Abstract
An investigation of new ways to activate brown adipose tissue (BAT) is highly valuable, as it is a possible tool for obesity prevention and treatment. The aim of our study was to evaluate the relationships between dietary intake and BAT activity. The study group comprised 28 healthy non-smoking males aged 21–42 years. All volunteers underwent a physical examination and 75-g OGTT and completed 3-day food intake diaries to evaluate macronutrients and fatty acid intake. Body composition measurements were assessed using DXA scanning. An FDG-18 PET/MR was performed to visualize BAT activity. Brown adipose tissue was detected in 18 subjects (67% normal-weight individuals and 33% overweight/obese). The presence of BAT corresponded with a lower visceral adipose tissue (VAT) content (p = 0.04, after adjustment for age, daily kcal intake, and DXA Lean mass). We noted significantly lower omega-6 fatty acids (p = 0.03) and MUFA (p = 0.02) intake in subjects with detected BAT activity after adjustment for age, daily average kcal intake, and DXA Lean mass, whereas omega-3 fatty acids intake was comparable between the two groups. BAT presence was positively associated with the concentration of serum IL-6 (p = 0.01) during cold exposure. Our results show that BAT activity may be related to daily omega-6 fatty acids intake.
Similar content being viewed by others
Introduction
Obesity and related metabolic complications are rising at a disturbingly fast rate worldwide1. Despite commonly known factors leading to weight gain, such as excessive food intake, a sedentary lifestyle, and the availability of pharmacological agents, the treatment of obesity remains challenging and is often unsuccessful. Obesity results in metabolic abnormalities, type 2 diabetes, and an increased risk of cardiometabolic complications2. Considering its negative effects on individuals, it is crucial to find new mechanisms to combat obesity.
Brown adipose tissue (BAT) has recently undergone investigation due to its unique ability to generate heat instead of storing energy3. In brown adipose tissue, lipids are packed in small droplets, unlike white adipose tissue (WAT), where triglycerides are stored in one large droplet inside the cell. BAT also has an abundance of large mitochondria and is more densely vascularized and innervated by the sympathetic nervous system compared to WAT4. Brown adipose tissue was initially thought to be present only in infants and children to maintain their core body temperature, but studies have also confirmed its presence in adults5—more commonly in lean individuals than in obese individuals6. The most known activators of brown adipose tissue are cold exposure and the agonist of the sympathetic nervous system (SNS)7,8. Brown adipose tissue can increase energy expenditure related to the function of unique uncoupling protein 1 (UCP1)4, which is considered to be a BAT indicator. A lower ambient temperature triggers the peripheral nervous system. Norepinephrine, released by the sympathetic nerve endings, induces intracellular triglyceride (TG) lipolysis during cold exposure. Brown adipocytes consume fatty acids and partially glucose, UCP1 is activated, and the process of non-shivering thermogenesis is initiated9.
In consideration of the uncomfortable and challenging aspects of cooling as well as the negative cardiovascular consequences of the pharmacological activation of SNS, the use of food ingredients is seemingly one of the more feasible methods of BAT activation10. Cold exerts its stimulatory effect on BAT through transient receptor potential channels, most of which are also receptors for various food products, such as capsaicin and its analogs, vanilloid11,12, which are representative agonists of the transient receptor potential vanilloid 113.
The exceptional function of brown adipose tissue to increase energy expenditure is reflected in its protective role against obesity and type 2 diabetes mellitus14,15. Although the amount of BAT is reduced in obese individuals, as well as it is not present or not detectable in some other individuals, its activation in adults is viewed as a means to treat obesity. The thermogenic effects, effectiveness, as well as safety of some of these ways of BAT activation must be considered, and the risk and benefits balance need further investigation. The impact of dietary factors on BAT activity is still not clear16. However, some dietary ingredients, which seem to be safe and without any serious adverse effects, may impact BAT activation. The experimental studies with tea catechins have shown an increase in energy expenditure17 which suggests that tea catechins can activate and recruit BAT in humans. Also, direct stimulation of BAT activity (measured by imaging methods such as FDG-PET) was observed after the acute or chronic consumption of other bioactives in humans18,19,20. Interesting seems to be recently published data that provided clinical evidence for the impact of monounsaturated fatty acids (MUFA) on BAT activity. After 4 weeks of dietary intervention with olive oil, a significant increase of blood monounsaturated fatty acid levels was accompanied by increased BAT activity in lean but not in overweight/obese volunteers21. Some previously published paper also showed the favorable influence of polyunsaturated fatty acids (PUFA) omega-3 supplementation on BAT activity with beneficial effect on glucose homeostasis and insulin sensitivity22,23,24. A Mediterranean diet, which is in general rich in unsaturated fatty acids, has a beneficial effect on human health through several mechanisms, with the lipid-lowering effect as the most prominent25. The fatty acids are the main source of fuel used by activated brown adipocytes26. Therefore, their daily intake may be associated with BAT metabolism and activity, which was the hypothesis of our preliminary study. Another ambiguous question regards the role of the interleukin-6 (IL-6) in BAT activity. Studies in experimental animals indicate that IL-6 in the central nervous system partly mediates the suppression of food intake and may influence body weight27. Very interesting is also observation that concentration of IL-6 may be modulated by dietary consumption of mono- and polyunsaturated fatty acids28,29,30,31,32,33,34. The interleukin 6, mainly known as a proinflammatory cytokine and an anti-inflammatory myokine, seems to affect the activation of brown adipocytes35. Therefore, also this possible association between BAT and IL-6 concentration was tested in our study.
The aim of our study was to evaluate the activity of BAT in healthy males and the potential associations between BAT, resting energy expenditure, IL-6 concentration, and dietary intake.
Results
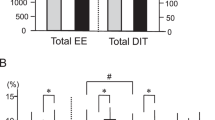
In the studied group, brown adipose tissue was detected in 18 volunteers (BAT-positive) (Table 1), with a mean age of 24 years and a mean BMI of 25 kg/m2. A total of 67% of BAT-positive subjects had normal BMI, whereas 33% were overweight or obese. In 10 volunteers who underwent a 2 h cold exposure test, we did not observe the activity of brown adipose tissue in PET/MR images. These subjects were included in the BAT negative group (BAT-negative) (Figs. 1, 2).
