Abstract
Persons with type 2 diabetes (T2D) have neutrophil dysfunction with a higher risk of infection than those without diabetes. We conducted this study aiming to compare the risk of pneumonia between metformin use and nonuse in persons with T2D. We identified 49,012 propensity score-matched metformin users and nonusers from Taiwan’s National Health Insurance Research Database between January 1, 2000, and December 31, 2017. We used the Cox proportional hazards model to compare the risks of pneumonia and respiratory death. The mean (SD) age of the participants was 57.46 (12.88) years, and the mean follow-up time for metformin users and nonusers was 5.47 (3.71) years and 5.15 (3.87) years, respectively. Compared with the nonuse of metformin, the adjusted hazard ratios (95% CI) for metformin use in bacterial pneumonia, invasive mechanical ventilation, and respiratory cause of death were 0.89 (0.84–0.94), 0.77 (0.73–0.82), and 0.64 (0.56–0.74), respectively. A longer cumulative duration of metformin use had further lower adjusted hazard ratios in these risks compared with nonuse. In patients with T2D, metformin use was associated with significantly lower risks of bacterial pneumonia, invasive mechanical ventilation, and respiratory cause of death; moreover, longer metformin use duration was associated with lower hazard ratios of these risks.
Similar content being viewed by others
Introduction
Polymorphonuclear neutrophils adhere to vascular endothelium and transmigrate through blood vessels in response to chemotactic gradients to phagocytose and kill invading pathogens1. Reports suggest that persons with type 2 diabetes (T2D) have neutrophil dysfunction and disturbed cytokine production due to cumulated hyperglycemia and excess oxidative stress2, with a higher risk of infection than those without diabetes3. Studies have demonstrated that persons with T2D have a higher risk of the following than those without T2D: 3.0- to 4.3-fold for urinary tract infections, 1.8- to 2.0-fold for cellulitis, 1.2- to 2.6-fold for pneumonia, and 2.0- to 3.3-fold for sepsis4. People with T2D have a higher risk of developing comorbidities and vascular complications, and the presence of infection can increase morbidity and mortality3,4. The diabetes guidelines for infection are scarce5.
Persons with T2D may have pulmonary microangiopathy and impaired lung function6, with an increased risk of respiratory failure or death due to pneumonia3. According to Taiwan’s Diabetes Atlas report (2019), the odds ratios for pneumonia hospitalization in patients with T2D versus non-diabetes ranged from 2.86 to 2.93 between 2005 and 2014, with a significantly increasing trend7. Pneumonia constituted 3.52–7.27% of the leading cause of death in patients with T2D from 2005 to 2014, with a significantly rising trend8.
Metformin has long been considered the first-line medication for T2D and a key regulator of metabolism. Activation of adenosine 5'-monophosphate-activated protein kinase (AMPK) by metformin can activate neutrophils and regulate the secretion of cytokines with anti-inflammatory and antibacterial effects9. In diabetic mice, pretreatment with metformin could modify glucose flux across the airway epithelium and limit hyperglycemia-induced bacterial growth10. Therefore, this study aimed to compare the risks of pneumonia and respiratory death between metformin users and nonusers.
Results
Participants
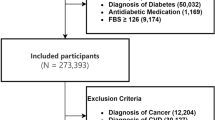
From January 1, 2000, to December 31, 2017, we identified 278,298 patients with newly diagnosed T2D. Of these, 176,556 were metformin users, and 101,742 were nonusers (Fig. 1). After excluding ineligible cases, 1:1 propensity score matching was used to construct 49012 pairs of metformin users and nonusers. In the matched cohorts (Table 1), 54.16% of patients were female; the mean (SD) age was 57.46 (12.88) years. The mean follow-up time for metformin users and nonusers was 5.47 (3.71) and 5.15 (3.87) years, respectively.
Main outcomes
In the matched cohorts (Table 2), 2133 (4.35%) metformin users and 2220 (4.53%) nonusers developed bacterial pneumonia during the follow-up period (incidence rate: 7.86 vs. 8.72 per 1000 person-years). In the multivariable model, metformin users showed a significantly lower risk of bacterial pneumonia than nonusers (aHR = 0.89, 95% CI = 0.84–0.94). Compared with nonusers, metformin users also showed significantly lower risks of IMV (aHR 0.77, 95% CI 0.73–0.82) and respiratory cause of death (aHR 0.64, 95% CI 0.56–0.74); however, metformin users demonstrated insignificant risks for all-cause pneumonia (aHR 1.01, 95% CI 0.96–1.06) and NIPPV (aHR 0.93, 95% CI 0.86–1.01) (Table 2).
