Abstract
Since the clinical outcome of patients with sarcoidosis is still unpredictable, a good prognostic biomarker is necessary. Autotaxin (ATX) and phosphatidylserine-specific phospholipase A1 (PS-PLA1) function as main enzymes to produce lysophospholipids (LPLs), and these enzymes are attracting attention as useful biomarkers for several chronic inflammatory diseases. Here, we investigated the relationships between LPLs-producing enzymes and the disease activity of sarcoidosis. In total, 157 patients with sarcoidosis (active state, 51%) were consecutively enrolled. Using plasma or urine specimens, we measured the values of LPLs-producing enzymes. Urine ATX (U-ATX) levels were significantly lower in the active state compared to those in the inactive state, while the plasma ATX (P-ATX) and PS-PLA1 levels showed no significant difference between these two states. Concerning the comparison with existing clinical biomarkers for sarcoidosis, U-ATX showed a weak negative correlation to ACE, P-ATX a weak positive correlation to both ACE and sIL-2R, and PS-PLA1 a weak positive one to sIL-2R. Notably, only the U-ATX levels inversely fluctuated depending on the status of disease activity whether OCS had been used or not. These findings suggest that U-ATX is likely to be a novel and useful molecule for assessing the disease activity of sarcoidosis.
Similar content being viewed by others
Introduction
Sarcoidosis is a systemic granulomatous disorder of unknown etiology. The clinical features of sarcoidosis are quite different among regions, races and ethnicities and the outcomes of this disease also greatly differ1,2,3,4. Because of the diversity in the pathogenesis of sarcoidosis, it is difficult to predict the clinical outcome of sarcoidosis, ranging from spontaneous remission to severe dysfunction of major organs3,5. Increased levels in several serum laboratory tests such as angiotensin converting enzyme (ACE), soluble interleukin-2 receptor (sIL-2R) and lysozyme were observed at the time of diagnosis. These molecules seem somewhat useful for monitoring the disease activity, but not enough to estimate the changes in severity or to predict the prognosis of sarcoidosis. Concerning the treatment for patients with severe sarcoidosis or severe dysfunction of major organs, oral corticosteroids (OCS) are commonly used as a first line therapy, but long-term use of OCS may cause many serious adverse effects including hyperglycemia, immunodeficiency, osteoporosis and so on. If we can find useful monitoring or prognostic biomarkers associated with the pathophysiology of sarcoidosis, it would be helpful not only for improving the management of patients with sarcoidosis, but also for developing novel treatment strategies.
Eicosanoid mediators such as prostaglandins and leukotrienes have long been studied as first-generation bioactive lipids. On the other hand, lysophospholipids (LPLs), such as lysophosphatidic acid (LPA), sphingosine 1-phosphate (S1P), and lysophosphatidylserine (LysoPS), have emerged and been attracting attention to researchers as second-generation bioactive lipids in recent decades6,7,8,9. Among these LPLs, LPA and LysoPS are produced extracellularly, whereas S1P is produced intracellularly and released extracellularly. Relatively high concentrations of LPLs are known to exist in body fluids such as blood (plasma, serum) and function through specific G protein-coupled receptors expressed on the surfaces of target cells. Although assays of these lipids themselves are possible, the regulation of the metabolism of LPL mediators is complicated and it is not LPLs themselves but the enzymes catalyzing their production, that are encoded by the genomic DNA. Therefore, LPLs-producing enzymes such as autotaxin (ATX) or phosphatidylserine-specific phospholipase A1 (PS-PLA1) and even a carrier such as apolipoprotein M (ApoM) are more promising as alternate biomarkers for LPA, LysoPS and S1P, respectively6,7,10,11,12. ATX is a secreted lysophospholipase D catalyzing the extracellular hydrolyses of lysophosphatidylcholine to LPA and is considered to be responsible for the synthesis of the majority of extracellular LPA13. ATX/LPA signaling plays important roles in the pathophysiology of several chronic inflammatory diseases such as interstitial lung diseases, cancer, liver cirrhosis and atherosclerosis14,15,16,17,18. It has been reported that ATX can bind to lymphocytes, and LPA or ATX/LPC strongly enhances the trans-endothelial migration of integrin-arrested T cells across an endothelial monolayer and promotes lymphocytes to enter lymphoid organs from the blood19,20. In mouse models with rheumatoid arthritis, ATX proteins are expressed on synovial fibroblasts and its expression is markedly stimulated by TNF, which is one of the crucial mediators for granuloma formation21. PS-PLA1 is one of the enzymes that is responsible for LysoPS production. Unlike the ATX/LPA axis, the biological roles of PS-PLA1/LysoPS in the pathogenesis of those diseases have not been well studied. Recent studies suggest that the PS-PLA1/LysoPS axis is partially involved in the pathogenesis of cancer, acute coronary syndrome, and hyperthyroidism22,23,24,25. However, although it has been reported that the PS-PLA1/LysoPS axis inhibits mitogen-induced T-cell activation in vitro26, the association between the LPLs-producing enzymes/LPLs signaling and the disease activity of sarcoidosis remains to be elucidated.