In the BAT-positive group, the mean volume of brown adipose tissue was estimated as 26 355 ± 43 202 mm3 and the mean activity of BAT was 19.1 ± 4.3 µmol × (100 g−1) × min−1. We noticed a lower mass and volume of visceral adipose tissue (p = 0.02, after adjustment for age, daily kcal intake, and DXA lean mass) in the BAT-positive group, as well as a lower percentage of VAT content (p = 0.007, after adjustment for age, daily average kcal intake, and DXA lean mass). No significant association was found linking BAT volume and 18F-FDG uptake with fat-free mass (FFM) (p = 0.57) and lean mass (lean mass) (p = 0.54). We did not observe any differences between studied groups in FFM, lean mass, fat mass, and OGTT results (data not shown).
We also observed that omega-6 fatty acids intake and MUFA intake was significantly lower in BAT-positive subjects (p = 0.03 and p = 0.02, respectively, Table 1) (Figs. 3, 4), compared to BAT-negative, after adjustment for age, daily average kcal intake, and DXA Lean Mass, whereas omega-3 fatty acids intake did not differ significantly between the studied groups. We did not discover any differences between groups BAT-positive and BAT-negative in the total kcal energy intake and consumption of carbohydrates, protein, and fat (data not presented). Also, BAT-positive group was characterized by a higher concentration of IL-6 during 2 h cold exposure (p = 0.01 after adjustment for age, daily average kcal intake, and DXA Lean mass) (Fig. 5).
Multiple linear regression models were applied to test independent determinants of the BAT-positive group. Generally, the presence of brown adipose tissue was negatively associated with omega-6 fatty acids intake (Est. − 2.96, R2 = 0.54, p = 0.003, after adjustment for age, daily kcal intake, and DXA Lean Mass), PUFA intake (Est. − 2.13, R2 = 0.39, p = 0.05), and MUFA intake (Est. − 7.42, R2 = 0.55, p = 0.03, after adjustment for age, daily average kcal intake, and DXA Lean mass). It was positively associated with the concentration of serum IL-6 (Est. 330,64, R2 = 0.32, p = 0.05, after adjustment for age, daily kcal intake, and DXA Lean mass) during cold exposure.
The BAT volume was positively associated with omega-3 fatty acids intake (Est. 0.00001, R2 = 0.69, p = 0.00007) and long-chain PUFA intake (Est. 0.0001, R2 = 0.38, p = 0.0005). It was negatively associated with an omega-6/omega-3 fatty acids ratio (Est. − 0.00002, R2 = 0.53, p = 0.001, after adjustment for age, daily kcal intake, and DXA Lean mass). We did not notice any differences in the AUC of REE measurements during cold exposure tests, except for the slight tendency (p = 0.07) of a higher Δ of REE between 0 and 120 min of the cold exposure test in BAT-positive subjects.
Discussion
In our study, we evaluated the relationship between dietary intake and the activity of brown adipose tissue in healthy males aged 21–42. Subjects in which the presence of BAT was found presented a lower daily consumption of MUFA and PUFA omega-6 fatty acids. Moreover, the BAT volume was related to higher omega-3 fatty acids intake, long-chain PUFA, and a lower ratio of omega-6/omega-3 fatty acids. A lower percentage of visceral adipose tissue was observed in subjects with detectable brown adipose tissue, along with a higher serum IL-6 concentration during the cold exposure test. This finding is consistent with previously published studies36. However, Monfort-Pires et al.21 reported that MUFA-rich olive intake induces the activation of BAT, which is in contrary to our observation; nevertheless, it has been noted only in lean subjects.
Our analysis of dietary omega-3 and omega-6 fatty acids intake produced valuable insight as well. Notably, omega-3 and omega-6 fatty acids belong to polyunsaturated fatty acids. The proportion between omega-3 and omega-6 fatty acids is crucial for health, and the proper ratio should be 1–4:1 of omega-6 to omega-3 fatty acids37. In all diets, especially Western diets, omega-6 fatty acids cover the majority of PUFAs food supply. Moreover, dietary changes over recent decades have resulted in a significant increase in the intake of omega-6 fatty acids, thus altering the omega-6 to omega-3 fatty acids ratio to around ~ 15:138. An imbalance in the omega-6/omega-3 fatty acids ratio may enhance an immune response. Omega-3 fatty acids are considered to be an anti-inflammatory agents, while omega-6 fatty acids have an opposite function. Derivatives from both polyunsaturated fatty acids—eicosapentaenoic acid (EPA), which belongs to omega-3 fatty acids, and arachidonic acid (ARA), which belongs to omega-6 fatty acids—compete for the same enzymes in the prostaglandin biosynthesis39. The long-chain omega-3 fatty acids depressed the production of proinflammatory prostaglandin40, while the omega-6 fatty acids are known for their proinflammatory properties. ARA can be metabolized to prostaglandins (A2, E2, I2, and thromboxane A2) by cyclooxygenases-2 (COX-2), while leukotrienes (B4, C4, and E4) are biosynthesized from ARA by lipoxygenases (5-LOX)41. The influence of omega-3 and omega-6 fatty acids on the immune system has been widely surveyed42. PUFA show also a significant impact on the regulation of adipocyte differentiation and their function. The beneficial role of the omega-6 and omega-3 fatty acids and long chain PUFA dietary intake on adipose tissue development and function has been already shown43 Diet enriched in particular in the omega-3 PUFA, may decrease adipose tissue content, however, the physiological and cellular effects of PUFA may depend on many factors, and it has been noted that omega-6 PUFAs may exert either an anti- or a proadipogenic effects44. Recently, studies on animal models reported that high-fat diets rich in PUFA affect the expression of uncoupling protein 1 mRNA in brown adipose tissue. The increase was more significant with the supplementation of omega-3 PUFA than with omega-6 PUFA45. In line with previous results, outcomes from the intervention study with supplementation with omega-3 long-chain PUFA showed that omega-3 fatty acids enhanced thermogenesis via the activation of brown adipose tissue (BAT)46,47. A recently published paper on metabolite profiling by liquid chromatography-mass spectrometry (LC–MS) in humans with detectable BAT showed a unique systemic PUFA and oxylipin profile with increased levels of anti-inflammatory omega-3 fatty acids48. The above-listed papers supported the relationship between PUFAs and brown adipose tissue. We did not notice any differences in omega-3 fatty acids intake between subjects with and without identified BAT activity, but the linear regression models showed that the BAT volume was positively associated with omega-3 fatty acids intake. Moreover, as mentioned above, the proper ratio between omega-6 and omega-3 fatty acids may also play a crucial role in metabolism49. A lower dietary omega-6/omega-3 fatty acids was noted to improve the thermogenic response of BAT and WAT under β3-adrenergic stimulation50. Based on our results, we can hypothesize that a lower amount of omega-6 fatty acids in a person’s diet may have a beneficial effect on brown adipose tissue activity, possibly due to the enzyme competition between omega-3 and omega-6 fatty acids, which need further investigation. Dietary omega-6 fatty acids intake could be one of the potential mechanisms underlying the activity of brown adipose tissue.
