked C-disaccharide as a case study. J. Org. Chem. 73(6), 2458–2461 (2008).
Khan, R. I. & Pitchumani, K. A pyridinium modified β-cyclodextrin: An ionic supramolecular ligand for palladium acetate in C–C coupling reactions in water. Green Chem. 18(20), 5518–5528 (2016).
Khan, R. I. & Pitchumani, K. Water-soluble palladium complex of N′-(pyridin-2-yl) propane-1, 3-diamine modified β-cyclodextrin: An efficient catalyst for transfer hydrogenation of carbonyl compounds. ACS Sustain. Chem. Eng. 6(12), 16130–16138 (2018).
Jicsinszky, L. et al. Nucleophilic substitutions of 6I-O-Monotosyl-β-cyclodextrin in a planetary ball mill. ACS Sustain. Chem. Eng. 4(3), 919–929 (2016).
Bircumshaw, L. L. & Everdell, M. H. The kinetics of the reaction between copper and iodine in various solutions. Part II. Solutions of iodine in organic solvents. J. Chem. Soc. (Resumed) 1947, 1119–1128 (2011).
Lobana, T. S., Sharma, R., Sharma, R. & Butcher, R. J. Metal derivatives of heterocyclic thioamides: Synthesis and crystal structures of copper complexes with 1-methyl-1, 3-imidazoline-2-thione and 1, 3-imidazoline-2-thione. Z. Anorg. Allg. Chem. 634(10), 1785–1790 (2008).
Bircumshaw, L. L. & Everdell, M. H. The kinetics of the reaction between copper and iodine in aqueous (potassium iodide) solution. Part I. J. Chem. Soc. 16, 598–604 (1942).
Das, J., Patil, S. N., Awasthi, R., Narasimhulu, C. P. & Trehan, S. An easy access to aryl azides from aryl amines under neutral conditions. Synthesis 2005(11), 1801–1806 (2005).
Kutonova, K. V., Trusova, M. E., Postnikov, P. S., Filimonov, V. D. & Parello, J. A simple and effective synthesis of aryl azides via arenediazonium tosylates. Synthesis 45(19), 2706–2710 (2013).
Islam, R. U., Taher, A., Choudhary, M., Witcomb, M. J. & Mallick, K. A polymer supported Cu (I) catalyst for the ‘click reaction’in aqueous media. Dalton Trans. 44(3), 1341–1349 (2015).
Lu, J. et al. One-pot three-component synthesis of 1, 2, 3-triazoles using magnetic NiFe 2 O 4–glutamate–Cu as an efficient heterogeneous catalyst in water. RSC Adv. 5(73), 59167–59185 (2015).
Shaabani, A., Afshari, R., Hooshmand, S. E., Tabatabaei, A. T. & Hajishaabanha, F. Copper supported on MWCNT-guanidine acetic acid@ Fe3O4: synthesis, characterization and application as a novel multi-task nanocatalyst for preparation of triazoles and bis (indolyl) methanes in water. RSC Adv. 6(22), 18113–18125 (2016).
Sun, N. et al. Synthesis of a heterogeneous Cu (OAc) 2-anchored SBA-15 catalyst and its application in the CuAAC reaction. New J. Chem. 42(3), 1612–1616 (2018).
Garg, A., Khupse, N., Bordoloi, A. & Sarma, D. Ag–NHC anchored on silica: an efficient ultra-low loading catalyst for regioselective 1, 2, 3-triazole synthesis. New J. Chem. 43(48), 19331–19337 (2019).
Chetia, M., Ali, A. A., Bordoloi, A. & Sarma, D. Facile route for the regioselective synthesis of 1, 4-disubstituted 1, 2, 3-triazole using copper nanoparticles supported on nanocellulose as recyclable heterogeneous catalyst. J. Chem. Sci. 129(8), 1211–1217 (2017).
Jafari, A. A., Mahmoudi, H. & Firouzabadi, H. A copper acetate/2-aminobenzenthiol complex supported on magnetite/silica nanoparticles as a highly active and recyclable catalyst for 1, 2, 3-triazole synthesis. RSC Adv. 5(130), 107474–107481 (2015).
Author information
Authors and Affiliations
Contributions
The work is a part of the PhD thesis of M.T. She made the experiments, collected the data, and wrote the main manuscript text. M.R.N.-J. was her supervisor and made corrections to the text and the scientific discussion.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tajbakhsh, M., Naimi-Jamal, M.R. Copper-doped functionalized β-cyclodextrin as an efficient green nanocatalyst for synthesis of 1,2,3-triazoles in water. Sci Rep 12, 4948 (2022). https://doi.org/10.1038/s41598-022-08868-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-08868-9
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.