Abstract
Individuals’ social contexts are broadly recognized to impact both their psychology and neurobiology. These effects are observed in people and in nonhuman animals who are the subjects for comparative and translational science. The social contexts in which monkeys are reared have long been recognized to have significant impacts on affective processing. Yet, the social contexts in which monkeys live as adults are often ignored and could have important consequences for interpreting findings, particularly those related to biopsychiatry and behavioral neuroscience studies. The extant nonhuman primate neuropsychological literature has historically tested individually-housed monkeys, creating a critical need to understand how social context might impact the outcomes of such experiments. We evaluated affective responding in adult rhesus monkeys living in four different social contexts using two classic threat processing tasks—a test of responsivity to objects and a test of responsivity to an unfamiliar human. These tasks have been commonly used in behavioral neuroscience for decades. Relative to monkeys with full access to a social partner, individually-housed monkeys had blunted reactivity to threat and monkeys who had limited contact with their partner were more reactive to some threatening stimuli. These results indicate that monkeys’ social housing contexts impact affective reactivity and point to the potential need to reconsider inferences drawn from prior studies in which the impacts of social context have not been considered.
Similar content being viewed by others
Introduction
Nonhuman animals have been used for decades in the service of understanding the biological mechanisms of phenomena like mood disorders and the basis of psychosocial behavior1,2,3,4. Nonhuman primates, specifically, are used extensively in psychological and psychiatric neuroscience research5,6,7 because of their psychological and biological homologies with humans8,9,10. Such studies often include neurobiological manipulations like lesioning particular brain regions or neural connections (e.g., see11 for a review of studies on the amygdala) and, more recently, genetic manipulations (e.g., see12 for a review). Typically, such experiments rely on a comparison between manipulated and unmanipulated animals (“control animals”), and the behavior or task performance of control animals is assumed to be “healthy” or species “normative”. Such approaches have historically ignored, or treated as inconsequential, aspects of the contexts in which animals are housed and tested. The human literature clearly demonstrates that isolated or limited social conditions impact affective processing, with social isolation and loneliness increasing levels of depression and introversion13,14,15,16,17. Following the logic that limiting social conditions could impact monkeys’ affective processing, it is possible that social contexts in which adults are living could influence how control animals behave and, thus, influence the interpretation of the effects of manipulations. Beyond this, the underlying source of social contextual effects may interact with experimental manipulations, complicating inferences drawn from ostensibly causal manipulations of biological mechanisms.
Neurobiological and psychobiological research conducted with animals has typically focused on psychosocial outcomes (e.g., anxiety-like behavior18) and measurements of specific behaviors thought to reflect these outcomes (e.g., freezing and cooing5). Such behavioral measures can be sensitive to the direct impact of, and interactions with, features of subjects’ environmental and social context (see19 for a review), sometimes in ways not immediately clear to experimenters. For example, in one rodent study the mere presence of a male vs. female experimenter (or even just the presence of clothing previously worn by a male experimenter) resulted in the differential inhibition of responses to painful stimuli20.
Social relationships and features of social context are widely recognized to play critical roles in human experience21 (see22,23 for reviews). In adult humans, social isolation and loneliness are associated with increased and earlier mortality17,24, chronic illness25, and depression26. Beyond physical health, social context and the strength of relationships with family and community correlate with evaluations of subjective wellbeing27. The interplay between human social relationships and psychosocial processes likely has deep evolutionary roots, seen in data gleaned with nonhuman primates, generally, and macaque monkeys, specifically. In macaques, rearing conditions interact significantly with genotypic features to produce behavioral differences28 and influence hormonal responses to threat29, among other differences (see30 for a review). Although much of the literature on the effects of social context in monkeys has focused on developmental context—that is, how infant and juvenile monkeys’ social environment influences psychosocial processing (see31 for an early review)—there is increasing evidence that variation in social housing impacts adult monkeys as well32,33.
In most cases, adult monkeys housed alone (without tactile contact with a social partner) are housed in rooms with other monkeys they can see and hear—so isolation in this case refers to physical features of social relationships. Often, experimenters will claim that these conditions do not actually reflect isolation, qualified by visual, auditory, and olfactory exposure to other monkeys (e.g.,34,35; see36 for a discussion). Despite this visual, auditory, and olfactory exposure, compared to socially-housed adult monkeys, individually-housed monkeys generate more aberrant behaviors, including self-injurious behaviors37, anxiety-like behaviors18, and depression-like behaviors38.
While the impact of adult social context on psychosocial outcomes is likely to be different from the impact of social context during rearing, the existing research on the impacts of adult social context is limited in scope. What studies exist are limited to assessing the impacts of social context on monkeys’ behavior at home (in their home cages)18,37,38,39,40,41,42,43,44 and physiological measures (e.g., cortisol levels, creatinine levels, absolute numbers of total T cells)45,46,47,48,49. While home cage behavior and physiology are critical measures for the successful maintenance of appropriate animal welfare in captive monkeys50,51,52, in order to best assess the potential impact of social context on past and future behavioral neuroscience experiments carried out on laboratory monkeys, it is essential that we directly assess the impact of this variable on the same outcome measures that these studies report, using task-based studies.
To assess the impact of varied social contexts on monkeys’ affective processing, we analyzed responses to threat in four cohorts of healthy adult rhesus macaques housed in various social configurations in the same laboratory, tested with identical experimental protocols, over several years. Monkeys completed a test of responsivity to ostensibly threatening objects (as in53,54,55,56) and a test of their responsivity to the presence of an unfamiliar human experimenter (the Human Intruder Test57). Importantly, the two tasks used here are widely used to characterize affective reactivity of monkeys’ natural or induced phenotypes (e.g., assessing the impact of amygdala53,55,58,59, hippocampus60,61,62, orbitofrontal cortex63,64,65, or anterior cingulate lesions54; assessing the impact of early adverse experience66; assessing differences in self-injurious monkeys67; determining neurobiological correlates of anxious temperament68) and are thought to provide a veridical representation of natural and induced variation in affective reactivity54. We capitalize upon methodological consistency across cohorts (including standardized protocols for observation, interrater reliability, etc.), but variation in housing condition as husbandry and laboratory standards changed across time. Subjects were individually-housed (no physical contact with any other monkey), grate-paired (access to a partner partially through a metal grate), intermittently-paired (access to a partner 7–8 h/day), or continuously-paired (access to a partner 24 h/day). Given the findings in the human mental health literature and nonhuman animal welfare literature, we expected social housing condition to impact animals’ affective behavior during tasks indexing threat sensitivity.
Methods
Experimental procedures were approved by the University of California, Davis Institutional Animal Care and Use Committee, the ethics board overseeing nonhuman animal research at the university. Procedures were conducted in accordance with the National Institutes of Health guidelines for the use of animals in research. All procedures were carried out at the California National Primate Research Center (CNPRC). The present reporting follows the recommendations in the ARRIVE guidelines69.