PUFA and their metabolites may have an impact not only on the BAT activity but on the conversion of white into brite adipocytes as well51. It was noted that diets rich in ARA favor WAT formation by preventing the “browning” process52. However, recently published results suggest no effect of dietary fish oil supplementation on the recruitment of brown and brite adipocytes in mice or humans under thermoneutral conditions53. The thermoneutral conditions could be an explanation of noted conflicting results, since different circulating PUFA and oxylipins (being the lipid mediators produced from PUFA) profiles in BAT-positive and BAT-negative subjects were noted54 and cold exposure significantly increased plasma lipid composition only in BAT-positive individuals, strongly supporting the relationship between BAT and PUFA. The presence of BAT was also characterized by increased concentrations of omega-3 fatty acids and their precursor molecules54.
We did not notice any differences between the studied groups in regard to total energy and macronutrient intake. Our results are in line with Sanchez-Delgato et al.’s study in which associations between BAT volume or 18F-FDG uptake and energy intake, assessed via either the ad libitum meal or the habitual dietary intake, were not observed55.
In our study, we also observed that subjects with detectable brown adipose tissue are characterized by lower visceral adipose tissue and lower BMI. The fact that individuals with identified BAT activity were significantly younger could also affect this observation. Nevertheless, our results are in line with Matsushita’s study, which similarly reported that subjects with BAT are younger and have less abdominal fat56. Several studies observed a relationship between body composition, adiposity-related parameters, such as BMI, central body fat distribution, and BAT, thus indicating a reduced amount of brown adipose tissue in obese subjects57,58,59. Obesity is characterized by a chronic low-grade inflammatory state in adipose tissue maintained by the secretion of a wide range of inflammatory proteins. Systemic inflammation, especially TNF alfa, suppresses the thermogenic activity of brown fat’s capacity to reduce energy expenditure60. Moreover, data suggested its contribution to the whitening of BAT that occurs after the prolonged consumption of high-fat foods61. In the 52-week-old insulin receptor knockout mice, a significant decrease of BAT mass was observed with a significant increase of visceral WAT mass compared to 33-week-old mice62.
We observed a slight tendency of higher Δ of the resting energy expenditure (REE) between baseline and 120 min of the cold exposure in BAT-positive subjects, but we did not observe any differences between BAT-positive and BAT-negative individuals in the REE during the cold exposure test. These results correspond with the outcomes of Orava et al.’s research59.
In our study, we noticed an increase of serum IL-6 during 2 h of cold exposure, which is in line with the results of other authors63. It may seem confusing, as IL-6 is known as a proinflammatory cytokine, while its effect and associations with BAT need more investigations since it is suggested that IL-6 may increase its activity64. The lack of IL-6 expression impaired the beneficial effects of BAT transplantation on metabolic health through the interaction with FGF2165. Additionally, IL-6 is indispensable for the induction of WAT browning in response to a cold environment66. Moreover, another anti-inflammatory role of interleukin-6 has also been shown. It is released by skeletal muscle in response to exercise and promotes insulin sensitivity67.
To the best of our knowledge, our study is one of the first to assess the relationship between daily nutrient intake assessed by the 3-day food diary and the activity of brown adipose tissue. The relationship between diet-induced thermogenesis has been evaluated in the interventional studies68,69,70. It is worth highlighting the significant outcomes from a systematic review (PROSPERO) and meta analyzes which showed no differences in standardized uptake value of BAT following a single meal or after 6 weeks of l-Arginine supplementation. Resting energy expenditure, however, was increased following a single meal and after supplementation of capsinoid and catechin when compared to a control condition16. The topic is still relevant and needs to be further investigated. The results from our study indicate an association between BAT volume/activity and omega-3 and omega-6 polyunsaturated fatty acids. Moreover, the results suggest that further attention should be directed toward the right balance between omega-6 and omega-3 fatty acids in brown adipose tissue activity. Researchers should evaluate whether polyunsaturated fatty acids directly influence the activation of BAT or if they indirectly do so through the beneficial effect of omega-3 fatty acids on body fat, weight loss, or the reduction of an inflammatory state71,72,73. Maintaining an adequate proportion of body fat with a normal body index may promote the activation of brown adipose tissue. Future studies should investigate how do omega-3 and omega-6 fatty acids activate BAT, if directly or through particular mechanisms.
It is worth to notice also, that previous research on BAT primarily used PET/CT as a tool for imaging human brown adipose tissue74. PET/MR is the preferred imaging source because of the lack of ionizing radiation, feasibility, and higher spatial resolution. PET/MR imaging has previously been used to detect the presence of BAT in adults as well as in children75,76.