To investigate potential diagnostic of prognostic biomarkers for sarcoidosis, we collected over 150 plasma and urine samples from patient with active or inactive sarcoidosis and analyzed the relationships between LPLs-producing enzymes and the disease activity. Because the present study aims to find novel clinically useful biomarkers and apply them to the daily examination in a clinical setting, we focused on the values of ATX and PS-PLA1, both of which are present in body fluids and have been used as an alternative for the value of LPA and LysoPS, respectively27.
Results
Clinical characteristics of subjects
During the inclusion period, 53 out of 211 patients with sarcoidosis were excluded because of ongoing OCS therapy at the time of enrollment and 1 patient because of chronic hepatitis, and then 157 patients were finally enrolled in this study. Because the plasma- ATX (P-ATX) levels are known to differ between genders28, we first analyzed the data among gender groups in addition to the whole subjects group. The clinical characteristics of subjects are summarized in Table 1. A subtotal of 100 (64%) patients were female. Female subjects were more likely to be older, and showed a lower smoking index and higher prevalence of Stage 1 lung lesions. The enrolled subjects had almost similar characteristics with a population in a previous sarcoidosis cohort study in Japan29,30. The disease activity was not significantly different between male and female groups.
The subjects were classified into two groups according to their disease activity, that is, 80 patients in an active state and 77 in an inactive state (Table 2). Subjects in an active state showed a lower body-mass index (BMI), a higher prevalence of eyes and cardiac involvement and higher values in ACE, sIL-2R and KL-6.
Association between LPLs-producing enzymes and the disease activity
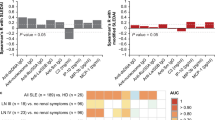
We first analyzed the association between LPLs-producing enzymes and the disease activity. As shown in Fig. 1a,g, neither the P-ATX nor PS-PLA1 levels showed significant differences between active and inactive states in all subjects (P-ATX: 0.96 [0.82–1.11] for active state and 0.91 [0.76–1.12] mg/L for inactive state, p = 0.28, PS-PLA1: 16.2 [13.5–18.8] for active state and 15.25 [12.4–17.7] ng/mL for inactive state, p = 0.13). On the other hand, subjects in an inactive state showed a significant increase in urine-ATX (U-ATX) (3.21 [1.34–6.15] for active state and 5.53 [3.41–8.57] \(\upmu\)g/gCre for inactive state, p < 0.001) (Fig. 1d).
Comparison of the values of LPLs-producing enzymes between patients with active state and inactive state. Comparison of box plot of P-ATX (a,b,c), U-ATX (d,e,f), and PS-PLA1 (g,h,i) levels between active and inactive state. Boxes indicate median and interquartile range, and whiskers indicate minimum–maximum range. Data were analyzed with Mann–Whitney U-test. Statistical significance was accepted as p < 0.05 and bold denotes values p < 0.05.
It is known that the P-ATX levels differ between genders28. The present study also showed a significant gender difference in the median values of P-ATX (0.79 [0.71–0.92] for males and 1.02 [0.89–1.20] mg/L for females, p < 0.001) and U-ATX (3.69 [1.78–5.31] for males and 5.35 [2.23–8.76] \(\upmu\)g/gCre for females, p = 0.01) (Table 1). Interestingly, the plasma levels of PS-PLA1 showed a reverse gender difference (16.5 [14.6–18.6] for males and 14.8 [12.0–17.6] ng/mL for females, p = 0.001) (Table 1). Because of these gender differences, we further compared these values between subjects in active and inactive states for each gender (Fig. 1). As in the case of all subjects, neither males nor females showed any differences between active and inactive states in the P-ATX values (p = 0.21 for males and p = 0.66 for females) (Fig. 1b,c). Notably, female subjects in an inactive state showed a significant increase in the U-ATX values compared with subjects in an active state (3.22 [1.24–7.75] for active state and 6.90 [4.09–10.8] \(\upmu\)g/gCre for inactive state, p < 0.001), while male subjects did not (3.20 [1.52–4.57] for active state and 4.29 [1.96–5.94] \(\upmu\)g/gCre for inactive state, p = 0.10) (Fig. 1e,f). While the PS-PLA1 levels did not show any difference between active and inactive states in all subjects, female subjects in an active state showed a significant increase in those values compared with subjects in an inactive state (14.9 [12.3–18.9] for active state and 13.5 [11.8–16.7] ng/mL for inactive state, p = 0.049) (Fig. 1g,h,i). These findings suggest that the levels of U-ATX and PS-PLA1 are associated with the disease inactivity of sarcoidosis, especially in female subjects. We further investigated the correlation between BMI and the levels of U-ATX or P-ATX, which mainly originate from adipose tissues31. As shown in supplemental Figure S1, both the P-ATX and U-ATX levels showed no significant correlation with BMI.