Subjects and living arrangements
Subjects were 22 adult male rhesus monkeys (Macaca mulatta; 5–10 years; Mean ± SD = 7.60 ± 1.37 years) that served as neurologically intact control animals for a series of behavioral neuroscience experiments over the course of 10 years (2001–2011) in the same laboratory53,54,70,71,72,73,74. They were born at CNPRC and raised by their mothers in large social groups in 0.2 hectare outdoor corrals, remaining there through adulthood. They were relocated to temperature-controlled indoor rooms (12-h light/dark cycle) where they were fed monkey chow (Ralston Purina, St. Louis, MO) twice daily supplemented with fresh fruit and vegetables twice per week and had ad libitum access to water and enrichment. Monkeys were housed in standard nonhuman primate caging (61 cm width × 66 cm depth × 81 cm height) in one of four possible configurations (see Table 1). Housing configurations were determined based on animal husbandry norms at the CNPRC at the time of testing and social compatibility with other study subjects or available partners. Some data were previously published (A1-653; D1-754) and the present report includes data from some monkeys involved in other previously published experiments (A1-670,73,74; B1-472; C1-570,71). All monkeys in the present report were relocated to indoor housing between 15 and 29 months (see Table 1) prior to completing the tasks described here.
Subjects were selected from large outdoor corrals at the CNPRC (total area: 0.2 hectare; 30.5 m width × 61 m depth × 2.44 m height) containing 60–120 monkeys following laboratory protocols to ensure that subjects were socially normal. This ensured that monkeys were raised in an environment most closely mirroring their natural environment as is possible in a captive setting. A series of 10 5-min focal observations were conducted to determine that all subjects were socially integrated and had no stereotypies or other abnormal behaviors prior to being enrolled in their respective experiments and relocated to indoor housing.
Behavioral experiments
Object responsivity test
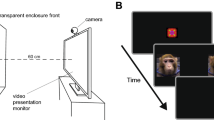
In the Object Responsivity Task (ORT), affective responses to threat were assessed by presenting monkeys with novel objects of varying complexity alongside a food reward. All 22 monkeys participated in one of four variations of the ORT (see Table 1). Only similar or identical trials were compared and only dependent variables that were identical across experiments were analyzed. The order of testing was counterbalanced across test day for each cohort and all testing occurred within the same time frame relative to meals (i.e., monkeys tested across variants should have been equally hungry). All monkeys were tested in the same lab care cage (80.01 cm × 83.32 cm × 101.6 cm) that was adapted to have a plexiglass front. The front had two vertical openings (25 cm × 5 cm), separated by 5.08 cm and centered 32.25 cm from the sides of the cage, which allowed the monkeys to interact with objects and retrieve the food reward. An opaque guillotine door lowered by a rope-and-pulley system blocked the plexiglass between trials. All trials were 30 s. Two observers sat diagonally to the right of the test cage (~ 2 m away) such that animals could view the observers when the opaque guillotine door was raised. A camera was also positioned directly in front of the test cage. No other monkeys were present in the room during testing.
Objects of varying complexity were used to evaluate changes in monkeys’ responses with variation in the affective value of stimuli (with simple objects being ostensibly less threatening than their complex counterparts; see discussion in75); complexity was varied across trials (see Table 2). All monkeys completed complex object trials and so the responses on these trials were compared. All monkeys were exposed to a combination of realistic (e.g., fake snake, alligator, lizard) and unrealistic (e.g., stuffed bear, stuffed lion, cheese grater) ostensibly threatening complex objects. Subject groups A, B, and D were exposed to both simple and complex object trials, so the responses on these trials were separately compared. All objects used were completely novel to the subjects.
Behavioral data collection was carried out by trained observers using The Observer (Noldus, Sterling, VA, USA; versions 1 through 5, depending on sample) and included: (1) latency to retrieve the food reward; (2) frequency of food retrieval; (3) frequency of species-typical affective behaviors that were measured across all experiments (i.e., facial behaviors: lipsmack and grimace; see Table S1). As there is considerable variance in how individual animals deploy different affective behaviors and in the contextual meanings of these behaviors76, affective behaviors were summed within and across bins to generate a continuous index of affective reactivity for each animal during each trial (as in77). Additional behaviors were recorded across each test administration but were not present in all data sets. All observers were trained to the lab standard of inter-rater reliability greater than 85%. As the present analyses (comparing monkeys across social housing conditions) were not planned at the time of data collection or scoring for each experimental group, no experimenters involved were aware of group allocation on the basis of housing condition.
Human intruder test
In the Human Intruder Test (HIT), affective responses to threat were indexed by presenting monkeys with an unfamiliar human experimenter in various combinations of spatial orientation and distance from the subjects. Eighteen monkeys participated in one of two variants of the HIT (see Table 1; Monkeys B1-4 did not participate in the HIT). Monkeys were relocated to a testing room and isolated in a standard primate cage (60 cm × 75 cm × 75 cm). After a one minute acclimation period they were exposed to an unfamiliar male experimenter in four different conditions, as described previously by our group (e.g.78) and others (e.g.79). The four conditions were presented in the following order: (1) profile far: person facing 90° away from the cage at 1 m distance; (2) profile near: profile at 0.3 m; (3) stare far: direct stare at the animal at 1 m; (4) stare near: stare at 0.3 m. Trials were 30 s (Variant 1) and 60 s (Variant 2). Tests were repeated across 5 days.
Behavioral data were scored live using a 1/0 sampling method by trained observers (as in53,54,78). In Variant 1, six 5 s bins were used across 30 s trials. In Variant 2, six 10 s bins were used across 60 s trials. Behaviors that occurred within a bin received a score of 1 and behaviors that did not occur received a 0. To standardize across variants for data analysis, only the first three bins were used for monkeys in Variant 2 (totaling 30 s). Position in cage and six affect-related behaviors (i.e., lipsmack, grimace, threat, cage shake, tooth grind, and yawn) were scored (see Table S2). Position in cage was scored based upon the location of the monkey’s head in the cage (i.e., if the head was present in the front half of the cage, even if the remainder of the body was in the rear portion, this was scored as being at the front). Affective behaviors were summed within and across bins to generate a continuous index of affective reactivity for each animal during each trial.
Statistical analysis
R version 4.0.480 was used for statistical analyses. Data were analyzed using linear mixed-effects (LME) models in lme481 fit by maximum likelihood (Laplace Approximation); subject ID was included as a random effect in all models. Follow-up LME models were fit to determine group differences in particular test conditions. Planned comparisons were made using emmeans82. To limit the number of comparisons, two were set a priori: (1) individually-housed monkeys were compared to all of the socially-housed monkeys (i.e., grate-, intermittently-, and continuously-paired) combined and (2) grate-paired monkeys were compared to all of the full-contact monkeys (i.e., intermittently- and continuously-paired) combined. A multi-variate t adjustment was applied. For models that included only three social housing conditions (i.e., ORT models that evaluated responses to complex objects, which did not include grate-paired monkeys), only one comparison was made: individually-housed monkeys were compared to socially-housed monkeys (i.e., intermittently- or continuously-paired). Latency data were analyzed using a survival analysis (mixed effects Cox model implemented in coxme83) with a censored latency of 30 s. Difference scores were computed and analyzed for each animal using the HIT stare near and stare far data to investigate group differences across conditions. Importantly, analyses carried out via these procedures are relatively insensitive to unequal sample sizes84.