The Kaplan–Meier analysis showed that the cumulative incidences of hospitalized bacterial pneumonia, IMV use, and respiratory cause of death were significantly lower in metformin users than in nonusers (Fig. 2).
Cumulative duration of metformin use
We investigated the association between the cumulative duration of metformin use and the risks of bacterial pneumonia, IMV, and respiratory cause of death (Table 3). We observed that a longer cumulative duration of metformin use was associated with lower risks of bacterial pneumonia, IMV, and respiratory cause of death, and if metformin use exceeded 364 days, all P values of lower aHR in these three outcomes were less than 0.001 (Table 3).
Additional analyses
The active comparison of metformin versus sulfonylureas disclosed that metformin use was associated with a lower risk of bacterial pneumonia (aHR 0.65, 95% CI 0.59–0.73. Table 4). The time-varying analysis of metformin exposure showed that metformin use was associated with reduced risk of bacterial pneumonia (aHR 0.92, 95% CI 0.855–0.988. P = 0.0213. Table 4). The subgroup analysis of three age groups of 20–40, 41–60, and 61–80 years revealed that metformin use was associated with a higher risk of bacterial pneumonia in patients with age of 20–40 years (aHR1.81, 95% CI 1.28–2.57), and with a lower risk of bacterial pneumonia in patients with age of 61–80 years (aHR 0.84, 95% 0.78–0.9). The stratified analysis of four different metformin daily dosage displayed that the higher daily dose of metformin use was associated with lower risks of bacterial pneumonia compared with metformin no-use (Table 4). The comparison of metformin use versus no-use exhibited that metformin was not significantly different in the risk of COPD exacerbation (aHR 0.97, 95% CI 0.92–1.02) in patients with T2D and COPD (Table 4).
Discussion
Our study showed that metformin use in persons with T2D was associated with significantly lower risks of bacterial pneumonia, IMV, and respiratory death than metformin nonuse. Moreover, a longer duration of metformin use tended to offer better protection against bacterial pneumonia and respiratory outcomes. Specifically, all the outcomes mentioned above had statistically significant protective effects when metformin use exceeded 364 days.
A study from the Emerging Risk Factors Collaboration revealed that cardiovascular disease and cancer were the main causes of death in persons with diabetes. However, infectious diseases [HR 2.39 (1.95–2.93)] and pneumonia [HR, 1.67 (1.45–1.92)] also accounted for a higher risk of death compared to non-diabetes persons11. Specifically, pneumonia is the most important infectious disease in patients with T2D3. Preclinical studies demonstrated that sputum and bronchial aspirates were enriched with bacteria in animals with hyperglycemia10. Diabetes may narrow the capillary lumen of the lung and impair pulmonary function6. Thus, diabetes may be a unique risk factor associated with an increased incidence of pneumonia1,4 and higher risks of hospitalization and mortality due to pneumonia than the non-diabetic state1,3. The American Diabetes Association guidelines recommend that patients with diabetes receive anti-influenza and pneumococcal vaccines to decrease the risks of influenza and pneumonia5. However, coverage rates for vaccines are inadequate, especially pneumococcal vaccination12. More efforts directed to prevent pneumonia are urgently needed to reduce the risk of progression to respiratory failure and death.
Studies show that metformin can prevent and mitigate tuberculosis infection13. A study on mice with diabetes showed that metformin could limit hyperglycemia-induced S. aureus growth in the airway10. One nested case-control study found no differences in the incidence of community-acquired pneumonia between oral antidiabetic drugs in monotherapy and metformin in patients with T2D14. However, a Danish population-based cohort study showed a lower risk of pneumonia hospitalization in patients with T2D and initiation with metformin therapy versus sulfonylurea or insulin15. Subgroup analyses conducted in the two studies mentioned above assessed the impact of metformin use in pneumonia risk. Yang et al. conducted a retrospective cohort study, aiming to compare the risk of pneumonia between metformin use and nonuse in individuals with T2D from the Hong Kong Diabetes Registry. This study disclosed that long-term use of metformin was associated with reduced risks of pneumonia and pneumonia-related death16. Our study is consistent with Yang’s study, showing that metformin use was associated with an 11% lower risk of bacterial pneumonia, and a longer duration and a higher dose of metformin use tended to lower the risk of bacterial pneumonia further. The comparative analysis of metformin versus sulfonylurea, and the time-varying analysis of metformin exposure also demonstrated that metformin use was associated with a lower risk of bacterial pneumonia. Additionally, the three age groups analysis revealed that younger metformin users (20–40 years) were associated with a higher risk of pneumonia (aHR1.81, 95% CI 1.28–2.57), probably because the number of younger metformin users was relatively small, and they may have suboptimal compliance of clinic visits and taking medications. However, metformin use seems to have a protective effect against pneumonia in older patients. For the older people (61–80 years), metformin users were less likely to suffer from bacterial pneumonia (aHR 0.84, 95% 0.78–0.9).