Association between LPLs-producing enzymes and the clinically used biomarkers for sarcoidosis
We next investigated whether these LPLs-producing enzymes are associated with existing clinically used biomarkers such as, serum ACE or serum sIL-2R (Fig. 2). The P-ATX levels showed weak positive correlations with both the ACE levels (r = 0.32, p < 0.0001) and sIL-2R levels (r = 0.21, p = 0.008) (Fig. 2a,b). The U-ATX levels showed a weakly significant negative correlation with the ACE levels (r = −0.17, p = 0.04) (Fig. 2c), but not with sIL-2R levels (r = −0.11, p = 0.17) (Fig. 2d). The PS-PLA1 levels showed weak positive correlations with the sIL-2R levels (r = 0.23, p = 0.005) (Fig. 2f), but not with the ACE levels (r = −0.002, p = 0.98) (Fig. 2e).
We next investigated whether U-ATX is independent of several clinical biomarkers for sarcoidosis (Table 3). Univariate logistic regression analysis with 5 variables revealed that U-ATX, ACE and sIL-2R were associated with the activity of sarcoidosis. After excluding explanatory variables with p > 0.05, multivariate logistic regression analysis revealed that U-ATX, ACE and sIL-2R were also independently associated with the activity of sarcoidosis. These findings suggest that the U-ATX levels are independent of the clinically used biomarker of sarcoidosis.
Association between U-ATX levels and urine N-acetyl-\({\varvec{\upbeta}}\)-D-glucosaminidase
It has been reported that U-ATX proteins are expressed in the proximal tubular epithelial cells32. We further investigated the relationships between the U-ATX levels and the urine N-acetyl-\(\upbeta\)-D-glucosaminidase (NAG), which is a popular clinical marker for renal tubular injury. The values of urine NAG did not show any correlation to U-ATX (r = 0.03, p = 0.71) (Fig. 3a). This finding suggests that the U-ATX levels are independent of the renal tubular injury. In line with a previous report showing that the U-ATX/Cre levels are positively correlated with the estimated glomerular filtration rate (eGFR)33, the U-ATX/Cre levels showed a weak positive correlation with the eGFR in this study (r = 0.34, p < 0.0001) (Fig. 3b). All subjects with sarcoidosis in this study showed normal eGFR values (Table 1) and the P-ATX levels did not correlate with the U-ATX levels (r = 0.14, p = 0.07) (Fig. 3c). These findings suggest that the changes in U-ATX levels are independent of the exudation of P-ATX into urine.
Association between LPLs-producing enzymes and the transition of disease activity for sarcoidosis
During the study periods, we could obtain plasma and/or urine samples repeatedly from 31 subjects every time their disease activities had changed. The clinical characteristics of subjects are summarized in supplemental Table S1. Six paired samples were obtained from sarcoidosis patients with disease progression, nine were from those with spontaneous remission and sixteen were from those with improvement by OCS administration. The associations between LPLs-producing enzymes and the transition of disease activity for sarcoidosis are summarized in Fig. 4.
Changes in the LPLs-producing enzymes before and after modification in the clinical status. The comparison of changes in the values of P-ATX (upper row), U-ATX (middle row) and PS-PLA1 (lower row) in paired-samples from the same patients with modification in their clinical status such as disease progression (a,d,g), spontaneous remission (b,e,h) and OCS administration (c,f,i). Data were analyzed by paired t-test. Bold denotes values p < 0.05.
Neither the P-ATX nor PS-PLA1 levels showed any meaningful change under disease progression and spontaneous remission (Fig. 4a,b,g,h). The U-ATX levels showed a significant increase after spontaneous remission (p = 0.03) and tended to decrease after disease progression, though the difference was not statistically significant (p = 0.11) (Fig. 4d,e).