Analysis of monkeys’ ages at the time of testing revealed that there were significant differences in age across social housing conditions at the beginning of HIT, but not at the beginning ORT (see Supplementary Results; Supplementary Fig. 1A,B). As a result, we included age in the HIT models. Although previous research has not investigated age-related effects on ORT outcomes per se, one study showed that social housing conditions, and not age, impacted monkeys’ tendency to manipulate novel objects85. Given this, and the lack of significant group differences in age for ORT, we did not include age in our ORT models. There were also significant differences across samples in terms of how much time they had spent indoors prior to testing (see Supplementary Results; Supplementary Fig. 1C,D) and so this variable was included as a fixed effect in all models to be certain that social housing effects could not be explained by other means. Neither age nor duration of previous indoor housing were significant predictors in any of our analyses (all p > 0.05). As such, we do not report these effects throughout the “Results”.
Results
Experiment 1: object responsivity test
Food retrieval
Retrieval frequencies during complex object trials were analyzed first as all subjects completed these trials. We fit a generalized linear model with housing condition and duration of previous indoor housing as fixed effects and trials nested within subjects as a random effect. There was a significant effect of housing condition on frequency of food retrieval (χ2(3) = 14.74, p = 0.002). Custom contrasts (see “Methods”) were used to compare the estimated marginal means across housing conditions and the multi-variate t adjustment was applied. Individually-housed monkeys retrieved food significantly more frequently than socially-housed monkeys (i.e., grate-, intermittently-, or continuously-paired; p = 0.009) and grate-paired monkeys retrieved food significantly less frequently than monkeys housed in full-contact (i.e., intermittently- or continuously-paired; p = 0.04) (see Fig. 1a).
Food retrieval during the Object Responsivity Test. (a) Retrieval frequency during complex object trials for all monkeys. Individually-housed monkeys retrieved food significantly more frequently than socially-housed monkeys and grate-paired monkeys retrieved food significantly less frequently than those housed in full-contact. (b) Retrieval frequency during simple (triangle) and complex (circle) object trials for monkeys who completed both trial types. Groups did not differ significantly in retrieval frequency when both levels of object complexity were included. Means ± adjusted 95% confidence intervals and individual data are shown. (c) Cumulative probability of food reward retrieval as a function of time elapsed in trial during complex object trials. Individually-housed monkeys retrieved food significantly faster than socially-housed monkeys and grate-paired monkeys retrieved food significantly slower than monkeys housed in full-contact. (d) Cumulative probability of food reward retrieval as a function of time elapsed in trial during simple (solid line) and complex (dashed line) object trials for monkeys who completed both trial types. Individually-housed monkeys retrieved food significantly faster than socially-housed monkeys in the presence of complex, but not simple, objects. Survival curves are shown. Retrieval latencies of 30 s (no retrieval) are right-censored.
Food retrieval frequencies during simple object (i.e., featureless objects like wooden blocks) and complex object (i.e., information-rich objects like animal figurines or household objects) trials were analyzed for monkeys who completed both trial types. We again fit a generalized linear model, this time with housing condition, object complexity (i.e., simple or complex), and duration of previous indoor housing as fixed effects, a housing condition × complexity interaction, and trials nested within subjects as a random effect. As only one grate-paired monkey experienced both trial types, this animal was excluded from all analyses including both simple and complex objects. There was a significant main effect of complexity (χ2(1) = 4.93, p = 0.03) on food retrieval; all monkeys retrieved food more frequently during simple vs. complex object trials. The effect of housing condition was not significant (χ2(2) = 3.48, p = 0.18) and the interaction between housing condition and complexity was not significant (χ2(2) = 1.31, p = 0.52). Although the effect of housing condition was not significant, visual inspection of the data suggested a possible impact of social housing, which we may have been underpowered to detect (see Fig. 1b). Although not significantly, individually-housed monkeys retrieved food more frequently during both simple (Mean ± SD = 0.94 ± 0.25) and complex (Mean ± SD = 0.83 ± 0.40) object trials than intermittently- (simple: Mean ± SD = 0.59 ± 0.53; complex: Mean ± SD = 0.50 ± 0.54) or continuously-paired (simple: Mean ± SD = 0.47 ± 0.54; complex: Mean ± SD = 0.40 ± 0.53) monkeys.
We fit a mixed-effects Cox proportional hazards model on latency to retrieve the food reward during complex object trials. Housing condition also significantly influenced food retrieval latency (χ2(3) = 16.39, p < 0.001) during these trials. Individually-housed monkeys retrieved food significantly faster than socially-housed monkeys (p = 0.007) and grate-paired monkeys retrieved food significantly slower than monkeys housed in full-contact (p = 0.02) (see Fig. 1c). We fit an additional mixed-effects proportional hazards model on latency to retrieve the food reward during both simple and complex object trials, including a housing condition × complexity interaction. When these trials were analyzed, there was a significant main effect of object complexity (χ2(1) = 38.40, p < 0.001). The effect of housing condition failed to reach the conventional level of significance (χ2(3) = 6.65, p = 0.08). There was, however, a significant interaction between housing condition and complexity (χ2(3) = 12.25, p = 0.007). Individually-housed monkeys retrieved food significantly faster than monkeys paired in full-contact in the presence of complex objects (p = 0.01), but not simple objects (p = 0.18). All groups retrieved the food faster during simple vs. complex object trials (see Fig. 1d).
Affective reactivity
As described in the Methods, affective reactivity was computed for each subject during each trial by summing the frequencies of individual affective behaviors generated. We then fit a generalized linear model with housing condition and duration of previous indoor housing as fixed effects and trials nested within subjects as a random effect to assess affective reactivity during complex object trials. There was a significant effect of housing condition on affective reactivity to complex objects (χ2(3) = 13.27, p = 0.004). Individually-housed monkeys were less reactive to complex objects than socially-housed monkeys (p = 0.005) and grate-paired monkeys did not differ significantly from monkeys housed in full-contact (p = 0.21) (see Fig. 2a). We fit an additional generalized linear model with housing condition, object complexity, and previous duration of indoor housing as fixed effects, a housing condition × complexity interaction, and trials nested within subjects as a random effect to assess responses to both simple and complex objects. The main effect of complexity (χ2(1) = 13.96, p < 0.001) was significant. The effect housing condition (χ2(2) = 4.98, p = 0.08) failed to reach the conventional level of significance and the interaction between housing condition and complexity was not significant (χ2(2) = 0.60, p = 0.74). Despite the effect of housing condition failing to reach the conventional level of significance, visual inspection of the data suggested a potential impact of housing condition that we were underpowered to detect (see Fig. 2b). Individually-housed monkeys were less reactive during both trial types (simple: Mean ± SD = 0.03 ± 0.18; complex: Mean ± SD = 0.08 ± 0.39) than intermittently- (simple: Mean ± SD = 0.88 ± 2.07; complex: Mean ± SD = 1.33 ± 2.67) or continuously-paired (simple: Mean ± SD = 0.69 ± 1.69; complex: Mean ± SD = 1.39 ± 2.46) monkeys. All groups showed greater reactivity to complex as compared to simple objects.
