Abstract
Selenium is a well-known essential element with important roles in human reproductive health mainly due to its antioxidant character. This study aimed to investigate the potential role of selenoproteins on gut microbiota and male reproductive health. A new assay for the absolute quantification of selenoproteins in testicular tissue based on two dimensional chromatography with inductively coupled plasma mass spectrometry was performed for the first time. The gut microbiota profile was obtained by 16S rRNA gene sequencing. Numerous associations were found between testicular selenoproteins and gut microbiota (e.g. Mucispirillum, related with sperm activity and testosterone, was associated with glutathione peroxidase (GPx) and selenoalbumin (SeAlb), while Escherichia/Shigella, related to sex hormones, correlated with GPx, selenoprotein P (SelP) and SeAlb). The effects of Se-supplementation on testicular selenoproteins only occur in conventional mice, suggesting a potential selenoproteins-microbiota interplay that underlies testicular function. The selenoproteins GPx and SelP have been quantified for the first time in the testicles, and the novel identification of SeAlb, a protein with nonspecifically incorporated Se, is also reported. These findings demonstrate the significant impact of Se-supplementation on gut microbiota and male reproductive health. In addition, the analytical methodology applied here in selenoprotein quantification in testicular tissue opens new possibilities to evaluate their role in gut microbiota and reproductive health axis.
Similar content being viewed by others
Introduction
Selenium (Se) is an essential trace element with important roles in immune function, the metabolism of thyroid hormones1 and cancer chemoprevention2. Se deficiency has been related to heart failure, nutritional myodegeneration (white muscle disease)3 and Keshan disease4 among other pathologies. Se dietary supplementation leads to the formation of specific selenoproteins, in which Se occupies the active center; hence, influencing the redox-regulated genes and helping the cell to convert with reactive oxygen species (ROS) into less reactive molecules5. Se is also important in reproductive health6, being essential for gonadal development, gametogenesis and fertilization7, likely as a result of its ability to modulate antioxidant defense mechanisms and redox sensitive pathways. There is clear evidence that a deficiency of Se and selenoproteins can lead to several reproductive health and obstetric complications as well as infertility, preeclampsia, miscarriage, preterm labor, fetal growth restriction, gestational diabetes and obstetric cholestasis6. Mammalian Se-containing proteins can be divided into three groups: (1) proteins containing non-specifically incorporated Se, in which sulfur is replaced by Se in amino acids such as methionine (SeMet) (e.g. selenoalbumin (SeAlb), which is not considered as a “real” selenoprotein), (2) specific Se-binding proteins (e.g. Se-binding protein 1, SBP1), in which Se is tightly associated with a cysteine (Cys) residue in the peptide but not as a component of selenocysteine (SeCys) and (iii) specific selenocysteine-containing selenoproteins (e.g. selenoprotein P (SELENOP))8. The role of Se in mammal spermatogenesis is mainly mediated by two selenoproteins, namely phospholipid hydroperoxide glutathione peroxidase (PHGPx/GPx4) related to sperm quality and male fertility and SELENOP, a plasma protein required for Se supply to the gonads where it is used as a reservoir of Se9. Other selenoprotein transcripts (~ tenfold lower level than PHGPx and the majority of them with unknown function) have also been identified in male gonads (Thioredoxin/Glutathione Reductase (TGR), selenoprotein V, selenoprotein W, selenoprotein K, selenoproteins 15 and selenoprotein S)9.
Recent studies have pointed out the potential role of dietary Se in shaping the gut microbiota and, subsequently, exerting effects on host metabolism and immunity10,11,12,13. Diet is considered a key regulator of gut microbiota with effects at local and systemic levels14,15 and also, on reproductive hormones levels16. Several reports have shown that high-fat diet induced gut dysbiosis can affect the system’s health even causing neurological disorders17,18 and spermatogenesis impairments19. Recent studies suggest the impact of gut microbiota on fertility and reproductive health in both, males and females20,21,22. However, little is known about the mechanisms underlying the shifts in gut microbiota across reproductive states. An increase in dietary Se intake has also been implicated in enhancing the antioxidant GPX activity, thereby improving male fertility23. The encouraging results in the last years suggest that the combination of Se with other essential micronutrients may improve reproductive efficiency in males23. However, to date there is not sufficient nor consistent findings upon which to draw solid conclusions. Indeed, previous studies regarding selenoproteins in the testicles applied non quantitative methods, such as transcriptomics or enzymatic assays9.
The aim of this work is to investigate the potential role of selenoproteins in the gut microbiota-reproductive health axis. For this purpose, the absolute quantification of selenoproteins by intact protein analysis will be performed using a metallomic approach based on inductively coupled plasma mass spectrometry (ICP-MS), a methodology used for analyses of serum, plasma24 and the liver25. To this end, mice testicular selenoproteome has been determined after Se-supplementation of conventional mice and mice with microbiota depleted by antibiotics. The total metal content in testicles has been also measured to evaluate the possible impact of Se-supplementation and microbiota on the homeostasis of elements in testicles, as well as their traffic.
Results and discussion
Preliminary observations and histopathology evaluation
This study analyzed the impact and effect of a Se-enriched diet on selenoproteins, total Se concentration and metal homeostasis in the testes for 8 week-old male Mus musculus mice as well as the relationships of these parameters with the gut microbiota composition. To study the influence on the gut microbiota, half of the mice received a cocktail of antibiotics (200 mg kg−1 per body weight (bw) of ampicillin, neomycin and metronidazole, 100 mg kg−1 bw of vancomycin and 2 mg kg−1 bw of amphotericin B)26 for one week, they were later fed with either a regular or a Se-supplemented diet for a further weeks. The Se-enriched diet provided the mice with a daily intake of 120 µg kg−1 bw, three times the regular mouse intake of Se27. This dose of Se is no-toxic, but able to modify some biological parameters28. The animals showed no external evidence of illness or discomfort, and all survived the treatment. No differences were found between the body weights of the mice at the end of the treatment. However, pretreatment with Abx caused a decrease in the testicular weight of mice (Fig. S2), albeit statistically non-significant one.