Several studies have suggested that preadmission metformin use may reduce mortality risk in patients with T2D and sepsis15. Experimental studies have demonstrated that metformin can alleviate acute lung injury and various sepsis-induced organ injuries17. Our study showed that metformin use was associated with a 23% lower risk for invasive mechanical ventilation than metformin nonuse in persons with T2D. Although metformin use in patients with hypoxia or critical condition may cause lactic acidosis17, these studies suggest that metformin use may have beneficial effects in such patients.
Metformin use was associated with significantly lower mortality risk in women with obesity or T2D who underwent hospitalization for COVID-19 infection18. Our study also showed that metformin use was associated with a 36% lower risk of respiratory causes of death than metformin nonuse in patients with T2D. This study has shown that metformin use was associated with lower risks of bacterial pneumonia and IMV use, contributing to the lower risk of respiratory death. In brief, metformin may reduce the risk of death from respiratory causes in patients with T2D.
The possible explanations for the role of metformin in preventing the development and progression of bacterial pneumonia are as follows: (1) Metformin inhibits mitochondrial respiratory-chain complex-1 and activates the liver kinase B1 (LKB1)/ AMPK pathway to facilitate neutrophil-dependent bacterial uptake and killing, and promote innate immune response18. (2) But it can suppress pro-inflammatory markers of high sensitivity C-reactive protein, interferon-α(IFN-α)19, tumor necrosis factor-α (TNF-α), and interleukin -6 (IL-6); and inhibits neutrophil activation and chemotaxis, improves neutrophil to lymphocyte ratio18, reduces B-cell intrinsic inflammation, increases antibody response, and stabilizes mast cells18. Metformin also can boost levels of the anti-inflammatory marker IL-1018. (3) The inhibition of mitochondrial complex-1 and electron transport can also suppress the energy production required for bacterial growth. Metformin inhibits bacterial gluconeogenesis, and the limited utilization of glycerol in the Kreb’s cycle reduces bacterial virulence; the anti-folate effect of metformin may inhibit the folate cycle of bacteria and limit bacterial growth20. Thus, metformin may attenuate the risk of bacterial pneumonia by its metabolic, immunologic, and antibacterial effects.
This study had some limitations. First, this dataset lacked information on family history, dietary patterns, physical activity, alcohol use, smoking habits, and vaccination status; it did not include data on hemoglobin A1C, biochemical tests, renal function, immune status, and pulmonary function test, precluding an accurate understanding of patient health status, and the severity of T2D. However, we matched the demographic information on age and sex to achieve a balance between the study and control groups; we matched the type and number of oral antidiabetic drugs, insulin use, and DCSI scores to balance the severity of T2D; we have matched the comorbidity of CKD to decrease the influence of renal function on outcomes, and further increase the comparability between the study and control groups. Second, metformin is contraindicated in patients with eGFR < 30 ml/min/1.73 m2. Because the NHIRD dataset lacks the information of renal function, we only excluded patients on dialysis or kidney transplant to avoid the effects of confounding by indication. Third, this study was conducted on Taiwanese people, and the results may not be generalizable to other ethnicities. Finally, cohort studies are likely to be influenced by some unknown and unmeasured confounding factors, and randomized controlled studies are warranted to confirm our results.
Standards of diabetes care have gradually become more comprehensive. A longer life expectancy in patients with T2D may contribute to the rise of several non-communicable and communicable diseases needing vigilance. Specifically, pneumonia is a critical communicable complication in patients with T2D; however, there are few recommendations on preventing pneumonia. Our study demonstrated that metformin was associated with lower risks of bacterial pneumonia, IMV use, and respiratory death. Metformin can play a beneficial role in reducing the risk and progression of pneumonia.