Affective reactivity during the Object Responsivity Test. (a) Affective reactivity (combined frequency of all affective behaviors) during complex object trials. Individually-housed monkeys were significantly less reactive than socially-housed monkeys. (b) Affective reactivity during simple (triangle) and complex (circle) object trials for monkeys who completed both trial types. All monkeys were significantly more reactive to complex vs. simple objects. Groups did not differ significantly when both levels of complexity were considered. Means ± adjusted 95% confidence intervals and individual data are shown.
Experiment 2: human intruder test
Position in cage
We analyzed position in cage—determined by the presence of the monkey’s head either in the front or rear half of the cage—using a generalized linear model with housing condition, task condition (profile far, profile near, stare far, stare near), test day (days 1–5), age, and previous duration of indoor housing as fixed effects, housing condition × test day and housing condition × task condition interactions, and trials nested within subjects as a random effect. There was a significant main effect of task condition on position at front of cage (χ2(3) = 14.81, p = 0.002) such that monkeys spent more time at the front of the cage during the profile as compared to the stare conditions. The effects of test day (χ2(4) = 1.26, p = 0.87) and housing condition (χ2(3) = 1.23, p = 0.75) were not significant. There was a significant interaction of housing condition and task condition (χ2(9) = 33.97, p < 0.001). Comparison of the estimated marginal means revealed significant differences between the individually-housed and socially-housed monkeys only in the stare near condition (p < 0.001; profile far: p = 0.37; profile near: p = 0.54; stare far: p = 0.50). Individually-housed monkeys spent less time at the front of the cage during this condition than socially-housed monkeys (see Fig. 3a). Grate-paired monkeys did not differ significantly from monkeys housed in full-contact in any of the conditions (profile far: p = 0.70; profile near: p = 0.37; stare far: p = 0.81; stare far: p = 0.79).
Responses during the Human Intruder Test. (a) Tendency to be present at the front of the cage (scored as the presence of the monkey’s head in the front or rear half of the cage) across condition for each group. Individually-housed monkeys spent less time at the front of the cage than socially-housed monkeys during stare near. (b) Affective reactivity (combined frequency of affective behaviors) across condition for each group. Groups did not differ significantly across conditions. (c) Affectivity reactivity difference scores (stare near–stare far) for each group. Individually-housed monkeys had significantly higher difference scores than socially-housed monkeys. Means ± adjusted 95% confidence intervals and individual data are shown.
Affective reactivity
We analyzed affective reactivity using an identical model to that specified for position in cage. There were significant main effects of test day (χ2(4) = 17.20, p = 0.002) and task condition (χ2(3) = 283.79, p < 0.001) on affective reactivity to the human intruder. The main effect of housing condition was not significant (χ2(3) = 0.22, p = 0.97). The interaction between test day and housing condition was not significant (χ2(12) = 19.79, p = 0.07). However, the interaction between housing condition and task condition was significant (χ2(9) = 29.27, p < 0.001) (see Fig. 3b). All groups showed declining reactivity across test days and increased reactivity in the stare conditions relative to the profile conditions.
To understand what was driving the significant interaction between housing condition and task condition, we analyzed responses in the two profile and two stare conditions separately, as monkeys are often lowly reactive during profile78. As expected, reactivity was low during profile, so there was not a significant effect of housing condition (χ2(3) = 2.55, p = 0.47), test day (χ2(4) = 2.29, p = 0.68) or distance (χ2(1) = 0.52, p = 0.47), nor was the interaction between housing condition and distance (χ2(3) = 1.71, p = 0.55) significant. There was a significant interaction between housing condition and test day (χ2(12) = 27.96, p = 0.006). Comparison of the estimated marginal means revealed that grate-paired monkeys were significantly less reactive on testing day 2 than monkeys housed in full-contact (p = 0.02). Grate-paired and full-contact groups did not differ significantly on any other days (all p > 0.05) and individually- and socially-housed monkeys did not differ significantly on any test day (all p > 0.05). When reactivity during stare trials was considered, there were significant main effects of test day (χ2(4) = 14.64, p = 0.006) and distance (χ2(1) = 37.19, p < 0.001), but not housing condition (χ2(3) = 0.14, p = 0.99). Critically, here, the interaction between housing condition and distance was significant (χ2(3) = 19.29, p < 0.001). Comparison of the estimated marginal means did not reveal a significant difference between individually- and socially-housed monkeys in either stare far (p = 0.87) or stare near (p = 0.84) and grate-paired monkeys also did not differ significantly from those housed in full-contact in either condition (stare far: p = 0.98; stare near: p = 0.97). Individually-housed monkeys appeared to be more reactive in stare near than stare far, while socially-housed monkeys showed similar reactivity across conditions. To investigate this, we computed difference scores between stare near and stare far responses for each monkey. Analysis of the difference scores revealed a significant effect of housing condition (χ2(3) = 12.81, p = 0.005). Individually-housed monkeys had significantly higher difference scores than socially-housed monkeys (p = 0.01). The difference scores of grate-paired monkeys did not differ significantly from monkeys housed in full-contact (p = 0.34) (see Fig. 3c).
Discussion
Despite many decades of use in neuropsychological experiments, the social contexts in which adult monkeys live and the impacts that these contexts may have on behavioral responses in tasks commonly used to assess psychosocial behavior have historically gone unacknowledged. Our data demonstrate that social context matters for affective reactivity. Adult monkeys housed in different social conditions responded differently in the very same tasks that behavioral neuroscientists and psychologists use to model neuropsychiatric disorders86,87,88,89,90 and determine the neural correlates of affective processing54,55,58,59,63,75,91. Adult monkeys housed individually during experiments generated different affective responses to threat compared to monkeys with social contact (either grate-, intermittently- or continuously-paired). Patterns of behaviors were consistent across experiments relative to housing condition.
When presented with ostensibly threating objects, individually-housed monkeys’ affective responses were dampened; they retrieved food rewards from beside objects faster, more frequently, and while generating fewer affective behaviors than socially-housed monkeys. Similarly, during HIT, isolated monkeys were less reactive and more willing to spend time at the front of the cage than socially-housed monkeys in three conditions. In the 4th condition, stare near, socially isolated monkeys exhibited the greatest affective reactivity, suggesting a different threshold for response than socially-housed monkeys. Importantly, there was variance in responses across ostensibly social contexts as well. Monkeys with restricted social access (grate-pairing) behaved differently from monkeys with full physical contact; they retrieved food rewards slower, less frequently, and while generating fewer behaviors during ORT. During HIT, however, grate-paired monkeys behaved comparably to those housed in full-contact, suggesting a more subtle impact on affective responding than isolation.