After OCS administration, the P-ATX levels showed a significant decrease (p < 0.0001), while the PS-PLA1 levels didn’t show any change (Fig. 4c,i). Because OCS reduce the synthesis of ATX, this change in P-ATX would partly reflect the reduction of ATX synthesis, not only the sarcoidosis activity34. Notably, the U-ATX levels showed a significant increase even after spontaneous remission (p = 0.04), which was similar to the changes after OCS administration (Fig. 4f).
Discussion
Clinical characteristics and prognosis greatly differ in subjects with sarcoidosis. Although several biomarkers were investigated to predict the disease activity or prognosis, no reliable biomarker has been established5,35,36. In the present study, subjects with active sarcoidosis showed a significant decrease in U-ATX levels compared to those with inactive sarcoidosis (Fig. 1d). Additionally, the U-ATX levels tended to decrease after disease progression (Fig. 4d) and increased significantly after the disease activity improved both after spontaneous remission and OCS administration (Fig. 4e,f). These findings suggest U-ATX reflect the disease activity of sarcoidosis. To our knowledge, this is a first report demonstrating a meaningful association between the U-ATX values and the disease activity of sarcoidosis.
The values of U-ATX and PS-PLA1 stratified by the disease activities showed different patterns according to the gender group. The U-ATX levels in whole and female subjects showed significant differences between the active and inactive states, while those in male subjects showed only a non-significant tendency to decrease in the active state. We guess this is because the number of male subjects was relatively smaller than that in females. Another possibility is that the clinical features of sarcoidosis are known to differ according to gender. For example, in Japan, female subjects have a higher incidence than males and biphasic pattern of age-specific incidence29. Symptom and organ involvement are also different between males and females29. Therefore, we think one cannot exclude the possibility that the gender difference in clinical features of sarcoidosis affects the levels of LPLs-producing enzyme. Further studies with a larger population, especially in male subjects matched in age or other clinical features with females, will be necessary in the future.
The exact origin of U-ATX production has not been determined. A previous report indicated U-ATX might be associated with serum ATX, proteinuria and renal tubular injury33. However, we could not find any association between the P-ATX and U-ATX levels (Fig. 3c). In line with our data, it was also reported that it is difficult for the ATX molecule in blood to pass through the normal glomerular filtration barrier due to its high molecular weight13,37, although only patients with severe proteinuria (> 3.5 g/gCre) showed an association with the U-ATX concentration33. In the present study, most subjects did not have severe glomerular injury and U-ATX showed a weak positive correlation with eGFR (Tables 1, 2 and Fig. 3b). Taken together, it was thought that the U-ATX levels were independent of the P-ATX levels. On the other hand, it was also reported that the expression of ATX was detected in the renal cortex, specifically the proximal tubular epithelial cells and the podocytes in the glomeruli32. However, the values of urine NAG did not show any correlation with U-ATX (Fig. 3a). We cannot identify the exact origin of U-ATX production yet, and further basic studies are necessary to elucidate the origin of U-ATX in sarcoidosis.
The present study revealed that the U-ATX levels were significantly lower in an active state than in an inactive state (Fig. 1d, Table 1), while both the serum ACE and sIL-2R were significantly higher in an active state than in an inactive state. We could not find a significant association between these clinical biomarkers and the U-ATX levels in the multivariate logistic regression analysis (Table 3). Likewise, although the values of KL-6 and the presence of cardiac or eye involvement showed significant differences between active and inactive sarcoidosis, we could not find any relationship between these clinical parameters and the U-ATX levels (Data not shown). It was reported that ATX in the local body fluid is independently regulated by unknown mechanisms38. Therefore, we guess that there might be some mediators regulating the U-ATX levels and such mediators might affect systemic organs other than kidneys. Since the underlying mechanisms of U-ATX in the pathophysiology of sarcoidosis and its regulatory factors are unkown, further studies will be necessary.