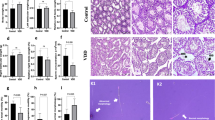
Studies in animal models clearly demonstrate the deleterious effects of antibiotics on testicular function29. The antibiotic cocktail we used here to deplete the intestinal microbiota included the aminoglycoside neomycin, an antibiotic that adversely affects spermatogenesis by cessation of meiosis at the level of primary spermatocytes30. Our histopathological study confirms this effect (Fig. 1), as we found a greater number of spermatogonia, the diploid undifferentiated germ cells, in the seminiferous tubules of the Abx group. Although the differences between the Abx group and the control group were not statistically significant, these results suggest a possible arrest of the cell division processes that convert spermatogonia into spermatozoa. The Abx group’s histological samples also showed an alteration of the normal structure of the seminiferous tubes with mild and punctual degenerative changes at the level of the basement membrane. This morphological layout is essential for the process of spermatogenesis since it is the structural and hormonal support of the spermatogonia in the different stages of the seminiferous epithelial cycle31 and abnormal basement membrane structures have been associated with spermatogenesis32. Although no significant decrease in the number of sperm per seminiferous tube was observed in any sample, our data suggests that Abx treatment may alter male fertility. The intake of a Se supplement after pretreatment with Abx (Abx-Se group) did not completely prevent the effect of Abx on the meiotic process but prevented the basement membrane abnormalities observed in the Abx group. These results suggest that Se supplementation likely improves the fertility of mice, in agreement with previous reports9.
(A) Photomicrograph showing the cross section of H&E staining of the testes of a representative mouse from groups Control, C-Se, Abx and Abx + Se. The basement membrane (bm), spermatogonia cells (sg), spermatocytes (sc), spermatids (st) and spermatozoids (sz) are indicated by arrows. (400 × magnification). (B) Bar plots showing the average counts for each germ cell type in the seminiferous tubule; the scheme illustrates the process of generation of the different types of germ cells during spermatogenesis.
Selenoproteins and total selenium in testicles
Selenoprotein extraction protocols from mammalian tissues requires high efficiency, avoiding interferences and protein degradation during the procedure. For total protein extraction we used here the CelLytic™ MT extraction reagent for total protein extraction, which contains a low concentration of a dialyzable mild detergent for minimal interference with protein interactions and biological activity33. Selenoproteins obtained from mice testicles from each study group were clearly identified in the typical mass flow chromatogram (Fig. 2). These chromatograms represent the mass of Se (µg) vs time (min), and, thus, they do not reflect the abundance of different selenoproteins, but the Se accounted for by each one. When converting chromatograms peaks to selenoproteins abundance, it must be taken into consideration that both mice and human SELENOP contains 10 selenocysteine molecules (C3H7NO2Se)34, while GPx has 4 g atoms of Se per mole35. In addition, we quantified SeAlb, which is not a “real selenoprotein”, but a protein that incorporates Se post-translationally in the form of selenomethionine (SeMet)36. Furthermore, the relative concentration of selenoprotein (in terms of Se) in the testicles is SELENOP > GPx + unretained (unr) ~ SeAlb (Fig. 2). It is noteworthy that GPx elutes in the void of the column and this peak should be assigned to GPx and other unretained selenoproteins (they have not been identified in mice testicles by organic mass spectrometry and will be the object of future work).
Significant changes in both selenoprotein and total Se concentrations were observed between groups (Fig. 3 and Table S1). The total Se content in the testicles is affected by Se-supplementation. In conventional mice fed with a Se-supplemented diet (C-Se group), the total Se content was similar to the control group, and there were no statistical differences between groups. In contrast, mice fed Se-supplemented diet after microbiota depletion (Abx + Se group) presented the highest concentrations of Se in the testicles, showing statistical differences with the control group (C, 1.24-fold↑, p = 0.01), with the Se-supplemented conventional mice (C-Se, 1.19-fold↑, p = 0.005) and with the microbiota-depleted mice that were fed a rodent diet (Abx, 1.15-fold↑, p = 0.01).
Selenoproteins (A) and total Se concentration (B) in the mouse testis. Data are expressed as mean ± SD (n = 10). (*) Statistically different in C-Se vs C comparison. (†) Statistically different in Abx + Se vs C comparison. (#) Statistically different in Abx + Se vs C-Se comparison. (‡) Statistically different in Abx + Se vs Abx comparison.
Regarding selenoproteins, the concentrations of SELENOP and SeAlb were highest in the testicles of conventional mice fed a Se-supplemented diet, while the concentration of GPx + unr was the lowest in this group. Thus, Se supplementation increases the concentration of SELENOP (1.61-fold↑, p = 0.000) and SeAlb (1.30-fold↑, p = 0.013) in the testicles of conventional mice (C-Se vs C), while decreasing the concentration of GPx + unr (2.32-fold↓, p = 0.000). The SeAlb concentration in the testicles was significantly different between Abx + Se and C groups (1.54-fold↓, p = 0.017). Se-supplementation of microbiota depleted mice (Abx + Se vs C-Se) increased the concentration of GPx + unr in testicles (2.22-fold↑, p = 0.000) and decreased that of SELENOP (1.85-fold↓, p = 0.000) and SeAlb (2.00-fold↓, p = 0.000). As mentioned in a previous study PHGPx/GPx4 (most abundant) and SELENOP have been previously identified in testicles along with other proteins with unknown function or lower abundance8. However, to our knowledge, this is the first identification of SeAlb in the testicles, likely due to either the methodologies previously used (transcriptomics and enzymatic activities37,38 or a lack of information about its presence and role in the testicles. Testosterone is produced by the Leydig cell and secreted into the interstitial fluid from where it is taken up by the Sertoli or diffuses into the interstitial capillaries to bind to albumin for transport through the body. If the presence of Se in albumin favors testosterone binding and distribution to other organs and tissues, this is an interesting issue to be addressed in further studies. In addition, the absolute quantification of selenoproteins in the testicles has not been reported before. Moreover, the selenoproteins determined in this work accounts for the highest Se content linked to proteins in the testicles as concluded from the mass flow chromatogram (Fig. 2). Selenometabolites (SeO42−, SeO32−, SeMet, SeCys, SeMetSeCys) that elute between GPx and SELENOP were under the detection limits in all analyzed samples (LD = 0.5 ng Se g−1).