Our data demonstrate that social housing conditions during adulthood shape affective responding in a way that may be consistent with some of the impacts of variation in socialization in early development. One of the most indelible findings in comparative psychology is the demonstration that social isolation in infancy causes lasting disruptions to normal patterns of social and exploratory behaviors, originally observed by Harlow and colleagues31,92,93. While many studies of social restriction in infancy focus on the impact of rearing baby monkeys in nurseries without their mothers (typically with humans, or with other caretakers, such as dogs in94), accumulating evidence demonstrates that infants raised alone with their mothers or with another mother-infant pair also have disrupted behavior and physiology (e.g., increased heart rates and lower respiratory sinus arrhythmia95; immune response reductions96). Just as we found blunted reactivity to threat in individually-housed adult monkeys, Gottlieb and Capitanio79 found that nursery-reared infants had significantly lower “aggression” (including threats and vocalizations) and “displacement” (including yawns and tooth grinding) scores than outdoor socially reared-infants when responses to a threatening human intruder were assessed. Consistent with this and our results, Shannon et al.97 also found that mother-reared infants exhibited higher cortisol levels than nursery-reared infants (i.e., those raised with inanimate surrogate mothers) in response to examination and handling by a human experimenter, suggesting that socially isolated rearing blunts reactivity. Additional research is needed in which identical methodologies are used to make behavioral assessments of monkeys socially isolated during rearing or during adulthood, but our data suggest that social contexts in adulthood may have similar impacts on psychosocial outcomes that have simply gone uninvestigated because of norms related to husbandry in behavioral neuroscience.
One interpretation of the behavioral differences that we see in individually-housed monkeys is that they may be exhibiting a depression-like phenotype, which is potentially consistent with the human literature indicating that loneliness is a significant variable impacting depression (e.g., see98 for a meta-analysis). The emotion context-insensitivity (ECI) hypothesis of depression99 posits that depressed individuals exhibit diminished emotional reactivity to negative stimuli100, as we show in individually-housed monkeys. Additional research is needed to determine whether individually-housed monkeys also exhibit diminished affective reactivity to positive stimuli, which represents the other major component of the ECI hypothesis. Human studies also indicate a relationship between depression and impulsivity101,102, with depressed individuals showing increased impulsivity across three dimensions: behavioral loss of control, non-planning, and cognitive impulsivity103. Such a relationship may explain why individually-housed monkeys in our sample retrieved food rewards from beside threatening objects quicker and more frequently than socially-housed monkeys. A limitation of our study is that we do not also have home cage behavioral observations from these monkeys. As such, we could not determine whether individually-housed monkeys exhibited depression-like behaviors in this setting, including hunched posture, laying down, and increased day time sleep—behaviors that have previously been shown to increase when monkeys were relocated from social to non-social housing43,44.
Our data also make clear that not all social contexts are created equal. Restricted social access (grate-pairing) appears to be different from social housing conditions permitting at least some full-contact with a partner. During complex object trials, grate-paired monkeys were most reactive and retrieved the food reward less frequently than other socially-housed monkeys. This was the opposite pattern of results generated by isolated monkeys. One hypothesis is that grate-pairing causes an anxiety-like phenotype while isolation causes a depression-like phenotype, causing animals in those conditions to behave differently from the mean (or “normal”) in different ways. Prior studies assessing the differences between monkeys housed in protected contact (i.e., with a barrier allowing limited tactile contact, similar to our grate-paired monkeys) and those housed in full-contact have demonstrated significantly higher abnormal and anxiety-related behaviors during home cage observations for protected contact monkeys104,105. An additional study showed that monkeys housed in protected contact (in this case, much less restrictive contact, permitting monkeys the ability to reach entire limbs through to the other side of the cage) also exhibited significantly more motor stereotypic behaviors than monkeys housed in full contact51. While future research is needed to determine the mechanism behind this behavioral phenotype, this may be due in part to increased stress associated with the ability of pairs to display agonistic behaviors (e.g., threatening, cage-shaking; which do not require contact) in close proximity while affiliative behaviors (e.g., grooming; which does require close contact) were more challenging.
Many neuropsychiatric and behavioral neuroscience studies have used individually-housed monkeys, including studies probing the neural basis of socioaffective processing60,62,64,70,106 and of addiction or drug-seeking behaviors107,108,109,110,111. Even studies intending to specifically address depression induced through means other than social isolation (e.g., administration of immune cytokines112; social stress113) and those intending to determine the impact of early social deprivation114 or stress115, have all used at least some socially isolated subjects. In light of the findings we present here, the documented effects of these manipulations may be severely confounded by the impact of restricted social contact. Considering the potential impact of social context in such studies may provide a beneficial opportunity to resolve conflicts in the literature (e.g., the role of the amygdala in social cognition, as discussed in 116). Further, the impact of adult social conditions may extend beyond the study of psychosocial processes to other domains of psychology, including cognition, for which monkeys have been proven an essential model for the advancement of biomedical research9,117.
Our analyses were made possible by access to data from experiments that were carried out using nearly identical protocols in the same laboratory. Given methodological consistencies across studies, it is unlikely that the effects are due solely to differences in experimental techniques. Importantly, our subjects were all raised in conditions most closely resembling the natural rearing conditions of macaques as are possible in a captive setting through adulthood (i.e., ~ 4 years of age118). Subjects were chosen for these experiments because they exhibited species-typical behaviors, without abnormalities, when observed in the large outdoor cages that they were raised in prior to being moved indoors. It is therefore striking that we observed as strong of an impact of adult social condition as we did. Nevertheless, as slight procedural and other changes may have occurred over the course of time and the experiments we describe here, future studies should replicate the present experiments in monkeys housed in different social contexts (both between and within subjects) and tested concurrently by the same experimenters. Future studies should also address the potential role of sex in the impact of social housing conditions. The present study is limited in that we only report data from male monkeys. While male monkeys have been used preferentially in the historical literature, as more work is done in female monkeys the potential for such differences will be important to understand.
Much of what we know about the neural basis of psychological functions come from causal manipulations in nonhuman animals’ brains. If control animals in these studies are behaviorally abnormal, then these subjects do not provide a faithful baseline for comparison of the experimental conditions. Further, the conditions that drive this altered phenotype may interact with experimental manipulations, leading to incorrect inferences about the manipulation’s impact. While we demonstrate the impact of social context, other aspects including diet, husbandry practices, and features of experimenters may drive similar differences. We aim to initiate a conversation regarding the potential impact of social context on the results of studies aiming to reveal the nature or pathophysiology of socioaffective processing, including the potential for rethinking existing inferences in the literature. We call on other researchers to increase the transparency and specificity with which they describe the social contexts their subjects are housed in, urge them to house animals in full-contact whenever possible, and compel individuals reviewing the published literature to carefully consider social context when assessing the strength or validity of prior studies and corresponding inferences.
Data availability
All data are available at: https://osf.io/n5ym2/.
References
Cryan, J. F. & Slattery, D. A. Animal models of mood disorders: Recent developments. Curr. Opin. Psychiatry 20, 1–7 (2007).
Nestler, E. J. & Hyman, S. E. Animal models of neuropsychiatric disorders. Nat. Neurosci. 13, 1161–1169 (2010).
Fernando, A. B. P. & Robbins, T. W. Animal models of neuropsychiatric disorders. Annu. Rev. Clin. Psychol. 7, 39–61 (2011).
Bliss-Moreau, E. & Rudebeck, P. H. Animal models of human mood. Neurosci. Biobehav. Rev. 120, 574–582 (2021).
Kalin, N. H. & Shelton, S. E. Nonhuman primate models to study anxiety, emotion regulation, and psychopathology. Ann. N. Y. Acad. Sci. 1008, 189–200 (2003).
Machado, C. J. & Bachevalier, J. Non-human primate models of childhood psychopathology: The promise and the limitations. J. Child Psychol. Psychiatry 44, 64–87 (2003).