Methods
Study population
The Taiwanese government formed the Bureau of National Health Insurance in 1995 to establish the National Health Insurance (NHI) program. This program is a compulsory insurance system. Until 2000, nearly 99% of Taiwan’s 23 million people have joined the NHI program21. All information of the insured people, including age, sex, residence, insurance premium, diagnosis, medical procedures, and medications, are recorded in the NHI Research Database (NHIRD). The diagnosis is based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). The NHIRD is linked to the National Death Registry to certify mortality information. We confirmed that all methods were performed in accordance to Declaration of Helsinki. This study was approved by the Research Ethics Committee of China Medical University and Hospital (CMUH109-109-REC2-031). The identifiable information of patients and caregivers was encrypted before release, and informed consent was waived by the Research Ethics Committee.
Study design
We identified patients newly diagnosed with T2D between January 1, 2000, and December 31, 2017, and followed them up until December 31, 2018. The diagnosis of T2D was based on the ICD-9-CM code 250.xx for at least 2 outpatient visits or one hospitalization record. A previous study in Taiwan has performed a validation using ICD codes to define T2D22. Patients were excluded (Fig. 1) if they were (1) younger than 20 or older than 80 years; (2) missing age or sex data; (3) diagnosed with type 1 diabetes (250.1x) or heart failure (398.91, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 428, 429.4), received dialysis (V56.0, V56.8, V45.1) or kidney transplant (V42.0x), had hepatic failure (570, 572.2, 572.4, 572.8); (4) diagnosed with T2D before January 1, 2000, to exclude prevalent diseases before 2000; (5) followed up for less than 6 months after the index date.
Procedures
We defined the date of metformin use as the index date. Patients who received metformin treatment ≧ 28 days were study cases and those who never received metformin served as controls. The index date of the metformin nonusers was assigned as the same date as their corresponding paired metformin users’ index date. Some related variates were assessed and matched between the metformin user and nonuser, including age, sex, comorbidities included hypertension (401–405 and A26), dyslipidemia (272), coronary artery disease (CAD; 410–414), stroke (430–438), atrial fibrillation (427), peripheral arterial occlusive disease (PAOD; 440.0, 440.20, 440.21, 440.22, 440.23, 440.24, 440.3, 440.4, 443.9, 443.81, 443.89), chronic kidney disease (250.4x, 403.xx, 404.xx, 585.xx, 586.xx, 581.8x, 791.0x, 593.9x), retinopathy (362.02, 362.07, 362.0), chronic obstructive pulmonary disease (COPD; 491, 492, 496), liver cirrhosis (571.5, 571.2, 571.6), cancers (140–239), psychosis (290–299), depression (311), and dementia (290, 290.4, 291.2, 292.82 and 331), diagnosed within 1 year before the index date; medications use, including oral antidiabetic drugs, insulin, statin, and aspirin, during the follow-up period. We used the Charlson Comorbidity Index (CCI), Diabetes Complication Severity Index (DCSI) score23,24, and the number of oral antidiabetic drugs to evaluate T2D severity.
Main outcomes
Hospitalization for all-cause pneumonia (480–486), bacterial pneumonia (481, 482.41, 482.8, 486), noninvasive positive pressure ventilation (NIPPV; 93.90 and 93.91), invasive mechanical ventilation (IMV; 96.7) use, and respiratory causes of death (460–466, 470–478, 480–488, 490–496, 500–508 and 510–519) were the main outcomes of this study. A previous study in Taiwan has validated the algorithm of using ICD-9 codes to define pneumonia, with a sensitivity of 92.3–94.7%25. We calculated the events and incidence rates for hospitalized all-cause pneumonia, bacterial pneumonia, NIPPV, IMV, and respiratory causes of death during the follow-up period. The cumulative incidences of bacterial pneumonia, IMV, and respiratory causes of death were also compared between metformin users and nonusers.
Statistical analysis
Propensity-score matching was used to optimize the related covariates between metformin users and nonusers26. We estimated the propensity score for each patient using non-parsimonious multivariable logistic regression, with metformin use as the dependent variable. We included 28 clinically relevant covariates as independent variables (Table 1). The nearest-neighbor algorithm was used to construct matched pairs, assuming the P value > 0.05 to be a negligible difference between the case and comparison cohorts.