Thus, we suggest that Se-supplementation in conventional mice influences the selenoprotome, but not the total concentration of Se in the testicles (Fig. 3). Indeed, SELENOP and SeAlb patterns are parallel, as they both increase in concentration after Se-supplementation. However, the concentration of GPx + unr decreased in the testicles in Se-supplemented conventional mice. Nevertheless, Se-supplementation of microbiota-depleted mice (Abx + Se vs. Abx) has no effect on the testicular selenoproteome, but the total concentration of Se is significantly increased. These findings suggest that the effect of Se-supplementation on the selenoproteome of the testicles could be influenced and mediated by microbiota although the exact mechanisms remain unknown.
Se-supplementation has proven the beneficial effects of Se in male fertility reproduction and testicular damage23,39,40,41.The main function of SELENOP is the transport and distribution of Se to other tissues, but it also possesses antioxidant action and it is involved in Se homeostasis42, while the GPx family are antioxidant selenoproteins43 and SeAlb is a Se transporter44. In the testicles, SELENOP is located in the Leydig cells45 and can influence sperm quality and, hence, male fertility38,46. PHGPx/GPx4 has also been related to sperm midpiece, mitochondrial sheath and sperm chromatin condensation8.
In previous work, the expression levels of GPx and SELENOP in the testicles of mice were not affected with dietary Se deficiency or excess selenomethionine47, however, in rats with Se deficiency the expression levels of SELENOP were decreased37. Other authors reported that Se-supplementation sharply increase the activity of testicular SELENOP46 and, in studies of co-exposure to Cd and Se, Se ameliorates the effects of Cd by increasing SelP and GPX4 gene expression48. The results obtained from studies of Se-supplementation in human prostate adenocarcinoma cells (F-9 and Du-145 cells) revealed an increase in mRNA expression levels on the glutathione peroxidases GPX1, GPX2 and GPX3, SelS and SEP15, while some selenoproteins located in the testes, such as SelW and SelV changes slightly and the TRXR3 selenoprotein decreased sharply49.
To summarize, our results suggest that the use of antibiotics (Abx) may affect the ability of the host to incorporate Se into SELENOP, which, as we have mentioned, may influence testicular activity and reproductive function, in good agreement with the above results.
Metals and metalloids are very important in biology since as one-third of all proteins in the human body require a metal cofactor for functionality50. Metallomics can be defined as the research field that elucidates the identification, distribution, dynamics, role and impact of metals and metalloids in biological systems51,52. The methodology for a metallomic analysis usually involve the use of an inductively coupled plasma mass spectrometer (ICP-MS) hyphenated to high performance liquid chromatography (HPLC), gas chromatography (GC–MS) or capillary electrophoresis (CE)) using the heteroelement (an atom different to C, H, N, O or F, e.g. Se) in the biomolecule as a “tag” (heteroatom-tagged proteomics)53. Thus, this approach, is more sensitive than the typical proteomic approaches which involve tryptic digestion and further analysis of peptides that are usually difficult to separate and it has not been previously applied for the absolute quantification of selenoproteins in testicular tissue54. Other techniques such as UV–Vis spectrophotometry allow determination of the total content of proteins or their activities, but not the absolute quantification of specific proteins.
Influence of selenium supplementation on testicular metal homeostasis
The concentration of toxic and essential metals (Al, V, Cr, Mn, Fe, Co, Cu, Zn, As, Mo Cd, Sb, Tl, and Pb) has been determined in the testicles of mice from the different groups to evaluate the metal homeostasis. The results are presented in Table 1. The concentrations of Cd, Sb, Tl and Pb in the testicles were all below the limit of detection (0.02–0.05 ng g−1) in all mice groups.
The statistical analysis showed numerous differences in the concentration of metals between groups. The significant differences for each comparison are summarized in Table S2. Se-supplementation of conventional mice (C-Se vs C) increased the levels of Cr (p = 0.004) and As (p = 0.008) and decreased the levels of Fe (p = 0.000), Cu (p = 0.003) and Zn (p = 0.020) in the testicles. The apparent paradox of Se increasing the As concentration in the testicles but contributing to As detoxification can be explained by the ability of Se to additionally increase the concentration of betaine in testicles, which sequester As in the non-toxic form of arsenobetaine55. The toxicity of Cr is determined by its chemical form, Cr(III) is essential while Cr(VI) is carcinogenic56. The effect of Se on metal homeostasis in the testicles was different after microbiota depletion. The concentration of Cr (p = 0.023), Cu (p = 0.013) and As (p = 0.009) were lower in the Abx + Se group when compared to the control group, while the concentration of Zn was higher (p = 0.013). However, when Abx + Se is compared with C-Se, the levels of Fe (p = 0.000), Co (p = 0.028), Zn (p = 0.004) and Mo (p = 0.024) increased and the levels of Al (p = 0.012), Cr (p = 0.001) and As (p = 0.003) decreased. Finally, Se-supplementation in microbiota depleted mice (Abx + Se vs Abx) reduces the concentrations of Al (p = 0.031) and Mo (p = 0.003) in the testicles whereas the concentration of Fe (p = 0.002) and Zn (p = 0.005) were augmented. The cytosol of most eukaryotic cells contains the enzyme superoxide dismutase (SOD), which contains Cu and Zn. After exposure to antibiotics, tissues are subjected to oxidative stress, which likely led to an increase in SOD and therefore, increased Zn levels57,58.