While only female subjects with inactive sarcoidosis showed a significant decrease in the levels of PS-PLA1, neither P-ATX in all, males and females nor PS-PLA1 in all and males showed an association with disease activity of sarcoidosis (Fig. 1a–c,g–i). Although ATX was found in most biological fluids, such as plasma, urine, bronchoalveolar fluid (BALF), synovial fluid and cerebrospinal fluid, the major source of P-ATX is in adipose tissue31,39. Therefore, even if ATX production in localized tissue is changed by granulomatous inflammation, the change might be undetectable. In fact, a previous report showed that the changes in the ATX levels in synovial or cerebrospinal fluid are not accompanied by changes in the serum38, and suggested that the ATX in urine could be regulated in an independent manner. In line with this speculation, we revealed that both the plasma and urine ATX levels were not correlated with BMI, which reflects the volume of adipose tissue in the whole body. In the present study, most participants showed relatively low values of BMI, which were around 20 to 25. Because these lower values of BMI are not well associated with percent body fat40, it is still unclear whether the ATX levels are affected by the amount of adipose tissues or not. Both P-ATX and PS-PLA1 had significant but very weak associations with the clinically used biomarkers, ACE and sIL-2R (Fig. 2a,b,f). Because these associations were too weak to determine the clinical significance, further studies investigating the relationships between LPLs-producing enzymes and existing biomarkers are necessary. In mouse models of rheumatoid arthritis, ATX expression in synovial fibroblasts was stimulated by TNF, which is one of the crucial mediators for granuloma formation in sarcoidosis21. Therefore, even though the P-ATX levels showed no significant difference between active and inactive sarcoidosis in the present study, we couldn’t exclude the possibility that P-ATX and PS-PLA1 have some relationship with the pathophysiology of sarcoidosis. Further studies will be required to reveal whether the local expression of ATX or PS-PLA1 changes in the affected organ or tissue.
Concerning the measurement of ATX in the body fluids, there are two procedures for the evaluation. One is the quantity of antigen levels (mg/L in plasma and μg/g\(\cdot\)Cre in urine), the other is the enzymatic activity (mol/mL/min). Since the serum ATX concentration is strongly correlated with the serum lysophospholipase D activity, it would be a more convenient and accurate method for clinical use28. The automated immunoassay system used in this study enables us to handle numerous samples simultaneously, and the results are reported very rapidly, within 22 min28. Therefore, we measured the ATX levels by the automated immunoassay system subject to the daily clinical practice.
There are several limitations in this study that should be addressed in the future. First, the present study lacks data from healthy controls and we cannot compare the LPLs-producing enzyme levels of heathy subjects to those of patients with sarcoidosis. However, based on a previous report by one of our colleagues, we think that the U-ATX levels in inactive subjects are distributed in almost the same range as those in control subjects when evaluated by completely identical procedures33. These findings suggest that the U-ATX levels decrease abnormally in active sarcoidosis compared with those of healthy or inactive sarcoidosis, and that the U-ATX levels would be considered as a novel marker of disease activity of sarcoidosis. Second, the sample size was relatively small and the follow-up period may not have been long enough. Although many patients with sarcoidosis resolve spontaneously for the first several years, a significant proportion of patients experience progressive exacerbations and remissions for a long time. Third, this is a discovery cohort study and a validation cohort is required to confirm the validity of U-ATX as a biomarker. Further prospective studies with larger samples and healthy controls are necessary to predict the activity of sarcoidosis.
Conclusion
In conclusion, the present study suggested the U-ATX levels as a novel and useful molecule for assessing the disease activity of sarcoidosis in daily practice. The measurement of U-ATX is likely to be helpful for clinical settings, because it is easy, less invasive and repeatable. Further study is required to elucidate the involvement of ATX in the pathophysiology of sarcoidosis and to develop novel treatment strategies for sarcoidosis.
Methods
Patient Population
All patients were diagnosed with sarcoidosis according to the American Thoracic Society (ATS)/European Respiratory Society (ERS)/ World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) statement on sarcoidosis41. Patients with a history of recent (within 1 month before study enrolment) or ongoing OCS and/or immunosuppressants therapy were excluded, because these agents may affect the disease activity and OCS is also known to reduce the synthesis of ATX34. Likewise, patients with chronic hepatitis were excluded, because the serum ATX level is known to be elevated in patients with hepatic fibrosis15. The disease activity of sarcoidosis was evaluated as active if (1) the patient was newly diagnosed with clinical symptoms such as cough, fever, fatigue and joint pain, (2) the asymptomatic patient had worsening organ involvement during the last 3–6 months follow-up or (3) the number of affected organs associated with sarcoidosis increased during the last 3–6 months follow-up. Inactive sarcoidosis was defined as subjects without clinical symptoms, worsening organ involvement or novel affected organs for at least 3 months. These activities were determined by two independent pulmonologists who were blinded to the measurement of the LPLs-producing enzymes. Patients’ characteristics and medical information including age, sex, smoking status, laboratory findings and radiological imaging data were obtained from the patients’ medical chart.