Crude and multivariable-adjusted Cox proportional hazards models were used to compare outcomes between metformin users and nonusers. The results are presented as hazard ratios (HRs) and 95% confidence intervals (CIs) for metformin users compared with nonusers. To calculate the investigated risks, we censored patients on the date of death, date of respective outcomes, or at the end of the follow-up on December 31, 2018, whichever came first. The Kaplan–Meier method and log-rank tests were used to compare the cumulative incidence of bacterial pneumonia, IMV, and respiratory cause of death during the follow-up period between metformin users and nonusers. We also assessed the cumulative duration of metformin use for the risks of bacterial pneumonia, IMV, and respiratory cause of death compared with metformin nonuse.
We have performed some additional analyses. (1) The comparative analysis of metformin versus sulfonylureas by matching demographics, comorbidities, medication use and disease stage, to provide an active comparison of metformin against sulfonylurea in the risk of bacterial pneumonia. (2) A time-varying analysis, assigning metformin exposure as a time-varying covariate, to decrease the biases of metformin discontinuation or immortal time bias. (3) A subgroup analysis of three age groups of 20–40, 41–60, and 61–80 year to investigate different effects of metformin use in different age groups. (4) A stratified analysis of four different metformin daily dosage to detect dose-response effects for metformin use. (5) A sensitive analysis to compare the incidence rates of acute COPD exacerbation (indicated by the prescription of systemic corticosteroids or antibiotics at outpatient department, hospitalization or an emergency room visit for COPD) of metformin use versus metformin no-use in patients with T2D and COPD.
SAS (version 9.4; SAS Institute, Cary, NC, USA) was used for statistical analysis; a two-tailed P value < 0.05 was considered significant.
References
Peleg, A. Y., Weerarathna, T., McCarthy, J. S. & Davis, T. M. E. Common infections in diabetes: Pathogenesis, management and relationship to glycaemic control. Diabetes Metab. Res. Rev. 23, 3–13. https://doi.org/10.1002/dmrr.682 (2007).
Gan, Y. H. Host susceptibility factors to bacterial infections in type 2 diabetes. PLoS Pathog. 9, e1003794. https://doi.org/10.1371/journal.ppat.1003794 (2013).
Pearson-Stuttard, J., Blundell, S., Harris, T., Cook, D. G. & Critchley, J. Diabetes and infection: Assessing the association with glycaemic control in population-based studies. Lancet Diabetes Endocrinol. 4, 148–158. https://doi.org/10.1016/S2213-8587(15)00379-4 (2016).
Luk, A. O. Y. et al. Temporal trends in rates of infection-related hospitalisations in Hong Kong people with and without diabetes, 2001–2016: A retrospective study. Diabetologia 64, 109–118. https://doi.org/10.1007/s00125-020-05286-2 (2021).
American Diabetes Association. 4. Comprehensive medical evaluation and assessment of comorbidities: Standards of medical care in diabetes-2021. Diabetes Care 44, S40–S52. https://doi.org/10.2337/dc20-S004 (2021).
Gläser, S., Krüger, S., Merkel, M., Bramlage, P. & Herth, F. J. F. Chronic obstructive pulmonary disease and diabetes mellitus: A systematic review of the literature. Respiration 89, 253–264. https://doi.org/10.1159/000369863 (2015).
Wang, J. S. et al. Hospitalization in patients with type 2 diabetes mellitus in Taiwan: A nationwide population-based observational study. J. Formos. Med. Assoc. 118, S90–S95. https://doi.org/10.1016/j.jfma.2019.06.017 (2019).
Li, H. Y. et al. Trends of mortality in diabetic patients in Taiwan: A nationwide survey in 2005–2014. J. Formos. Med. Assoc. 118, S83–S89. https://doi.org/10.1016/j.jfma.2019.07.008 (2019).
Zmijewski, J. W. et al. Mitochondrial respiratory complex I regulates neutrophil activation and severity of lung injury. Am. J. Respir. Crit. Care Med. 178, 168–179. https://doi.org/10.1164/rccm.200710-1602OC (2008).
Garnett, J. P. et al. Metformin reduces airway glucose permeability and hyperglycaemia-induced Staphylococcus aureus load independently of effects on blood glucose. Thorax 68, 835–845. https://doi.org/10.1136/thoraxjnl-2012-203178 (2013).