Essential metals like Mn, Cu and Zn are crucial for maintaining male reproductive functions, as they are involved in spermatogenesis and sperm motility59,60. Moreover, their interaction with toxic elements (As, Cd, Hg, Pb, and others) may change the toxicity of these metals61. The synergistic/antagonistic interactions between elements through metal traffic and homeostasis in the different organs and tissues have been reported62. The antagonistic role of Se has been proven with toxic elements such as Hg63, Cd64 and As65 and also with organic pollutants66.
Selenoproteins and Gut Microbiota
It is well-known that gut microbiota play important roles in host health, modulating physiological, immunological and metabolic functions, but they also participate in the regulation of hormones related to reproductive functions16 through the hypothalamic-pituitary–testicular axis67. Recently, the subject of the effects of dietary and supplemented Se on gut microbiota is has been receiving growing attention, but the interplay between testicular selenoproteins and microbiota has not been previously reported. As detailed elsewhere28, Se-supplementation shape the gut microbiota composition as well as the effect of the antibiotics treatment (Fig. S3). In brief, Se-supplemented groups showed an increase in members of the Lachnospiraceae and Ruminococcaceae families as well as Christensenellaceae family and Lactobacillus genus.
The microbial richness (Chao1 index) and diversity (Shannon index) indeces were associated with selenoproteins in the testicles despite the impact of Abx on the microbial composition. In control group, higher microbial diversity (R = 0.67, p ≤ 0.05) and richness (R = 0.71, p ≤ 0.05) were associated with higher levels of SeAlb. In the Se-supplemented group, higher microbial diversity was also associated to SeAlb (R = 0.81, p ≤ 0.05), but not with microbial richness. Furthermore, the relationship between SeAlb and microbial diversity disappear after the Abx treatment; with one exception: in the Abx + Se group, Chao1 index showed a positive association with SELENOP (R = 0.64, p ≤ 0.05). These data suggest a potential effect of the microbiota on the specific selenoproteins in mice testes. To further explore the gut microbiota-testicular selenoproteome interplay, specific associations at the genus level in each group were determined (Fig. 4). The associations at phylum and family levels are detailed in Tables S3–S6.
Heatmap showing the correlations between the testicular selenoproteome, total Se content and gut microbiota composition at genus level in the groups: Control (A), C-Se (B), Abx (C), Abx + Se (D). The colors range from blue (positive correlation) to red (negative correlation) and (*) indicates a p-value ≤ 0.05.
As shown in Fig. 4, an elevated number of correlations between selenoproteins in the testicles and microbiota composition appeared after Se-supplementation of conventional mice (C-Se) and/or microbiota depleted mice (Abx + Se). It is also noteworthy that the correlations between total Se and SELENOP with microbiota were highly similar in the control mice, and always in the opposite manner than those of GPx + unr. Moreover, this behavior was dependent on the group/treatment. As previously discussed, these findings suggest that the effect of Se-supplementation on the selenoproteome of testicles is influenced by microbiota. In the control group, a higher total Se concentration was positively linked to several groups from the Ruminococcaceae and Lachnospiraceae families as well as the Butyricoccus genus, all known as short chain fatty acids (SCFAs) producers with beneficial impacts on intestinal homeostasis and promoting health benefits. Furthermore, members from the Lachnospiraceae and Ruminococcaceae families were also found to be associated with testicular functions68. Indeed, after the supplementation with Se, some SCFA producers such as Faecalibacterium genus as well as Lachnospiraceae_UCG001 were correlated with SELENOP and Ruminococceae_UCG009 was correlated with SeAlb. Moreover, in the Abx group these correlations were lost and most of the relations between microbiota and selenoproteome components were found with the total Se and SeAlb (Tables S4–S7). However, in the Abx + Se group, several new associations between all components of selenoproteome were observed, including positive associations between previously mentioned families such as Lachnospiraceae groups and Ruminococcus_1 with Total Se and SeAlb (Fig. 4). It is noteworthy that in the control group these bacteria correlated positively with total Se, while in Se-supplemented mice groups they correlated with specific selenoproteins such as Mucispirillim. This observation may indicate that Se-supplementation aids specific functions such as transport (SeAlb and SELENOP) or sperm quality and male fertility (SELENOP)38,46.
A higher relative abundance of the Mucispirillum genus was associated with higher total Se content in the control group. However, after Se-supplementation, higher relative abundance of this genus was associated with lower GPx + unr and higher SeAlb concentrations in the testicular tissue of conventional mice. A decrease in the level of this genus in mice fed with supranutritional Se has been previously reported69. Other authors indicated numerous associations between bacterial taxa and testicular function, but specially showed that Mucispirillum were positively correlated with testosterone and sperm activity68. Escherichia/Shigella has been related with sex hormones in the reproductive endocrine system70. This genus correlated positively with SELENOP and negatively with total Se in conventional mice after Se supplementation, while correlating positively with SeAlb in the Abx group. After Se supplementation of this group (Abx + Se), Escherichia/Shigella correlated positively with GPx + unr and SELENOP, and negatively with SeAlb.
In summary, Se-supplementation has an impact on the selenoproteome and mineral homeostasis in the testes, and also, on the gut microbiota, suggesting a pivotal key interplay between Se-microbiota and male reproductive health. The metallomic analytical approach, based on the quantification of selenoproteins using an atomic spectrometric detector such as ICP-MS coupled to HPLC, allowed for the first time the absolute quantification of the selenoproteins containing most of the bonded Se in the testicles as well as the novel identification of SeAlb in testicles. Our data indicate that Se-supplementation of conventional mice did not change the total level of Se in the testicles, but significantly changed the selenoproteome profile. Moreover, the opposite situation was observed in microbiota depleted mice suggesting that the effect of Se-supplementation on the selenoproteome of the testicles could be influenced by microbiota. Specific associations between selenoproteins in the testicles and gut microbiota composition and diversity have been observed, some of them related to sperm activity and sex hormones, demonstrating the interplay of Se supplementation with microbiota and the impact on reproductive health. More studies are needed to ascertain the mechanisms behind the Se-microbiota-reproductive health.