Study design
This was a single-center, cross-sectional survey that targeted sarcoidosis patients. The ethics committee of Tohoku University Graduate School of Medicine approved this study (approval number, 2014-1-401) and all participants provided informed written consent. In total, 157 outpatients with sarcoidosis in Tohoku University Hospital were consecutively enrolled in this study from October 1, 2014 to December 31, 2018. The study was carried out in accordance with the Declaration of Helsinki.
Measurement of plasma ATX, urine ATX and plasma PS-PLA1 levels
Both plasma and urine samples were obtained from patients with sarcoidosis simultaneously and were immediately stored at −80 °C until use. The samples were collected twice or more from some patients whose disease activity had changed spontaneously or by OCS administration. The ATX and PS-PLA1 antigen levels were measured using specific 2-site enzyme immunoassay with TOSOH AIA system (TOSOH, Tokyo, Japan)28,33,42,43. Briefly, primary antibody coated with magnetic beads were placed in the reaction cup and alkaline phosphatase-labeled secondary antibody in assay buffer was added to the reaction cup. The ATX and PS-PLA1 assay reagent was prepared by a freeze-dry procedure of the reaction cap. All procedures, such as samples dispensation, incubation of reaction cap, washing and fluorometric detection, were automatically performed by an automated immunoassay analyzer (TOSOH AIA system). Because the ATX antigen levels in urine were lower than the lower limit of the standard ATX measurement, the U-ATX levels were measured using a slightly modified reagent containing a more sensitive anti-ATX monoclonal antibody as compared to the reagent used in the standard assay. The details and performances of the high-sensitive ATX measurement have been described by us previously33. On the other hand, the urinary PS-PLA1 levels were not measured because PS-PLA1 in the urine sample was not detectable in the preliminary data.
Statistical analysis
Data of age, ACE, sIL-2R, KL-6 and pulmonary function were expressed as median and interquartile range (IQR, [Q1-Q3]). The clinical characteristics were compared using Mann–Whitney U-test or Fisher’s exact test. Using a Spearman’s rank test, univariate correlations analyses were assessed, and multivariate logistic regression analyses were performed to investigate the relationship between several biomarkers and LPLs-producing enzymes. Statistical significance was accepted as p < 0.05. All statistical analyses were performed using JMP Pro 14 software (SAS Institute, Inc, Cary, NC, USA).
Data availability
The datasets generated during and/or analysed during the present study are available from the corresponding author on reasonable request.
References
Hunninghake, G. W. et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis. Vasc. Diffuse Lung Dis. 16, 149–173 (1999).
Iannuzzi, M. C., Rybicki, B. A. & Teirstein, A. S. Sarcoidosis. N. Engl. J. Med. 357, 2153–2165. https://doi.org/10.1056/NEJMra071714 (2007).
Nagai, S. et al. Outcome of sarcoidosis. Clin. Chest Med. 29(565–574), x. https://doi.org/10.1016/j.ccm.2008.03.006 (2008).
Iannuzzi, M. C. & Fontana, J. R. Sarcoidosis: Clinical presentation, immunopathogenesis, and therapeutics. JAMA 305, 391–399. https://doi.org/10.1001/jama.2011.10 (2011).
Chopra, A., Kalkanis, A. & Judson, M. A. Biomarkers in sarcoidosis. Expert. Rev. Clin. Immunol. 12, 1191–1208. https://doi.org/10.1080/1744666X.2016.1196135 (2016).
Yatomi, Y. et al. Lysophospholipids in laboratory medicine. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 94, 373–389. https://doi.org/10.2183/pjab.94.025 (2018).
Aikawa, S., Hashimoto, T., Kano, K. & Aoki, J. Lysophosphatidic acid as a lipid mediator with multiple biological actions. J. Biochem. 157, 81–89. https://doi.org/10.1093/jb/mvu077 (2015).
Chun, J., Hla, T., Lynch, K. R., Spiegel, S. & Moolenaar, W. H. International Union of Basic and Clinical Pharmacology. LXXVIII. Lysophospholipid receptor nomenclature. Pharmacol. Rev. 62, 579–587. https://doi.org/10.1124/pr.110.003111 (2010).
Hecht, J. H., Weiner, J. A., Post, S. R. & Chun, J. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J. Cell Biol. 135, 1071–1083. https://doi.org/10.1083/jcb.135.4.1071 (1996).