Nelson, E. E. & Winslow, J. T. Non-human primates: Model animals for developmental psychopathology. Neuropsychopharmacology 34, 90–105 (2009).
Capitanio, J. P. & Emborg, M. E. Contributions of non-human primates to neuroscience research. Lancet 371, 1126–1135 (2008).
Phillips, K. A. et al. Why primate models matter. Am. J. Primatol. 76, 801–827 (2014).
Harding, J. D. Nonhuman primates and translational research: Progress, opportunities, and challenges. ILAR J. 58, 141–150 (2017).
Living without an amygdala. (The Guilford Press, 2016).
Qiu, Z. & Li, X. Non-human primate models for brain disorders—Towards genetic manipulations via innovative technology. Neurosci. Bull. 33, 247–250 (2017).
Cacioppo, J. T. & Cacioppo, S. The growing problem of loneliness. Lancet 391, 426 (2018).
Ge, L., Yap, C. W., Ong, R. & Heng, B. H. Social isolation, loneliness and their relationships with depressive symptoms: A population-based study. PLoS ONE 12, e0182145 (2017).
Courtin, E. & Knapp, M. Social isolation, loneliness and health in old age: A scoping review. Health Soc. Care Community 25, 799–812 (2017).
Schrempft, S., Jackowska, M., Hamer, M. & Steptoe, A. Associations between social isolation, loneliness, and objective physical activity in older men and women. BMC Public Health 19, 74 (2019).
Steptoe, A., Shankar, A., Demakakos, P. & Wardle, J. Social isolation, loneliness, and all-cause mortality in older men and women. Proc. Natl. Acad. Sci. 110, 5797–5801 (2013).
Baker, K. C. et al. Benefits of pair housing are consistent across a diverse population of rhesus macaques. Appl. Anim. Behav. Sci. 137, 148–156 (2012).
Bush, N. R. & Boyce, W. T. Differential sensitivity to context: Implications for developmental psychopathology. In Developmental Psychopathology (ed. Cicchetti, D.) 1–31 (Wiley, 2016). https://doi.org/10.1002/9781119125556.devpsy203.
Sorge, R. E. et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat. Methods 11, 629–632 (2014).
House, J., Landis, K. & Umberson, D. Social relationships and health. Science 241, 540–545 (1988).
Cohen, S. Social relationships and health. Am. Psychol. 59, 676–684 (2004).
Uchino, N., Cacioppo, J. T. & Kiecolt-Glaser, J. K. The relationship between social support and physiological processes: A review with emphasis on underlying mechanisms and implications for health. Psychol. Bull. 119, 488–531 (1996).
Cacioppo, J. T., Cacioppo, S., Capitanio, J. P. & Cole, S. W. The neuroendocrinology of social isolation. Annu. Rev. Psychol. 66, 733–767 (2015).
Alpass, F. M. & Neville, S. Loneliness, health and depression in older males. Aging Ment. Health 7, 212–216 (2003).
Catalano, G. et al. Anxiety and depression in hospitalized patients in resistant organism isolation. South. Med. J. 96, 141–145 (2003).
Helliwell, J. F. & Putnam, R. D. The social context of well–being. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 359, 1435–1446 (2004).
Karere, G. M. et al. What is an “adverse” environment? Interactions of rearing experiences and MAOA genotype in rhesus monkeys. Biol. Psychiatry 65, 770–777 (2009).
Barr, C. S. et al. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biol. Psychiatry 55, 733–738 (2004).
Stevens, H. E., Leckman, J. F., Coplan, J. D. & Suomi, S. J. Risk and resilience: Early manipulation of macaque social experience and persistent behavioral and neurophysiological outcomes. J. Am. Acad. Child Adolesc. Psychiatry 48, 114–127 (2009).
Harlow, H. F. & Suomi, S. J. Induced depression in monkeys. Behav. Biol. 12, 273–296 (1974).
Hannibal, D. L., Bliss-Moreau, E., Vandeleest, J., McCowan, B. & Capitanio, J. Laboratory rhesus macaque social housing and social changes: Implications for research: Macaque Laboratory Housing Changes and Research. Am. J. Primatol. 79, e22528 (2017).
DiVincenti, L. & Wyatt, J. D. Pair housing of macaques in research facilities: A science-based review of benefits and risks. J. Am. Assoc. Lab. Anim. Sci. 50, 856–863 (2011).
Mello, N. K. et al. The effects of cocaine on gonadal steroid hormones and LH in male and female rhesus monkeys. Neuropsychopharmacology 29, 2024–2034 (2004).
Turner, P. V. & Grantham, L. E. Short-term effects of an environmental enrichment program for adult cynomolgus monkeys. Contemp. Top. Lab. Anim. Sci. 41, 5 (2002).
Shively, C. A., Clarkson, T. B. & Kaplan, J. R. Social deprivation and coronary artery atherosclerosis in female cynomolgus monkeys. Atherosclerosis 77, 69–76 (1989).
Novak, M. A. Self-injurious behavior in rhesus monkeys: New insights into its etiology, physiology, and treatment. Am. J. Primatol. 59, 3–19 (2003).
Li, X. et al. Depression-like behavioral phenotypes by social and social plus visual isolation in the adult female Macaca fascicularis. PLoS ONE 8, e73293 (2013).
Hannibal, D. L. et al. Intermittent pair-housing, pair relationship qualities, and HPA activity in adult female rhesus macaques. Am. J. Primatol. 80, e22762 (2018).
Gilbert, M. H. & Baker, K. C. Social buffering in adult male rhesus macaques (Macaca mulatta): Effects of stressful events in single vs. pair housing: Social buffering in male rhesus macaques. J. Med. Primatol. 40, 71–78 (2011).
Schapiro, S. J., Bloomsmith, M. A., Porter, L. M. & Suarez, S. A. Enrichment effects on rhesus monkeys successively housed singly, in pairs, and in groups. Appl. Anim. Behav. Sci. 48, 159–171 (1996).
Doyle, L. A., Baker, K. C. & Cox, L. D. Physiological and behavioral effects of social introduction on adult male rhesus macaques. Am. J. Primatol. 70, 542–550 (2008).
Hennessy, M. B., Chun, K. & Capitanio, J. P. Depressive-like behavior, its sensitization, social buffering, and altered cytokine responses in rhesus macaques moved from outdoor social groups to indoor housing. Soc. Neurosci. 12, 65–75 (2017).
Hennessy, M. B., McCowan, B., Jiang, J. & Capitanio, J. P. Depressive-like behavioral response of adult male rhesus monkeys during routine animal husbandry procedure. Front. Behav. Neurosci. 8, 309–309 (2014).
Capitanio, J. P. & Cole, S. W. Social instability and immunity in rhesus monkeys: The role of the sympathetic nervous system. Philos. Trans. R. Soc. B Biol. Sci. 370, 20140104 (2015).
Gust, D. A., Gordon, T. P. & Hambright, M. K. Response to removal from and return to a social group in adult male rhesus monkeys. Physiol. Behav. 53, 599–602 (1993).
Xie, L. et al. Effect of living conditions on biochemical and hematological parameters of the cynomolgus monkey: Living conditions affect macaque blood. Am. J. Primatol. 76, 1011–1024 (2014).