Materials and methods
Animal experimental design
After a three day acclimation period, forty mice (male Mus musculus BALB/c, 8 weeks old) were randomly divided into two groups, one receiving water and the other, receiving water with a mixture of antibiotics (ampicillin 1%, metronidazole 1%, neomycin 1%, vancomycin 0.5%) and an antifungal (amphotericin B, 10 mg/L) for one week. After this pretreatment time, half of the mice in each group (n = 10) were fed for an additional two weeks (treatment period) with the same regular diet used in the previous days, and the other half, were fed with a Se-enriched diet (0.65 mg/kg of sodium selenite). The four groups, C (control), C-Se (Se-enriched diet during treatment); Abx (antibiotics in the water during pretreatment) and Abx + Se (Antibiotics in the water during pretreatment and Se-enriched diet during treatment) were caged in pairs, with free access to water and food, which were changed every other day. Figure S1 summarizes the design of the experiment. At the end of the experiment, mice were anesthetized (isoflurane) and sacrificed by cervical dislocation, and organs were immediately removed, cleaned in NaCl (0.9% w/w) solution, cryo-homogenized in liquid nitrogen and stored at -80ºC until analysis.
Ethics statement
The experimental procedures were carried out at the Animal Experimentation Service of the University of Cordoba (SAEX-UCO), after approval by the bioethics committee of the university and the regional government (Code Num. 02-01-2019-001), in accordance with current European Union regulations. Furthermore, the study was carried out in compliance with the ARRIVE Guideline.
Histopathological evaluation
Testicle tissues were fixed in 10% neutral buffered formalin for 24 h and then embedded in paraffin wax. 4 µm-thick paraffin serial sections were obtained using a rotary microtome (SAKURA Tissue Tek Accu Cut SRM 119 200) and stained with hematoxylin–eosin according to routine protocols. Photomicrographs were obtained with a Nikon Eclipse E400 photomicroscope at 400 magnifications.
Speciation of selenoproteins in mice testicles
To isolate selenoproteins, testicles were cryo-homogenized with a mortar and pestle in the presence of liquid nitrogen. Selenoproteins were extracted using the CelLytic™ MT extraction reagent (Sigma-Aldrich, Steinheim, Germany) (3 Ml g−1) into a glass/teflon homogenizer at 4 °C. Protease inhibitor cocktail (Sigma-Aldrich, Steinheim, Germany) was added to CelLytic MT reagent to avoid protein degradation. Then, the mixture was centrifuged at 15,500g for 20 min at 4 °C. The supernatant was collected and filtered through low protein absorption Iso-Disc poly(vinylidene difluoride) filters (PVDF, 25 mm diameter, 0.45 µm pore size). A preconcentration step is necessary due to the low concentration of selenospecies. To this end, the extracts were completely evaporated under a nitrogen stream and re-dissolved in 0.1 mL of MilliQ water prior to the analysis.
Selenoproteins were separated from the obtained extracts using a previously described method71. Briefly, the chromatographic separation of GPx, SELENOP and SeAlb were performed with an ultra-high performance liquid chromatograph (model 1260 Infinity Quaternary LC, Agilent Technologies) using two size exclusion columns (5 ml HiTrap ®Desalting Columns, GE Healthcare, Uppsala, Sweden) and two different affinity chromatography columns (AFC, GE Healthcare, Uppsala, Sweden) with stationary phases of heparine-sepharose column (HEP-HP) and blue-sepharose column (BLUE-HP). The HEP-HP column retains only SELENOP, while the BLUE-HP column retains SELENOP and SeAlb. The column switching method allows the simultaneous separation of selenoproteins and selenometabolites: GPx and selenometabolites elute when the column switching system is in position 1 (0–20 min), while SELENOP and SeAlb are retained in the AFC columns. The valve switches to position 2 (20–24 min) to elute the SELENOP, and then returns to position 1 for the elution of SeAlb. Polyether ether ketone (PEEK) tubing (30 cm × 0.6 mm i.d.) and a T-connector were used to connect the eluent of the chromatograph to the Micromist nebulizer (Glass Expansion, Switzerland) of the triple quadrupole inductively coupled plasma mass spectrometer (ICP-QqQ-MS, model Agilent 8800 Triple Quad, Agilent Technologies, Tokyo, Japan) (2D-SEC-SEC-AFxAF-ICP-MS)74. Se (Cambridge Isotope Laboratories, Andover, MA, USA) was also introduced into the system via a T-connector for isotope dilution analysis. The absolute quantification of selenoproteins by 2D-SEC-AF-SUID-ICP-MS was carried out using the operational conditions as summarized in Table S7.
Total elements determination in mice testicles
For total elemental analysis, testicular tissue samples from mice in each group were pooled, and approximately, 0.1000 g of sample were digested in a microwave reaction system MARS 6 (CEM Corporation, Matthews, NC, USA) with a mixture of nitric acid and hydrogen peroxide (4:1, v/v). The mineralization was carried out from room temperature to 160 °C over 15 min, then maintaining at 400 W for 40 min. Then, the samples were diluted fivefold in 5% HNO3 containing 100 µg L−1 of rhodium, and filtered using 0.45 µm PTFE syringe filters prior to the analysis by ICP-QqQ-MS. The operational conditions for ICP-QqQ-MS are listed in Table S7. The validation of the methodology was carried out using a fish protein certified reference material for trace element DORM-4 (National Research Council of Canada) (Table S8).