Aoki, J., Inoue, A. & Okudaira, S. Two pathways for lysophosphatidic acid production. Biochim Biophys Acta 1781, 513–518. https://doi.org/10.1016/j.bbalip.2008.06.005 (2008).
Choi, J. W. et al. LPA receptors: subtypes and biological actions. Annu. Rev. Pharmacol. Toxicol. 50, 157–186. https://doi.org/10.1146/annurev.pharmtox.010909.105753 (2010).
Skoura, A. & Hla, T. Lysophospholipid receptors in vertebrate development, physiology, and pathology. J. Lipid Res. 50(Suppl), S293-298. https://doi.org/10.1194/jlr.R800047-JLR200 (2009).
Umezu-Goto, M. et al. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J. Cell Biol. 158, 227–233. https://doi.org/10.1083/jcb.200204026 (2002).
Magkrioti, C. et al. Autotaxin and chronic inflammatory diseases. J. Autoimmun. 104, 102327. https://doi.org/10.1016/j.jaut.2019.102327 (2019).
Nakagawa, H. et al. Autotaxin as a novel serum marker of liver fibrosis. Clin. Chim. Acta 412, 1201–1206. https://doi.org/10.1016/j.cca.2011.03.014 (2011).
Ninou, I., Magkrioti, C. & Aidinis, V. Autotaxin in pathophysiology and pulmonary fibrosis. Front. Med. (Lausanne) 5, 180. https://doi.org/10.3389/fmed.2018.00180 (2018).
Park, G. Y. et al. Autotaxin production of lysophosphatidic acid mediates allergic asthmatic inflammation. Am. J. Respir. Crit. Care Med. 188, 928–940. https://doi.org/10.1164/rccm.201306-1014OC (2013).
Valdes-Rives, S. A. & Gonzalez-Arenas, A. Autotaxin-lysophosphatidic acid: From inflammation to cancer development. Mediators Inflamm. 2017, 9173090. https://doi.org/10.1155/2017/9173090 (2017).
Kanda, H. et al. Autotaxin, an ectoenzyme that produces lysophosphatidic acid, promotes the entry of lymphocytes into secondary lymphoid organs. Nat. Immunol. 9, 415–423. https://doi.org/10.1038/ni1573 (2008).
Zhang, Y., Chen, Y. C., Krummel, M. F. & Rosen, S. D. Autotaxin through lysophosphatidic acid stimulates polarization, motility, and transendothelial migration of naive T cells. J. Immunol. 189, 3914–3924. https://doi.org/10.4049/jimmunol.1201604 (2012).
Nikitopoulou, I. et al. Autotaxin expression from synovial fibroblasts is essential for the pathogenesis of modeled arthritis. J. Exp. Med. 209, 925–933. https://doi.org/10.1084/jem.20112012 (2012).
Iwata, Y. et al. Higher serum levels of autotaxin and phosphatidylserine-specific phospholipase A1 in patients with lupus nephritis. Int. J. Rheum. Dis. https://doi.org/10.1111/1756-185X.14031 (2020).
Kurano, M. et al. Association between serum autotaxin or phosphatidylserine-specific phospholipase A1 levels and melanoma. J. Dermatol. 45, 571–579. https://doi.org/10.1111/1346-8138.14278 (2018).
Nakawatari, K. et al. Elevated phosphatidylserine-specific phospholipase A1 level in hyperthyroidism. Clin. Chim. Acta 503, 99–106. https://doi.org/10.1016/j.cca.2020.01.011 (2020).
Sawada, T. et al. Serum phosphatidylserine-specific phospholipase A1 as a novel biomarker for monitoring systemic lupus erythematosus disease activity. Int. J. Rheum. Dis. 22, 2059–2066. https://doi.org/10.1111/1756-185X.13689 (2019).
Bellini, F. & Bruni, A. Role of a serum phospholipase A1 in the phosphatidylserine-induced T cell inhibition. Febs. Lett. 316, 1–4. https://doi.org/10.1016/0014-5793(93)81724-e (1993).
Hosogaya, S. et al. Measurement of plasma lysophosphatidic acid concentration in healthy subjects: strong correlation with lysophospholipase D activity. Ann. Clin. Biochem. 45, 364–368. https://doi.org/10.1258/acb.2008.007242 (2008).
Nakamura, K. et al. Validation of an autotaxin enzyme immunoassay in human serum samples and its application to hypoalbuminemia differentiation. Clin. Chim. Acta 388, 51–58. https://doi.org/10.1016/j.cca.2007.10.005 (2008).