Our study has some limitations. The major limitation is a relatively small sample size, but the number of subjects enrolled in the study is comparable to the previous survey77. The main reason of limitation for conducting a large-scale trial is the high costs associated with PET/MR scanning and a tracer purchase. Therefore, if possible, our findings should be further tested in a larger population and different ethnic groups. The other important fact is that in our study, only the BAT glucose uptake was measured, and it is important to note that the main source of energy for BAT are fatty acids78,79. Therefore, we overlook the possibility that some of the BAT-negative subjects, defined by the glucose rate, might have a significant fatty acid uptake by the BAT tissue. Moreover, FDG allow to analyze and to localize BAT but it could be less informative for BAT activity. Indeed, BAT thermogenic activity, as mentioned above, is mainly due to fatty acid oxidation and uptake77. Because of the difficulties associated with obtaining a tracer to investigate fatty acids metabolism in humans, we used 18F-FDG in our study. Moreover, brown adipocytes are interspersed within white adipose tissue. Therefore, through PET detection, BAT regions could contain both, BAT and some white adipocytes57. It is also possible that the cooling was not optimal for some of the subjects, especially for those who were obese, thus resulting in false-negative results related to BAT activity. In our study, the water-perfused blankets were used, and the many cold exposures to large skin areas, such as via water-perfused suits or vests, seem to demonstrate minor variation in BAT activation80,81, what also should be considered.
Conclusions
In conclusion, we noted lower visceral fat accumulation in subjects with identified BAT, which confirms the protective role of brown adipose tissue and indicates that BAT shows strong potential as a means to combat obesity and its metabolic consequences. Moreover, our results suggest that both, dietary MUFA, as well as omega-3 and omega-6 fatty acods intake, may be associated with the volume and activity of BAT in healthy males aged 21–42, which deserve further investigations.
Materials and methods
Study participants
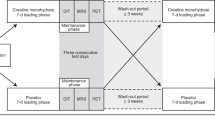
The study group comprised of 28 healthy, non-smoking Caucasian males aged 21–42 years (mean age 26.75 ± 5.11 years old). Sixteen participants had normal body weight (BMI < 25 kg/m2) and 12 were obese/overweight (BMI > 25 kg/m2). Volunteers to this study were recruited from the other cohort study group, described in detail previously82,83. The participants in this study were without any comorbidities (e.g., hypo- or hyperthyroidism, asthma, cardiovascular disease, renal or liver failure, and any acute or chronic diseases) and were not taking any medications (e.g., beta-blockers) or dietary supplements that could have had an impact on the results. Outside and shift workers were excluded from the study as well. Subjects were enrolled in the study, and all study procedures were performed during the October–April periods of 2016–2018.
Screening of subjects
During the screening visit, the medical history and metabolic status of all volunteers were reviewed. They underwent a physical examination, routine laboratory tests (hematology, TSH, creatinine, liver enzymes, Na, K, CRP), blood pressure measurement, an electrocardiogram (ECG), and an oral glucose tolerance test (OGTT). The OGTTs were performed according to World Health Organization (WHO) recommendations, with a 75-g glucose load.
Dietary assessments
All subjects completed a 3-day food diary. Subjects were asked to compare their portion sizes with each portion size’s color photographs from “Album of Photographs of Food Products and Dishes” developed by the National Food and Nutrition Institute84 and weigh food, if possible. Subjects were asked to record the amount and the type of fats and oils used for cooking as well. Daily total energy, macronutrients, monounsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA), and omega-3 and omega-6 fatty acids intake were analyzed using Dieta 6 software (National Food and Nutrition Institute, Warsaw, Poland), which was developed and which is continuously updated by the National Food and Nutrition Institute (Warsaw, Poland). This software is used to calculate the nutritional value of food and diets based on tables of the nutritional value of local food products and dishes and is commonly used to evaluate the fatty acid dietary intake85.
Anthropometric measurements
The body height and weight of participants were measured using a standardized method. Bodyweight was measured in a standard way (InBody 220, Biospace, Korea). Body composition and body fat distribution measurements were assessed using DXA scanning (enCORE™, iDXA Lunar GE Healthcare). In further analyses, the following parameters were evaluated: visceral adipose tissue mass (VAT mass), visceral adipose tissue volume (VAT volume), the visceral adipose tissue percentage of body weight (VAT BW %), the visceral adipose tissue percentage of adipose tissue (VAT AT%), the android fat to gynoid fat ratio (DXA A/G ratio), free fat mass (FFM), and lean mass (Lean mass).
Cold exposure and PET/MR scanning
During the second visit, in the fasting state, all volunteers underwent 2 h of cold exposure. Water perfused blankets were used as part of the applied protocol for cooling. Blood samples were also taken in the 60th and 120th min of cooling. After this procedure, a fluorodeoxyglucose F 18 injection (18F-FDG) (4 MBq/kg of body mass) was given, and a PET/MRI scan (Biograph mMR 3 T, Siemens Healthcare, Erlangen, Germany) of the whole body was performed during the autumn and winter periods.
Regions of interest (ROIs) were manually outlined in fusion images composed of a summed dynamic 18F-FDG PET image and magnetic resonance (MR). The software Carimas, developed at the Turku PET Centre in Finland, was applied for the image analyses. ROIs were drawn in image planes with a defined structure of brown adipose tissue and in the aortic arch in the time frame with the highest first-pass concentration of the tracer. Regional time-activity curves (TACs) were generated, and glucose uptake rate data for the regions were assessed. The influx rate constant (Ki) of FDG-F18 for BAT was determined using the Gjedde-Patlak model. A lumped constant (LC) value of 1.1486 was used for all adipose tissues. The glucose uptake rate was calculated as follows: plasma glucose concentration × Ki × LC−1. The activation of BAT was defined as a glucose uptake rate higher than 2.0 µmol × (100 g−1) × min−1, which was chosen after a visual interpretation of PET images and the determination of the BAT glucose uptake rate at warm conditions, where it was always lower than 1.7 µmol × (100 g−1) × min−187. Individuals in which BAT was detected were matched to the BAT positive group (BAT-positive), while subjects without detectable BAT in PET/MR images were classified as BAT negative (BAT-negative).