Gut microbiota analysis
Fecal samples were collected from the colon and immediately frozen in liquid nitrogen. DNA from fecal samples (approx. 100 mg) was obtained with the Master-Pure DNA extraction kit (Epicentre, Madison, WI, United States) following the manufacturer’s instructions. Specific modifications were included as described elsewhere72. DNA concentration was measured using a Qubit® 2.0 Fluorometer (Life Technology, Carlsbad, CA, United States). A specific 16S rRNA amplicon (V3-V4 variable region of the 16S rRNA gene) was amplified and sequenced following Illumina protocols. Briefly, a multiplexing step was conducted using the NextEra Index Kit (Illumina, San Diego, CA, United States) and amplicons were checked with a Bioanalyzer DNA 1000 chip (Agilent Technologies, Santa Clara, CA, United States). Libraries were sequenced (2 × 300 bp paired-end run, MiSeq Reagent kit v3) on a MiSeq-Illumina platform (FISABIO sequencing service, Valencia, Spain). Controls (DNA extraction procedure and libraries amplification) were included. A DADA2 pipeline was used to achieve quality filtering, sequence joining and chimera removal73. Taxonomy assignment was performed using Silva v132 database74,75. Sample with less than 1000 reads as well as specific taxa present at levels less than 0.01% and those present less than 3 times in at least 20% of the samples were filtered and removed from the analysis. Furthermore, sequences classified as Chloroplast and Cyanobacteria were filtered from the final dataset as they are associated with potential contaminants.
Statistical analysis
Statistical analysis was performed using Minitab16 Statistical Software (State College, PA, United States) and STATISTICA 8 Software. Firstly, Anderson–Darling normality test was used to determine whether or not data are normally distributed. Differences between groups were tested using the Kruskal–Wallis test (non-parametric statistics) and one-way ANOVA (parametric statistics). The Spearman correlation test was performed for correlation analysis between gut microbiota abundance and selenoproteins concentrations. The level of p < 0.05 was considered statistically significant. Heatmaps were generated in R Project software (version 4.0.2) (R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria). For the microbiota analyses, total sum normalization (TSS) for the statistical analysis, multivariate test and data mining were performed with Calypso web platform v. 8.5676. Alpha- diversity metrics (Chao1 and Shannon indeces) and beta diversity analysis (based on Bray Curtis distance) were obtained. Briefly, Permutational multivariate analysis of variance (ADONIS) and Redundancy Discriminant Analysis (RDA) were obtained. Relative abundance (%) differences between groups at different taxonomical levels were tested using the Krustal-Wallis test with False discovery test rate (FDR) for multiple test correction. Alpha diversity indexes were obtained after a rarefaction to 93,525 sequences (minimum number of reads per sample). The level of statistical significance for all tests was fixed to p < 0.05.
References
Winther, K. H., Rayman, M. P., Bonnema, S. J. & Hegedüs, L. Selenium in thyroid disorders—essential knowledge for clinicians. Nat. Rev. Endocrinol. 16, 165–176 (2020).
Lü, J. et al. Cancer chemoprevention research with selenium in the post-SELECT era: Promises and challenges. Nutr. Cancer 68, 1–17 (2016).
Fairweather-Tait, S. J., Collings, R. & Hurst, R. Selenium bioavailability: current knowledge and future research requirements. Am. J. Clin. Nutr. 91, 1484S-1491S (2010).
Rayman, M. P. Selenium and human health. Lancet (London, England) 379, 1256–1268 (2012).
Hugejiletu, H. et al. Selenium supplementation alters gene expression profiles associated with innate immunity in whole-blood neutrophils of sheep. Biol. Trace Elem. Res. 154, 28–44 (2013).
Mistry, H. D., Broughton Pipkin, F., Redman, C. W. G. & Poston, L. Selenium in reproductive health. Am. J. Obstet. Gynecol. 206, 21–30 (2012).
Mirone, M., Giannetta, E. & Isidori, A. M. Selenium and reproductive function: A systematic review. J. Endocrinol. Invest. 36, 28–36 (2013).
Behne, D. & Kyriakopoulos, A. Mammalian selenium-containing proteins. Annu. Rev. Nutr. 21, 453–473 (2001).
Boitani, C. & Puglisi, R. Selenium, a key element in spermatogenesis and male fertility. Adv. Exp. Med. Biol. 636, 65–73 (2008).
Derrien, M., Belzer, C. & de Vos, W. M. Akkermansia muciniphila and its role in regulating host functions. Microb. Pathog. 106, 171–181 (2017).
Plovier, H. et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 23, 107–113 (2017).
Presley, L. L., Wei, B., Braun, J. & Borneman, J. Bacteria associated with immunoregulatory cells in mice. Appl. Environ. Microbiol. 76, 936–941 (2010).
Liu, W. et al. Diet- and Genetically-Induced Obesity Produces Alterations In The Microbiome, Inflammation and Wnt pathway in the intestine of Apc(+/1638N) mice: Comparisons and contrasts. J. Cancer 7, 1780–1790 (2016).
Sherwin, E., Bordenstein, S. R., Quinn, J. L., Dinan, T. G. & Cryan, J. F. Microbiota and the social brain. Science (80-. ). 366, eaar2016 (2019).
Dowd, J. B. & Renson, A. ‘Under the skin’ and into the gut: Social epidemiology of the microbiome. Curr. Epidemiol. Reports 5, 432–441 (2018).
Hussain, T. et al. Relationship between gut microbiota and host-metabolism: Emphasis on hormones related to reproductive function. Anim. Nutr. 7, 1–10 (2021).
Moos, W. H. et al. Microbiota and neurological disorders: A gut feeling. Biores. Open Access 5, 137–145 (2016).
Wu, W. et al. Targeting gut microbiota dysbiosis: Potential intervention strategies for neurological disorders. Engineering 6, 415–423 (2020).
Ding, N. et al. Impairment of spermatogenesis and sperm motility by the high-fat diet-induced dysbiosis of gut microbes. Gut 69, 1608–1619 (2020).
Zhang, C. et al. Rescue of male fertility following faecal microbiota transplantation from alginate oligosaccharide-dosed mice. Gut https://doi.org/10.1136/gutjnl-2020-323593 (2020).
Guo, L. et al. Gut microbiological disorders reduce semen utilization rate in duroc boars. Front. Microbiol. 11, 2493 (2020).
Komiya, S. et al. Characterizing the gut microbiota in females with infertility and preliminary results of a water-soluble dietary fiber intervention study. J. Clin. Biochem. Nutr. 67, 105–111 (2020).
Qazi, I. H. et al. Role of selenium and selenoproteins in male reproductive function: a review of past and present evidences. Antioxidants 8, (2019).