Seshasai, R. K. S. et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N. Engl. J. Med. 364, 829–841. https://doi.org/10.1056/NEJMoa1008862 (2011).
Smith, S. A. & Poland, G. A. Use of influenza and pneumococcal vaccines in people with diabetes. Diabetes Care 23, 95–108. https://doi.org/10.2337/diacare.23.1.95 (2000).
Tseng, C. H. Metformin decreases risk of tuberculosis infection in type 2 diabetes patients. J. Clin. Med. 7, 264. https://doi.org/10.3390/jcm7090264 (2018).
Gorricho, J. et al. Use of oral antidiabetic agents and risk of community-acquired pneumonia: A nested case–control study. Br. J. Clin. Pharmacol. 83, 2034–2044. https://doi.org/10.1111/bcp.13288 (2017).
Mor, A., Petersen, I., Sørensen, H. T. & Thomsen, R. W. Metformin and other glucose-lowering drug initiation and rates of community-based antibiotic use and hospital-treated infections in patients with type 2 diabetes: A Danish nationwide population-based cohort study. BMJ Open 6, e011523. https://doi.org/10.1136/bmjopen-2016-011523 (2016).
Yang, A. et al. Long-term metformin use and risk of pneumonia and related death in type 2 diabetes: A registry-based cohort study. Diabetologia 64, 1760–1765. https://doi.org/10.1007/s00125-021-05452-0 (2021).
Liang, H. et al. Association of preadmission metformin use and mortality in patients with sepsis and diabetes mellitus: A systematic review and meta-analysis of cohort studies. Crit. Care 23, 50. https://doi.org/10.1186/s13054-019-2346-4 (2019).
Bramante, C. T. et al. Metformin and risk of mortality in patients hospitalised with COVID-19: A retrospective cohort analysis. Lancet Healthy Longev. 2, e34–e41. https://doi.org/10.1016/S2666-7568(20)30033-7 (2021).
Saenwongsa, W. et al. Metformin-induced suppression of IFN-α via mTORC1 signalling following seasonal vaccination is associated with impaired antibody responses in type 2 diabetes. Sci. Rep. 10, 3229. https://doi.org/10.1038/s41598-020-60213-0 (2020).
Maniar, K. et al. A story of metformin-butyrate synergism to control various pathological conditions as a consequence of gut microbiome modification: Genesis of a wonder drug?. Pharmacol. Res. 117, 103–128. https://doi.org/10.1016/j.phrs.2016.12.003 (2017).
Cheng, T. M. Taiwan’s new national health insurance program: Genesis and experience so far. Health Aff (Millwood). 22, 61–76. https://doi.org/10.1377/hlthaff.22.3.61 (2003).
Lin, C. C., Lai, M. S., Syu, C. Y., Chang, S. C. & Tseng, F. Y. Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J. Formos. Med. Assoc. 104, 157–163 (2005).
Meduru, P. et al. Chronic illness with complexity: Implications for performance measurement of optimal glycemic control. J. Gen. Intern. Med. 22, 408–418. https://doi.org/10.1007/s11606-007-0310-5 (2007).
Young, B. A. et al. Diabetes complications severity index and risk of mortality, hospitalization, and health care utilization. Am. J. Manag. Care 14, 15–23 (2008).
Su, V. Y. et al. Sleep apnea and risk of pneumonia: A nationwide population-based study. CMAJ 186, 415–421. https://doi.org/10.1503/cmaj.131547 (2014).
D’Agostino, R. B. Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat. Med. 17, 2265–2281. https://doi.org/10.1002/(SICI)1097-0258(19981015)17:19%3C2265::AID-SIM918%3E3.0.CO;2-B (1998).
Acknowledgements
We are grateful to the Health Data Science Center of China Medical University Hospital for providing administrative, technical, and funding support.
Author information
Authors and Affiliations
Contributions
F.-S.Y., C.-C.H. and C.-M.H. conceived the study. F.-S.Y., J.C.-C.W. and Y.-H.S. conducted the research. C.-C.H., C.-M.H., Y.-H.S., and J.C.-C.W. analysed the results. F.-S.Y., Y.-H.S., and C.-C.H. wrote this paper. All authors reviewed and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yen, FS., Wei, J.CC., Shih, YH. et al. Metformin use and the risk of bacterial pneumonia in patients with type 2 diabetes. Sci Rep 12, 3270 (2022). https://doi.org/10.1038/s41598-022-07294-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-07294-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.