Resting metabolic rate measurements
During the cold exposure, whole-body resting energy expenditure (REE) was assessed using a computed open-circuit indirect calorimetry method based on the consumption of O2 and the production of CO2. The 30-min long measurements of resting oxygen uptake and resting carbon dioxide production were performed using a ventilated canopy Vmax Encore 29n system (Viasys HealthCare, Yorba Linda, CA, USA) at the baseline (− 30 to 0 min) and every 30 min until 120 min of cold exposure.
Blood collection and biochemical measurements
During the cold exposure, blood samples were collected and stored at − 60 °C for further analyses. The serum IL-6 concentration was determined using an enzyme-linked immunosorbent assay (ELISA) (ELISA Kit for Human Interleukin 6 (Human IL-6); R&D Systems, Inc., Minneapolis, MN 55413, Canada, D6050) according to the manufacturer’s protocol and based on observing the principles of internal laboratory control for the performed determinations. The serum glucose level was measured using the colorimetric methods of the Cobas c111 analyzer (Roche Diagnostics, Basel, Switzerland). Samples and controls were measured in the same run using the blind analysis method.
Statistical analyses
Numerical data were summarized with a number of observations (N), arithmetic means, and standard deviations (SD). For categorical data, the number of observations and frequencies were presented. Study participants were divided into two groups based on the presence of brown adipose tissue: BAT-positive and BAT-negative. Continuous parameters were examined for normality with the Shapiro–Wilk test and thorough visual inspection. The homogeneity of variance across groups was studied using the Levene test. Non-parametric tests were used for response variables that failed these two tests. The differences between the selected responses and BAT groups were then compared using an analysis of variance (ANOVA) or the Kruskal–Wallis test for numerical variables, with, respectively, a Tukey or Dunn post hoc test with a Holm p-value adjustment (in case multiple pairwise tests were performed or when there were multiple grouping variables). In order to study the hypothesis that there is a significant association between the presence of brown adipose tissue and body composition, as well as the hypothesis that the average daily consumption of omega-3 and omega-6 fatty acids can significantly alter brown adipose tissue activation, we studied its association using multivariate linear regression models. In all two-group comparisons and regression models, an adjustment for age, daily average energy intake (kcal/day), and DXA lean mass) was made to eliminate the potential effect of the covariates. The statistical significance level was set at 0.05 for all two-sided tests. All calculations were prepared in R (R version 4.0.2)88.
Ethics
The local Ethics Committee of Medical University of Bialystok, Poland (R-I-002/233/2015) approved the study protocol, and written informed consent was obtained from all participants. The procedures were performed in accordance with the Helsinki Declaration of 1975 as revised in 1983.
References
Chooi, Y. C., Ding, C. & Magkos, F. The epidemiology of obesity. Metabolism 92, 6–10. https://doi.org/10.1016/j.metabol.2018.09.005 (2019).
Maliszewska, K., Adamska-Patruno, E. & Krętowski, A. The interplay between muscle mass decline, obesity, and type 2 diabetes. Pol. Arch. Intern. Med. 129, 809–816. https://doi.org/10.20452/pamw.15025 (2019).
Cinti, S. The role of brown adipose tissue in human obesity. Nutr. Metab. Cardiovasc. Dis. 16, 569–574. https://doi.org/10.1016/j.numecd.2006.07.009 (2006).
Cannon, B. & Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 84, 277–359. https://doi.org/10.1152/physrev.00015.2003 (2004).
Virtanen, K. A. et al. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 360, 1518–1525. https://doi.org/10.1056/NEJMoa0808949 (2009).
Worku, M. G., Seretew, W. S., Angaw, D. A. & Tesema, G. A. Prevalence and associated factor of brown adipose tissue: Systematic review and meta-analysis. Biomed. Res. Int. 2020, 9106976. https://doi.org/10.1155/2020/9106976 (2020).
van der Lans, A. A. et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J. Clin. Invest. 123, 3395–3403. https://doi.org/10.1172/JCI68993 (2013).
Lee, P. & Greenfield, J. R. Non-pharmacological and pharmacological strategies of brown adipose tissue recruitment in humans. Mol. Cell Endocrinol. 418(Pt 2), 184–190. https://doi.org/10.1016/j.mce.2015.05.025 (2015).
Boon, M. R. & van MarkenLichtenbelt, W. D. Brown adipose tissue: A human perspective. Handb. Exp. Pharmacol. 233, 301–319. https://doi.org/10.1007/164_2015_11 (2016).
Villarroya, F. & Vidal-Puig, A. Beyond the sympathetic tone: The new brown fat activators. Cell Metab. 17, 638–643. https://doi.org/10.1016/j.cmet.2013.02.020 (2013).
Yoneshiro, T., Aita, S., Kawai, Y., Iwanaga, T. & Saito, M. Nonpungent capsaicin analogs (capsinoids) increase energy expenditure through the activation of brown adipose tissue in humans. Am. J. Clin. Nutr. 95, 845–850. https://doi.org/10.3945/ajcn.111.018606 (2012).
Nirengi, S. et al. Assessment of human brown adipose tissue density during daily ingestion of thermogenic capsinoids using near-infrared time-resolved spectroscopy. J. Biomed. Opt. 21, 091305. https://doi.org/10.1117/1.JBO.21.9.091305 (2016).
Saito, M., Yoneshiro, T. & Matsushita, M. Activation and recruitment of brown adipose tissue by cold exposure and food ingredients in humans. Best Pract. Res. Clin. Endocrinol. Metab. 30, 537–547. https://doi.org/10.1016/j.beem.2016.08.003 (2016).
Chondronikola, M. et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 63, 4089–4099. https://doi.org/10.2337/db14-0746 (2014).
Villarroya, F., Cereijo, R., Villarroya, J. & Giralt, M. Brown adipose tissue as a secretory organ. Nat. Rev. Endocrinol. 13, 26–35. https://doi.org/10.1038/nrendo.2016.136 (2017).