García-Sevillano, M. A., García-Barrera, T. & Gómez-Ariza, J. L. Development of a new column switching method for simultaneous speciation of selenometabolites and selenoproteins in human serum. J. Chromatogr. A 1318, 171–179 (2013).
García-Sevillano, M. A., Rodríguez-Moro, G., García-Barrera, T., Navarro, F. & Gómez-Ariza, J. L. Biological interactions between mercury and selenium in distribution and detoxification processes in mice under controlled exposure: Effects on selenoprotein. Chem. Biol. Interact. 229, 82–90 (2015).
D’Amato, A. et al. Faecal microbiota transplant from aged donor mice affects spatial learning and memory via modulating hippocampal synaptic plasticity- and neurotransmission-related proteins in young recipients. Microbiome 8, 140 (2020).
Raines, A. M. & Sunde, R. A. Selenium toxicity but not deficient or super-nutritional selenium status vastly alters the transcriptome in rodents. BMC Genomics 12, 26 (2011).
Callejón-Leblic, B., Selma-Royo, M., Collado, M. C., Abril, N. & García-Barrera, T. Impact of antibiotic-induced depletion of gut microbiota and selenium supplementation on plasma selenoproteome and metal homeostasis in a mice model. J. Agric. Food Chem. 69, 7652–7662 (2021).
Singh, V. J. & Sharma, S. Effect of antibiotic therapy on sperm quality. Eur. J. Mol. Clin. Med. 7, 4398–4403 (2020).
Samplaski, M. K. & Nangia, A. K. Adverse effects of common medications on male fertility. Nat. Rev. Urol. 12, 401–413 (2015).
Shetty, G. & Meistrich, M. L. Hormonal approaches to preservation and restoration of male fertility after cancer treatment. JNCI Monogr. 2005, 36–39 (2005).
Lehmann, D. et al. Role of immunological factors in male infertility Immunohistochemical and serological evidence. Lab. Invest. 57, 21–28 (1987).
Raman, A. V. et al. Selenoprotein W expression and regulation in mouse brain and neurons. Brain Behav. 3, 562–574 (2013).
Hill, K. E., Lloyd, R. S., Yang, J. G., Read, R. & Burk, R. F. The cDNA for rat selenoprotein P contains 10 TGA codons in the open reading frame. J. Biol. Chem. 266, 10050–10053 (1991).
Flohe, L., Günzler, W. A. & Schock, H. H. Glutathione peroxidase: A selenoenzyme. FEBS Lett. 32, 132–134 (1973).
Rayman, M. P., Infante, H. G. & Sargent, M. Food-chain selenium and human health: Spotlight on speciation. Br. J. Nutr. 100, 238–253 (2008).
Wang, Q. et al. Low-Se Diet Can Affect Sperm Quality and Testicular Glutathione Peroxidase-4 activity in Rats. Biol. Trace Elem. Res. 4–10 (2021).https://doi.org/10.1007/s12011-020-02515-y.
Michaelis, M. et al. Selenoprotein P in seminal fluid is a novel biomarker of sperm quality. Biochem. Biophys. Res. Commun. 443, 905–910 (2014).
Ibrahim, H. A. M. et al. Selenium-enriched probiotics improves murine male fertility compromised by high fat diet. Biol. Trace Elem. Res. 147, 251–260 (2012).
Moslemi, M. K. & Tavanbakhsh, S. Selenium-vitamin E supplementation in infertile men: effects on semen parameters and pregnancy rate. Int. J. Gen. Med. 4, 99–104 (2011).
Benvenga, S. et al. Effects of Myo-inositol alone and in combination with Seleno-Lmethionine on cadmium-induced testicular damage in mice. Curr. Mol. Pharmacol. 12, 311–323 (2019).
Burk, R. F. & Hill, K. E. Selenoprotein P-expression, functions, and roles in mammals. Biochim. Biophys. Acta 1790, 1441–1447 (2009).
Björnstedt, M., Xue, J., Huang, W., Akesson, B. & Holmgren, A. The thioredoxin and glutaredoxin systems are efficient electron donors to human plasma glutathione peroxidase. J. Biol. Chem. 269, 29382–29384 (1994).
Suzuki, Y. et al. Selenium metabolism and excretion in mice after injection of (82)Se-enriched selenomethionine. Metallomics 5, 445–452 (2013).
Nishimura, K. et al. Association of selenoprotein P with testosterone production in cultured Leydig cells. Arch. Androl. 47, 67–76 (2001).
Zhou, J.-C. et al. Dietary selenium deficiency or excess reduces sperm quality and testicular mRNA abundance of nuclear glutathione peroxidase 4 in rats. J. Nutr. 147, 1947–1953 (2017).
Akahoshi, N. et al. Dietary selenium deficiency or selenomethionine excess drastically alters organ selenium contents without altering the expression of most selenoproteins in mice. J. Nutr. Biochem. 69, 120–129 (2019).
Messaoudi, I., Banni, M., Saïd, L., Saïd, K. & Kerkeni, A. Involvement of selenoprotein P and GPx4 gene expression in cadmium-induced testicular pathophysiology in rat. Chem. Biol. Interact. 188, 94–101 (2010).
Kuznetsova, Y. P., Goltyaev, M. V., Gorbacheva, O. S. & Novoselov, S. V. Influence of Sodium Selenite on the mRNA Expression of the Mammalian Selenocysteine-Containing Protein Genes in Testicle and Prostate Cancer Cells. 480, 131–134 (2018).
Andreini, C., Bertini, I., Cavallaro, G., Holliday, G. L. & Thornton, J. M. Metal ions in biological catalysis: from enzyme databases to general principles. JBIC J. Biol. Inorg. Chem. 13, 1205–1218 (2008).
Haraguchi, H. Metallomics as integrated biometal science. J. Anal. At. Spectrom. 19, 5–14 (2004).
Williams, R. J. P. Chemical selection of elements by cells. Coord. Chem. Rev. 216–217, 583–595 (2001).