Morimoto, T. et al. Epidemiology of sarcoidosis in Japan. Eur Respir J 31, 372–379. https://doi.org/10.1183/09031936.00075307 (2008).
Tamada, T. et al. The clinical features of patients with sarcoidosis and malignant diseases in Japan. Intern. Med. 60, 209–216. https://doi.org/10.2169/internalmedicine.5441-20 (2021).
Dusaulcy, R. et al. Adipose-specific disruption of autotaxin enhances nutritional fattening and reduces plasma lysophosphatidic acid. J. Lipid Res. 52, 1247–1255. https://doi.org/10.1194/jlr.M014985 (2011).
Zhang, M. Z. et al. Lysophosphatidic acid receptor antagonism protects against diabetic nephropathy in a type 2 diabetic model. J. Am. Soc. Nephrol. 28, 3300–3311. https://doi.org/10.1681/ASN.2017010107 (2017).
Morita, Y. et al. Urinary autotaxin concentrations are associated with kidney injury. Clin. Chim. Acta 509, 156–165. https://doi.org/10.1016/j.cca.2020.06.019 (2020).
Sumida, H. et al. Decrease in circulating autotaxin by oral administration of prednisolone. Clin. Chim. Acta 415, 74–80. https://doi.org/10.1016/j.cca.2012.10.003 (2013).
Danila, E., Jurgauskiene, L. & Malickaite, R. BAL fluid cells and pulmonary function in different radiographic stages of newly diagnosed sarcoidosis. Adv. Med. Sci. 53, 228–233. https://doi.org/10.2478/v10039-008-0014-z (2008).
Paone, G. et al. Use of discriminant analysis in assessing pulmonary function worsening in patients with sarcoidosis by a panel of inflammatory biomarkers. Inflamm. Res. 62, 325–332. https://doi.org/10.1007/s00011-012-0585-9 (2013).
Stracke, M. L. et al. Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J. Biol. Chem. 267, 2524–2529 (1992).
Nakamura, K. et al. Autotaxin enzyme immunoassay in human cerebrospinal fluid samples. Clin. Chim. Acta 405, 160–162. https://doi.org/10.1016/j.cca.2009.04.025 (2009).
Barbayianni, E., Kaffe, E., Aidinis, V. & Kokotos, G. Autotaxin, a secreted lysophospholipase D, as a promising therapeutic target in chronic inflammation and cancer. Prog. Lipid Res. 58, 76–96. https://doi.org/10.1016/j.plipres.2015.02.001 (2015).
Meeuwsen, S., Horgan, G. W. & Elia, M. The relationship between BMI and percent body fat, measured by bioelectrical impedance, in a large adult sample is curvilinear and influenced by age and sex. Clin. Nutr. 29, 560–566. https://doi.org/10.1016/j.clnu.2009.12.011 (2010).
Crouser, E. D. et al. Diagnosis and detection of sarcoidosis: An official American thoracic society clinical practice guideline. Am. J. Respir. Crit. Care Med. 201, 26–51. https://doi.org/10.1164/rccm.202002-0251ST (2020).
Honjo, M. et al. Role of the autotaxin-LPA pathway in dexamethasone-induced fibrotic responses and extracellular matrix production in human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 59, 21–30. https://doi.org/10.1167/iovs.17-22807 (2018).
Nakamura, K. et al. A novel enzyme immunoassay for the determination of phosphatidylserine-specific phospholipase A(1) in human serum samples. Clin. Chim. Acta 411, 1090–1094. https://doi.org/10.1016/j.cca.2010.04.006 (2010).
Acknowledgements
The authors gratefully acknowledged Mr. Brent K. Bell for reading the manuscript. This research was funded by Japan Society for Science and Promotion of Science (No.15K19410 for K.M. and No.18K08137 for T.T.) and GSK Japan Research Grant 2017 (E.M.).
Author information
Authors and Affiliations
Contributions
K.M.: study design, samples collection, analysis, interpretation of the data and drafting of the manuscript. T.T.: study concept and design, sample collection, analysis, interpretation of the data and drafting of the manuscript. D.S.: study design and interpretation of the data. E.M.: interpretation of the data. M.N.: sample collection. M.I.: study supervision. M.K.: acquisition of the data. Y.Y.: interpretation of the data. H.S.: study supervision. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Murakami, K., Tamada, T., Saigusa, D. et al. Urine autotaxin levels reflect the disease activity of sarcoidosis. Sci Rep 12, 4372 (2022). https://doi.org/10.1038/s41598-022-08388-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-08388-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.