Heenan, K. A. et al. Effects of nutrition/diet on brown adipose tissue in humans: A systematic review and meta-analysis. Nutrients https://doi.org/10.3390/nu12092752 (2020).
Nomura, S. et al. Tea catechins enhance the mRNA expression of uncoupling protein 1 in rat brown adipose tissue. J. Nutr. Biochem. 19, 840–847. https://doi.org/10.1016/j.jnutbio.2007.11.005 (2008).
Yoneshiro, T. et al. Recruited brown adipose tissue as an antiobesity agent in humans. J. Clin. Invest. 123, 3404–3408. https://doi.org/10.1172/JCI67803 (2013).
Sugita, J. et al. Grains of paradise (Aframomum melegueta) extract activates brown adipose tissue and increases whole-body energy expenditure in men. Br. J. Nutr. 110, 733–738. https://doi.org/10.1017/S0007114512005715 (2013).
Matsushita, M. et al. Kaempferia parviflora extract increases whole-body energy expenditure in humans: Roles of brown adipose tissue. J. Nutr. Sci. Vitaminol. (Tokyo) 61, 79–83. https://doi.org/10.3177/jnsv.61.79 (2015).
Monfort-Pires, M. et al. Short dietary intervention with olive oil increases brown adipose tissue activity in lean but not overweight subjects. J. Clin. Endocrinol. Metab. 106, 472–484. https://doi.org/10.1210/clinem/dgaa824 (2021).
Kim, M. et al. Fish oil intake induces UCP1 upregulation in brown and white adipose tissue via the sympathetic nervous system. Sci. Rep. 5, 18013. https://doi.org/10.1038/srep18013 (2015).
Pahlavani, M. et al. Eicosapentaenoic acid regulates brown adipose tissue metabolism in high-fat-fed mice and in clonal brown adipocytes. J. Nutr. Biochem. 39, 101–109. https://doi.org/10.1016/j.jnutbio.2016.08.012 (2017).
Kim, J. et al. Eicosapentaenoic acid potentiates brown thermogenesis through FFAR4-dependent up-regulation of miR-30b and miR-378. J. Biol. Chem. 291, 20551–20562. https://doi.org/10.1074/jbc.M116.721480 (2016).
Tosti, V., Bertozzi, B. & Fontana, L. Health benefits of the mediterranean diet: Metabolic and molecular mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 73, 318–326. https://doi.org/10.1093/gerona/glx227 (2018).
Shinde, A. B., Song, A. & Wang, Q. A. Brown adipose tissue heterogeneity, energy metabolism, and beyond. Front. Endocrinol. (Lausanne) 12, 651763. https://doi.org/10.3389/fendo.2021.651763 (2021).
Mishra, D. et al. Parabrachial interleukin-6 reduces body weight and food intake and increases thermogenesis to regulate energy metabolism. Cell Rep. 26, 3011-3026.e3015. https://doi.org/10.1016/j.celrep.2019.02.044 (2019).
Galland, L. Diet and inflammation. Nutr. Clin. Pract. 25, 634–640. https://doi.org/10.1177/0884533610385703 (2010).
Ravaut, G., Légiot, A., Bergeron, K. F. & Mounier, C. Monounsaturated fatty acids in obesity-related inflammation. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22010330 (2020).
Chrysohoou, C., Panagiotakos, D. B., Pitsavos, C., Das, U. N. & Stefanadis, C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: The ATTICA Study. J. Am. Coll. Cardiol. 44, 152–158. https://doi.org/10.1016/j.jacc.2004.03.039 (2004).
Li, K., Huang, T., Zheng, J., Wu, K. & Li, D. Effect of marine-derived n-3 polyunsaturated fatty acids on C-reactive protein, interleukin 6 and tumor necrosis factor α: A meta-analysis. PLoS One 9, e88103. https://doi.org/10.1371/journal.pone.0088103 (2014).
Paniagua, J. A. et al. Monounsaturated fat-rich diet prevents central body fat distribution and decreases postprandial adiponectin expression induced by a carbohydrate-rich diet in insulin-resistant subjects. Diabetes Care 30, 1717–1723. https://doi.org/10.2337/dc06-2220 (2007).
Devaraj, S., Kasim-Karakas, S. & Jialal, I. The effect of weight loss and dietary fatty acids on inflammation. Curr. Atheroscler. Rep. 8, 477–486. https://doi.org/10.1007/s11883-006-0023-y (2006).
Basu, A., Devaraj, S. & Jialal, I. Dietary factors that promote or retard inflammation. Arterioscler. Thromb. Vasc. Biol. 26, 995–1001. https://doi.org/10.1161/01.ATV.0000214295.86079.d1 (2006).
Severinsen, M. C. K. & Pedersen, B. K. Muscle-organ crosstalk: The emerging roles of myokines. Endocr. Rev. https://doi.org/10.1210/endrev/bnaa016 (2020).
Villarroya, J., Cereijo, R. & Villarroya, F. An endocrine role for brown adipose tissue?. Am. J. Physiol. Endocrinol. Metab. 305, E567-572. https://doi.org/10.1152/ajpendo.00250.2013 (2013).
Kim, S. C., Adesogan, A. T., Badinga, L. & Staples, C. R. Effects of dietary n-6:n-3 fatty acid ratio on feed intake, digestibility, and fatty acid profiles of the ruminal contents, liver, and muscle of growing lambs. J. Anim. Sci. 85, 706–716. https://doi.org/10.2527/jas.2006-289 (2007).
Patterson, E., Wall, R., Fitzgerald, G. F., Ross, R. P. & Stanton, C. Health implications of high dietary omega-6 polyunsaturated Fatty acids. J. Nutr. Metab. 2012, 539426. https://doi.org/10.1155/2012/539426 (2012).
Araujo, P. et al. The effect of omega-3 and omega-6 polyunsaturated fatty acids on the production of cyclooxygenase and lipoxygenase metabolites by human umbilical vein endothelial cells. Nutrients https://doi.org/10.3390/nu11050966 (2019).