Sanz-Medel, A. ‘Heteroatom-tagged’ quantification of proteins via ICP-MS. Anal. Bioanal. Chem. 408, 5393–5395 (2016).
Calderón-Celis, F., Sanz-Medel, A. & Encinar, J. R. Universal absolute quantification of biomolecules using element mass spectrometry and generic standards. Chem. Commun. 54, 904–907 (2018).
Fowler, B. A. & Jones, R. L. C h a p t e r 19. (2007). doi:https://doi.org/10.1016/B978-0-12-369413-3.50074-4
Anderson, R. A. Chromium as an essential nutrient for humans. Regul. Toxicol. Pharmacol. 26, S35–S41 (1997).
Muller, F. L. et al. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic. Biol. Med. 40, 1993–2004 (2006).
Elchuri, S. et al. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene 24, 367–380 (2005).
Li, Y., Wu, J., Zhou, W. & Gao, E. Effects of manganese on routine semen quality parameters: results from a population-based study in China. BMC Public Health 12, 919 (2012).
Guzikowski, W., Szynkowska, M. I., Motak-Pochrzęst, H., Pawlaczyk, A. & Sypniewski, S. Trace elements in seminal plasma of men from infertile couples. Arch. Med. Sci. 11, 591–598 (2015).
Goyer, R. A. Toxic and essential metal interactions. Annu. Rev. Nutr. 17, 37–50 (1997).
García-Barrera, T. et al. Biological responses related to agonistic, antagonistic and synergistic interactions of chemical species. Anal. Bioanal. Chem. 403, 2237–2253 (2012).
Li, X., Yin, D., Li, J. & Wang, R. Protective Effects of Selenium on Mercury Induced Immunotoxic Effects in Mice by Way of Concurrent Drinking Water Exposure. Arch. Environ. Contam. Toxicol. 67, 104–114 (2014).
Rodríguez-Moro, G., Roldán, F. N., Baya-Arenas, R. & Arias-Borrego, A. Metabolic impairments , metal traffic , and dyshomeostasis caused by the antagonistic interaction of cadmium and selenium using organic and inorganic mass spectrometry. 1762–1775 (2020).
García-Sevillano, M. A., Jara-Biedma, R., González-Fernández, M., García-Barrera, T. & Gómez-Ariza, J. L. Metal interactions in mice under environmental stress. Biometals 26, 651–666 (2013).
Ungvári, É. et al. Protective effects of meat from lambs on selenium nanoparticle supplemented diet in a mouse model of polycyclic aromatic hydrocarbon-induced immunotoxicity. Food Chem. Toxicol. an Int. J. Publ. Br. Ind. Biol. Res. Assoc. 64, 298–306 (2014).
Al-Asmakh, M. et al. The gut microbiota and developmental programming of the testis in mice. PLoS One 9, e103809 (2014).
Tian, X. et al. Lactobacillus plantarum TW1-1 alleviates diethylhexylphthalate-induced testicular damage in mice by modulating gut microbiota and decreasing inflammation. Front. Cell. Infect. Microbiol. 9, 1–16 (2019).
Zhai, Q. et al. Effects of dietary selenium supplementation on intestinal barrier and immune responses associated with its modulation of gut microbiota. Environ. Sci. Technol. Lett. 5, 724–730 (2018).
Xu, J. et al. Fertility factors affect the vaginal microbiome in women of reproductive age. Am. J. Reprod. Immunol. 83, e13220 (2020).
Callejón-Leblic, B. et al. Absolute quantification of selenoproteins and selenometabolites in lung cancer human serum by column switching coupled to triple quadrupole inductively coupled plasma mass spectrometry. J. Chromatogr. A 1619, 460919 (2020).
Sanguinetti, E. et al. Microbiota signatures relating to reduced memory and exploratory behaviour in the offspring of overweight mothers in a murine model. Sci. Rep. 9, 12609 (2019).
Callahan, B. J. et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
Quast, C. et al. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 41, 590–596 (2013).
Yilmaz, P. et al. The SILVA and ‘All-species Living Tree Project (LTP)’ taxonomic frameworks. Nucleic Acids Res. 42, D643–D648 (2014).
Zakrzewski, M. et al. Calypso: a user-friendly web-server for mining and visualizing microbiome-environment interactions. Bioinformatics 33, 782–783 (2017).
Funding
This article was funded by the projects PGC 2018- 096608-B-C21 from the Spanish Ministry of Science and innovation (MCI) (Generación del Conocimiento. MCI/AEI/FEDER, EU “Una manera de hacer Europa”) and UHU-1256905 from the FEDER Andalusian Operative Program 2014-2020 (Ministry of Economy, Knowledge, Business and Universities, Regional Government of Andalusia, Spain). S.R.A. thanks the Spanish Ministry of Economy and Competitiveness for a PhD scholarship (BES-2016-076364). The authors are grateful to FEDER (European Community) for financial support, Grant UNHU13-1E-1611. The authors would like to acknowledge the support from The Ramón Areces Foundation (ref. CIVP19A5918).
Author information
Authors and Affiliations
Contributions
S.R.A: formal analysis, investigation, data curation, writing-original draft, writing—review and editing, visualization. M.S.R.: formal analysis, investigation, data curation, software, writing—review and editing, visualization. M.C.C.: conceptualization, methodology, resources, supervision, writing—reviewing and editing. F.N.R.: conceptualization, investigation, writing—review and editing. N.A.: conceptualization, methodology, data curation, writing—original draft, writing—review and editing. T.G.B.: conceptualization, methodology, resources, writing—original draft, writing—review and editing, visualization, supervision, funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ramírez-Acosta, S., Selma-Royo, M., Collado, M.C. et al. Selenium supplementation influences mice testicular selenoproteins driven by gut microbiota. Sci Rep 12, 4218 (2022). https://doi.org/10.1038/s41598-022-08121-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-08121-3
This article is cited by
-
Association between Sperm Mitochondrial DNA Copy Number and Concentrations of Urinary Cadmium and Selenium
Biological Trace Element Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.