Schapiro, S. J., Bloomsmith, M. A., Kessel, A. L. & Shively, C. A. Effects of enrichment and housing on cortisol response in juvenile rhesus monkeys. Appl. Anim. Behav. Sci. 37, 251–263 (1993).
Gordon, T. P. et al. Social separation and reunion affects immune system in juvenile rhesus monkeys. Physiol. Behav. 51, 467–472 (1992).
Baker, K. C. & Dettmer, A. M. The well-being of laboratory non-human primates: The well-being of laboratory primates. Am. J. Primatol. 79, e22520 (2017).
Gottlieb, D. H., Maier, A. & Coleman, K. Evaluation of environmental and intrinsic factors that contribute to stereotypic behavior in captive rhesus macaques (Macaca mulatta). Appl. Anim. Behav. Sci. 171, 184–191 (2015).
Truelove, M. A., Martin, J. E., Langford, F. M. & Leach, M. C. The identification of effective welfare indicators for laboratory-housed macaques using a Delphi consultation process. Sci. Rep. 10, 20402 (2020).
Charbonneau, J. A., Bennett, J. L. & Bliss-Moreau, E. Amygdala or hippocampus damage only minimally impacts affective responding to threat. Behav. Neurosci. 136(1), 30–45 (2021).
Bliss-Moreau, E., Santistevan, A. C., Bennett, J., Moadab, G. & Amaral, D. G. Anterior cingulate cortex ablation disrupts affective vigor and vigilance. J. Neurosci. 41, 8075–8087 (2021).
Mason, W. A., Capitanio, J. P., Machado, C. J., Mendoza, S. P. & Amaral, D. G. Amygdalectomy and responsiveness to novelty in rhesus monkeys (Macaca mulatta): Generality and individual consistency of effects. Emotion 6, 73–81 (2006).
Bernstein, S. & Mason, W. A. The effects of age and stimulus conditions on the emotional responses of rhesus monkeys: Responses to complex stimuli. J. Genet. Psychol. 101, 279–298 (1962).
Kalin, N. H. & Shelton, S. E. Defensive behaviors in infant rhesus monkeys: Environmental cues and neurochemical regulation. Science 243, 1718–1721 (1989).
Kalin, N. H., Shelton, S. E., Davidson, R. J. & Kelley, A. E. The primate amygdala mediates acute fear but not the behavioral and physiological components of anxious temperament. J. Neurosci. 21, 2067–2074 (2001).
Aggleton, J. P. & Passingham, R. E. Syndrome produced by lesions of the amygdala in monkeys (Macaca mulatta). J. Comp. Physiol. Psychol. 95, 961–977 (1981).
Machado, C. J. & Bachevalier, J. The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta). Behav. Neurosci. 120, 761–786 (2006).
Machado, C. J. & Bachevalier, J. Behavioral and hormonal reactivity to threat: Effects of selective amygdala, hippocampal or orbital frontal lesions in monkeys. Psychoneuroendocrinology 33, 926–941 (2008).
Chudasama, Y., Wright, K. S. & Murray, E. A. Hippocampal lesions in rhesus monkeys disrupt emotional responses but not reinforcer devaluation effects. Biol. Psychiatry 63, 1084–1091 (2008).
Pujara, M. S., Rudebeck, P. H., Ciesinski, N. K. & Murray, E. A. Heightened defensive responses following subtotal lesions of macaque orbitofrontal cortex. J. Neurosci. 39, 4133–4141 (2019).
Izquierdo, A. Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. J. Neurosci. 25, 8534–8542 (2005).
Kalin, N. H., Shelton, S. E. & Davidson, R. J. Role of the primate orbitofrontal cortex in mediating anxious temperament. Biol. Psychiatry 62, 1134–1139 (2007).
Howell, B. R. et al. Early adverse experience increases emotional reactivity in juvenile rhesus macaques: Relation to amygdala volume: Adverse Caregiving Increases Emotional Reactivity. Dev. Psychobiol. 56, 1735–1746 (2014).
Peterson, E. J. et al. Rhesus macaques (Macaca mulatta) with self-injurious behavior show less behavioral anxiety during the human intruder test. Am. J. Primatol. 79, e22569 (2017).
Kalin, N. H., Shelton, S. E., Fox, A. S., Oakes, T. R. & Davidson, R. J. Brain regions associated with the expression and contextual regulation of anxiety in primates. Biol. Psychiatry 58, 796–804 (2005).
Kilkenny, C., Browne, W., Cuthill, I. C., Emerson, M. & Altman, D. G. Animal research: Reporting in vivo experiments: The ARRIVE guidelines: Animal research: reporting in vivo experiments the ARRIVE guidelines. Br. J. Pharmacol. 160, 1577–1579 (2010).
Antoniadis, E. A., Winslow, J. T., Davis, M. & Amaral, D. G. Role of the primate amygdala in fear-potentiated startle: Effects of chronic lesions in the rhesus monkey. J. Neurosci. 27, 7386–7396 (2007).
Antoniadis, E. A., Winslow, J. T., Davis, M. & Amaral, D. G. The nonhuman primate amygdala is necessary for the acquisition but not the retention of fear-potentiated startle. Biol. Psychiatry 65, 241–248 (2009).
Babineau, B. A. et al. Context–specific social behavior is altered by orbitofrontal cortex lesions in adult rhesus macaques. Neuroscience 179, 80–93 (2011).
Banta Lavenex, P., Amaral, D. G. & Lavenex, P. Hippocampal lesion prevents spatial relational learning in adult macaque monkeys. J. Neurosci. 26, 4546–4558 (2006).
Emery, N. J., Capitanio, J. P., Mason, W. A., Machado, C. J. & Mendoza, S. P. The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta). Behav. Neurosci. 115, 515–544 (2001).
Bliss-Moreau, E., Toscano, J. E., Bauman, M. D., Mason, W. A. & Amaral, D. G. Neonatal amygdala or hippocampus lesions influence responsiveness to objects. Dev. Psychobiol. 52, 487–503 (2010).
Bliss-Moreau, E. & Moadab, G. The faces monkeys make. Sci. Facial Expr. (Oxf., 2017).
Bliss-Moreau, E., Bauman, M. D. & Amaral, D. G. Neonatal amygdala lesions result in globally blunted affect in adult rhesus macaques. Behav. Neurosci. 125, 848–858 (2011).
Bliss-Moreau, E. & Baxter, M. G. Estradiol treatment in a nonhuman primate model of menopause preserves affective reactivity. Behav. Neurosci. 132, 224–229 (2018).
Gottlieb, D. H. & Capitanio, J. P. Latent variables affecting behavioral response to the human intruder test in infant rhesus macaques (Macaca mulatta). Am. J. Primatol. 75, 314–323 (2013).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2019).
Bates, D., Maechler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
Lenth, R. V. emmeans: Estimated marginal means, aka least-squares means. (2021).
Therneau, T. M. coxme: Mixed effects cox models. (2020).
Snijders, T. A. B. Power and sample size in multilevel modeling. Encycl. Stat. Behav. Sci. 3, 1570–1573 (2005).
Novak, M. A. et al. Old, socially housed rhesus monkeys manipulate objects. Zoo Biol. 12, 285–298 (1993).