Wall, R., Ross, R. P., Fitzgerald, G. F. & Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 68, 280–289. https://doi.org/10.1111/j.1753-4887.2010.00287.x (2010).
Saini, R. K. & Keum, Y. S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—a review. Life Sci. 203, 255–267. https://doi.org/10.1016/j.lfs.2018.04.049 (2018).
Calder, P. C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 45, 1105–1115. https://doi.org/10.1042/BST20160474 (2017).
Pisani, D. & Ailhaud, G. Involvement of polyunsaturated fatty acids in the control of energy storage and expenditure. Lipids Health 26, 9 (2019).
Madsen, L., Petersen, R. K. & Kristiansen, K. Regulation of adipocyte differentiation and function by polyunsaturated fatty acids. Biochim. Biophys. Acta 1740, 266–286. https://doi.org/10.1016/j.bbadis.2005.03.001 (2005).
Takahashi, Y. & Ide, T. Dietary n-3 fatty acids affect mRNA level of brown adipose tissue uncoupling protein 1, and white adipose tissue leptin and glucose transporter 4 in the rat. Br. J. Nutr. 84, 175–184 (2000).
You, M., Fan, R., Kim, J., Shin, S. H. & Chung, S. Alpha-linolenic acid-enriched butter promotes fatty acid remodeling and thermogenic activation in the brown adipose tissue. Nutrients https://doi.org/10.3390/nu12010136 (2020).
Bargut, T. C., Silva-e-Silva, A. C., Souza-Mello, V., Mandarim-de-Lacerda, C. A. & Aguila, M. B. Mice fed fish oil diet and upregulation of brown adipose tissue thermogenic markers. Eur. J. Nutr. 55, 159–169. https://doi.org/10.1007/s00394-015-0834-0 (2016).
Dieckmann, S. et al. Fatty acid metabolite profiling reveals oxylipins as markers of brown but not brite adipose tissue. Front. Endocrinol. (Lausanne) 11, 73. https://doi.org/10.3389/fendo.2020.00073 (2020).
Simopoulos, A. P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 56, 365–379. https://doi.org/10.1016/s0753-3322(02)00253-6 (2002).
Ghandour, R. A. et al. Impact of dietary ω3 polyunsaturated fatty acid supplementation on brown and brite adipocyte function. J. Lipid Res. 59, 452–461. https://doi.org/10.1194/jlr.M081091 (2018).
Kuda, O., Rossmeisl, M. & Kopecky, J. Omega-3 fatty acids and adipose tissue biology. Mol. Aspects Med. 64, 147–160. https://doi.org/10.1016/j.mam.2018.01.004 (2018).
Pisani, D. F. et al. The ω6-fatty acid, arachidonic acid, regulates the conversion of white to brite adipocyte through a prostaglandin/calcium mediated pathway. Mol. Metab. 3, 834–847. https://doi.org/10.1016/j.molmet.2014.09.003 (2014).
Maurer, S. F. et al. No effect of dietary fish oil supplementation on the recruitment of brown and brite adipocytes in mice or humans under thermoneutral conditions. Mol. Nutr. Food Res. 65, e2000681. https://doi.org/10.1002/mnfr.202000681 (2021).
Kulterer, O. C. et al. The presence of active brown adipose tissue determines cold-induced energy expenditure and oxylipin profiles in humans. J. Clin. Endocrinol. Metab. https://doi.org/10.1210/clinem/dgaa183 (2020).
Sanchez-Delgado, G. et al. Brown adipose tissue volume and 18F-fluorodeoxyglucose uptake are not associated with energy intake in young human adults. Am. J. Clin. Nutr. 111, 329–339. https://doi.org/10.1093/ajcn/nqz300 (2020).
Matsushita, M. et al. Impact of brown adipose tissue on body fatness and glucose metabolism in healthy humans. Int. J. Obes. (Lond.) 38, 812–817. https://doi.org/10.1038/ijo.2013.206 (2014).
Leitner, B. P. et al. Mapping of human brown adipose tissue in lean and obese young men. Proc. Natl. Acad. Sci. USA 114, 8649–8654. https://doi.org/10.1073/pnas.1705287114 (2017).
Vijgen, G. H. et al. Brown adipose tissue in morbidly obese subjects. PLoS One 6, e17247. https://doi.org/10.1371/journal.pone.0017247 (2011).
Orava, J. et al. Blunted metabolic responses to cold and insulin stimulation in brown adipose tissue of obese humans. Obesity (Silver Spring) 21, 2279–2287. https://doi.org/10.1002/oby.20456 (2013).
Lorenzo, M. et al. Insulin resistance induced by tumor necrosis factor-alpha in myocytes and brown adipocytes. J. Anim. Sci. 86, E94-104. https://doi.org/10.2527/jas.2007-0462 (2008).
Omran, F. & Christian, M. Inflammatory signaling and brown fat activity. Front. Endocrinol. (Lausanne) 11, 156. https://doi.org/10.3389/fendo.2020.00156 (2020).
Gómez-Hernández, A. et al. Brown fat lipoatrophy and increased visceral adiposity through a concerted adipocytokines overexpression induces vascular insulin resistance and dysfunction. Endocrinology 153, 1242–1255. https://doi.org/10.1210/en.2011-1765 (2012).
Burýsek, L. & Houstek, J. beta-Adrenergic stimulation of interleukin-1alpha and interleukin-6 expression in mouse brown adipocytes. FEBS Lett. 411, 83–86. https://doi.org/10.1016/s0014-5793(97)00671-6 (1997).
Li, G. et al. Induction of uncoupling protein 1 by central interleukin-6 gene delivery is dependent on sympathetic innervation of brown adipose tissue and underlies one mechanism of body weight reduction in rats. Neuroscience 115, 879–889. https://doi.org/10.1016/s0306-4522(02)00447-5 (2002).
Stanford, K. I. et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Invest. 123, 215–223. https://doi.org/10.1172/JCI62308 (2013).
Knudsen, J. G. et al. Role of IL-6 in exercise training- and cold-induced UCP1 expression in subcutaneous white adipose tissue. PLoS One 9, e84910. https://doi.org/10.1371/journal.pone.0084910 (2014).
Ikeda, S. I. et al. Exercise-induced increase in IL-6 level enhances GLUT4 expression and insulin sensitivity in mouse skeletal muscle. Biochem. Biophys. Res. Commun. 473, 947–952. https://doi.org/10.1016/j.bbrc.2016.03.159 (2016).
Kozak, L. P. Brown fat and the myth of diet-induced thermogenesis. Cell Metab. 11, 263–267. https://doi.org/10.1016/j.cmet.2010.03.009 (2010).
van Baak, M. A. Meal-induced activation of the sympathetic nervous system and its cardiovascular and thermogenic effects in man. Physiol. Behav. 94, 178–186. https://doi.org/10.1016/j.physbeh.2007.12.020 (2008).
Rothwell, N. J. & Stock, M. J. A role for brown adipose tissue in diet-induced thermogenesis. Nature 281, 31–35. https://doi.org/10.1038/281031a0 (1979).
Martínez-Fernández, L., Laiglesia, L. M., Huerta, A. E., Martínez, J. A. & Moreno-Aliaga, M. J. Omega-3 fatty acids and adipose tissue function in obesity and metabolic syndrome. Prostaglandins Other Lipid Mediat. 121, 24–41. https://doi.org/10.1016/j.prostaglandins.2015.07.003 (2015).
Kalupahana, N. S., Claycombe, K. J. & Moustaid-Moussa, N. (n-3) Fatty acids alleviate adipose tissue inflammation and insulin resistance: Mechanistic insights. Adv. Nutr. 2, 304–316. https://doi.org/10.3945/an.111.000505 (2011).
Albracht-Schulte, K. et al. Omega-3 fatty acids in obesity and metabolic syndrome: A mechanistic update. J. Nutr. Biochem. 58, 1–16. https://doi.org/10.1016/j.jnutbio.2018.02.012 (2018).
Steinberg, J. D., Vogel, W. & Vegt, E. Factors influencing brown fat activation in FDG PET/CT: A retrospective analysis of 15,000+ cases. Br. J. Radiol. 90, 20170093. https://doi.org/10.1259/bjr.20170093 (2017).
Johannesen, H. H. et al. Identification and characterization of human brown adipose tissue (BAT) content and metabolism in adults using [(18)F]-FDG PET/MR—a pilot study. EJNMMI Phys. 1, A68. https://doi.org/10.1186/2197-7364-1-S1-A68 (2014).
Gariani, K. et al. Hybrid PET/MRI as a tool to detect brown adipose tissue: Proof of principle. Obes. Res. Clin. Pract. 9, 613–617. https://doi.org/10.1016/j.orcp.2015.05.004 (2015).
Blondin, D. P. et al. Dietary fatty acid metabolism of brown adipose tissue in cold-acclimated men. Nat. Commun. 8, 14146. https://doi.org/10.1038/ncomms14146 (2017).
Ma, S. W. & Foster, D. O. Uptake of glucose and release of fatty acids and glycerol by rat brown adipose tissue in vivo. Can. J. Physiol. Pharmacol. 64, 609–614. https://doi.org/10.1139/y86-101 (1986).
Maliszewska, K. & Kretowski, A. Brown adipose tissue and its role in insulin and glucose homeostasis. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22041530 (2021).
Cypess, A. M. et al. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc. Natl. Acad. Sci. USA 109, 10001–10005. https://doi.org/10.1073/pnas.1207911109 (2012).
Haman, F. et al. Effect of cold exposure on fuel utilization in humans: Plasma glucose, muscle glycogen, and lipids. J. Appl. Physiol. 1985(93), 77–84. https://doi.org/10.1152/japplphysiol.00773.2001 (2002).
Maliszewska, K. et al. The role of muscle decline in type 2 diabetes development: A 5-year prospective observational cohort study. Nutrients https://doi.org/10.3390/nu11040834 (2019).
Bauer, W. et al. Dietary macronutrient intake may influence the effects of TCF7L2 rs7901695 genetic variants on glucose homeostasis and obesity-related parameters: A cross-sectional population-based study. Nutrients https://doi.org/10.3390/nu13061936 (2021).
Szponar, L., Wolnicka, K., Rychlik & E. Album of Photographs of Food Products and Dishes. (2011).
Bzikowska-Jura, A. et al. The concentration of omega-3 fatty acids in human milk is related to their habitual but not current intake. Nutrients https://doi.org/10.3390/nu11071585 (2019).
Virtanen, K. A. et al. Human adipose tissue glucose uptake determined using [(18)F]-fluoro-deoxy-glucose ([(18)F]FDG) and PET in combination with microdialysis. Diabetologia 44, 2171–2179. https://doi.org/10.1007/s001250100026 (2001).
Orava, J. et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab. 14, 272–279. https://doi.org/10.1016/j.cmet.2011.06.012 (2011).
Team, R. C. (2020).
Acknowledgements
We thank anonymous reviewers for their valuable comments.
Author information
Authors and Affiliations
Contributions
Conceptualization, K.M.; methodology, K.M., M.M.; software, W.B., K.M.; validation, E.A.-P., and K.M..; formal analysis, W.B.; investigation, K.M..; resources, K.M..; data curation, K.M..; writing—original draft preparation, K.M.; writing—review and editing, E.A.-P..; visualization, E.A.-P., K.M..; supervision, A.K.; project administration, K.M..; funding acquisition, A.K.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maliszewska, K., Adamska-Patruno, E., Miniewska, K. et al. PET/MRI-evaluated brown adipose tissue activity may be related to dietary MUFA and omega-6 fatty acids intake. Sci Rep 12, 4112 (2022). https://doi.org/10.1038/s41598-022-08125-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-08125-z
This article is cited by
-
Biorefining of essential polyunsaturated fatty acids from microbial sources: current updates and prospects
Systems Microbiology and Biomanufacturing (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.