Shackman, A. J. et al. Neural mechanisms underlying heterogeneity in the presentation of anxious temperament. Proc. Natl. Acad. Sci. 110, 6145–6150 (2013).
Fox, A. S. et al. Intergenerational neural mediators of early-life anxious temperament. Proc. Natl. Acad. Sci. 112, 9118–9122 (2015).
Kalin, N. H., Shelton, S. E. & Davidson, R. J. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J. Neurosci. 24, 5506–5515 (2004).
Rogers, J., Shelton, S. E., Shelledy, W., Garcia, R. & Kalin, N. H. Genetic influences on behavioral inhibition and anxiety in juvenile rhesus macaques. Genes Brain Behav. 7, 463–469 (2008).
Teng, T. et al. Chronic unpredictable mild stress produces depressive-like behavior, hypercortisolemia, and metabolic dysfunction in adolescent cynomolgus monkeys. Transl. Psychiatry 11, 126 (2021).
Chudasama, Y., Izquierdo, A. & Murray, E. A. Distinct contributions of the amygdala and hippocampus to fear expression: Effects of amygdala and hippocampal lesions on emotion. Eur. J. Neurosci. 30, 2327–2337 (2009).
Harlow, H. F., Dodsworth, R. O. & Harlow, M. K. Total social isolation in monkeys. Proc. Natl. Acad. Sci. 54, 90–97 (1965).
Harlow, H. F. & Suomi, S. J. Production of depressive behaviors in young monkeys. J. Autism Child. Schizophr. 1, 246–255 (1971).
Mason, W. A. & Green, P. C. The effects of social restriction on the behavior of rhesus monkeys: IV. Responses to a novel environment and to an alien species. J. Comp. Physiol. Psychol. 55, 363–368 (1962).
Bliss-Moreau, E., Moadab, G. & Capitanio, J. P. Maternal rearing environment impacts autonomic nervous system activity. Dev. Psychobiol. 59, 551–556 (2017).
Lubach, G., Coe, C. & Ershler, W. Effects of early rearing environment on immune responses of infant rhesus monkeys. Brain. Behav. Immun. 9, 31–46 (1995).
Shannon, C., Champoux, M. & Suomi, S. J. Rearing condition and plasma cortisol in rhesus monkey infants. Am. J. Primatol. 46, 311–321 (1998).
Erzen, E. & Çikrikci, Ö. The effect of loneliness on depression: A meta-analysis. Int. J. Soc. Psychiatry 64, 427–435 (2018).
Rottenberg, J. & Gotlib, I. H. Socioemotional functioning in depression. In Mood Disorders: A Handbook of Science and Practice 61–77 (2004).
Rottenberg, J., Gross, J. J. & Gotlib, I. H. Emotion context insensitivity in major depressive disorder. J. Abnorm. Psychol. 114, 627–639 (2005).
Clarke, D. Impulsivity as a mediator in the relationship between depression and problem gambling. Personal. Individ. Differ. 40, 5–15 (2006).
Swann, A. C., Steinberg, J. L., Lijffijt, M. & Moeller, F. G. Impulsivity: Differential relationship to depression and mania in bipolar disorder. J. Affect. Disord. 106, 241–248 (2008).
Corruble, E., Benyamina, A., Bayle, F., Falissard, B. & Hardy, P. Understanding impulsivity in severe depression? A psychometrical contribution. Prog. Neuropsychopharmacol. Biol. Psychiatry 27, 829–833 (2003).
Baker, K. C. et al. Pair housing for female longtailed and rhesus macaques in the laboratory: Behavior in protected contact versus full contact. J. Appl. Anim. Welf. Sci. 15, 126–143 (2012).
Baker, K. C. et al. Comparing options for pair housing rhesus macaques using behavioral welfare measures: Options for Pair Housing Rhesus Macaques. Am. J. Primatol. 76, 30–42 (2014).
Taubert, J. et al. Amygdala lesions eliminate viewing preferences for faces in rhesus monkeys. Proc. Natl. Acad. Sci. 115, 8043–8048 (2018).
Claghorn, J. L., Ordy, J. M. & Nagy, A. Spontaneous opiate addiction in rhesus monkeys. Sci. New Ser. 149, 440–441 (1965).
Foltin, R. W. & Evans, S. M. A novel protocol for studying food or drug seeking in rhesus monkeys. Psychopharmacology 132, 209–216 (1997).
Negus, S. S. & Mello, N. K. Effects of chronic methadone treatment on cocaine- and food-maintained responding under second-order, progressive-ratio and concurrent-choice schedules in rhesus monkeys. Drug Alcohol Depend. 74, 297–309 (2004).
Gasior, M., Paronis, C. A. & Bergman, J. Modification by dopaminergic drugs of choice behavior under concurrent schedules of intravenous saline and food delivery in monkeys. J. Pharmacol. Exp. Ther. 308, 249–259 (2004).
Nader, M. et al. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: Initial and chronic exposure. Neuropsychopharmacology 27, 35–46 (2002).
Felger, J. C. et al. Effects of interferon-alpha on rhesus monkeys: A nonhuman primate model of cytokine-induced depression. Biol. Psychiatry 62, 1324–1333 (2007).
Shively, C. A. et al. Social stress-associated depression in adult female cynomolgus monkeys (Macaca fascicularis). Biol. Psychol. 69, 67–84 (2005).
Lewis, M. H., Gluck, J. P., Petitto, J. M., Hensley, L. L. & Ozer, H. Early social deprivation in nonhuman primates: Long-term effects on survival and cell-mediated immunity. Biol. Psychiatry 47, 119–126 (2000).
Mathew, S. J. et al. A magnetic resonance spectroscopic imaging study of adult nonhuman primates exposed to early-life stressors. Biol. Psychiatry 54, 727–735 (2003).
Bachevalier, J. & Málková, L. The amygdala and development of social cognition: Theoretical comment on Bauman, Toscano, Mason, Lavenex, and Amaral (2006). Behav. Neurosci. 120, 989–991 (2006).
Upright, N. A. & Baxter, M. G. Prefrontal cortex and cognitive aging in macaque monkeys. Am. J. Primatol. https://doi.org/10.1002/ajp.23250 (2021).
Fooden, J. Systematic Review of the Rhesus Macaque, Macaca Mulatta (Field Museum of Natural History, 2000).
Acknowledgements
The authors wish to thank Jeffrey Bennett for his help finding archival data records. This work was funded by R37MH57502, R01NS16980, R01MH41479, and R01MH75702 to DGA and F32MH087067 to EBM. The work was also supported by OD011107 to the California National Primate Research Center. The authors have no competing financial interests or potential conflicts of interest to declare.
Author information
Authors and Affiliations
Contributions
J.A.C., E.B.M., and D.G.A. were responsible for the study concept and design. J.A.C. and E.B.M. assisted with data analysis and interpretation of findings. J.AC. drafted the manuscript. E.B.M. and D.G.A. provided critical revision of the manuscript for important intellectual content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Charbonneau, J.A., Amaral, D.G. & Bliss-Moreau, E. Social housing status impacts rhesus monkeys’ affective responding in classic threat processing tasks. Sci Rep 12, 4140 (2022). https://doi.org/10.1038/s41598-022-08077-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-08077-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.