Abstract
The experience of the early nationwide COVID-19 pandemic in South Korea led to an early shortage of medical resources. For efficient resource allocation, accurate prediction of the prognosis or mortality of confirmed patients is essential. Therefore, the aim of this study was to develop an accurate model for predicting COVID-19 mortality using epidemiolocal and clinical variables and for identifying a high-risk group of confirmed patients. Clinical and epidemiolocal variables of 4049 patients with confirmed COVID-19 between January 20, 2020 and April 30, 2020 collected by the Korean Disease Control and Prevention Agency were used. Among the 4049 total confirmed patients, 223 patients died, while 3826 patients were released from isolation. Patients who had the following risk factors showed significantly higher risk scores: age over 60 years, male sex, difficulty breathing, diabetes, cancer, dementia, change of consciousness, and hospitalization in the intensive care unit. High accuracy was shown for both the development set (n = 2467) and the validation set (n = 1582), with AUCs of 0.96 and 0.97, respectively. The prediction model developed in this study based on clinical features and epidemiological factors could be used for screening high-risk groups of patients and for evidence-based allocation of medical resources.
Similar content being viewed by others
Introduction
Coronavirus disease-2019 (COVID-19) has become a global pandemic that is threatening far more than a health crisis. It also affects societies and economics1,2,3. As the number of confirmed patients has explosively increased, there is a need for risk stratification both for preventing (i.e., home quarantine, social distancing) and for treating confirmed patients (i.e., hospitalization vs. community isolation). Although the COVID-19 pandemic has caused more than 5,000,000 death worldwide, there are also a significant number of asymptomatic patients who become infected and recover asymptomatically4. Identification of high-risk confirmed patients is required to allow better allocation of existing available medical resources. According to the Centers for Disease Control and Prevention (CDC), confirmed patients who are over 65 years old, who live in nursing homes, and who have at least one of the following conditions, chronic lung disease, serious heart conditions, severe obesity, diabetes, liver disease, and immunocompromised status, are at a high risk of death due to COVID-194. Although the CDC guideline has been used as a reference for overall patients, more precise prediction using patient multivariable data is required to evaluate individualized risk and to establish evidence for risk stratification5. In this context, an accurate model for predicting COVID-19 mortality and identifying risk factors could help stratify management strategies for patients who have a high risk of death. Previous mortality prediction studies reported in the early period of COVID-19 pandemic used relatively few variables and showed lower predictive performance6.
Since the COVID-19 outbreak started in Hubei Province of the People’s Republic of China, the majority of early prediction studies were based on Chinese data7,8,9,10.
Barda et al. established a prediction model by combining the development of a baseline respiratory infection risk predictor and a postprocessing method using Israel data11. However, since they did not have individual data, they were not able to test its prediction performance. According to Wynants’ review of early reported prediction models regarding COVID-19, these proposed models are poor with a high risk of bias due to the lack of external validation of models5.
Since South Korea is geopolitically close to China, it is one of the countries most affected by COVID-19 during the early stage of the pandemic. In reality, Korea experienced an explosive outbreak in the first two months since the first confirmed patient was detected on January 2012. A mortality prediction model using a machine method based on sociodemographic and medical information of national health insurance data has been proposed13. However, it was focused on socioeconomic variables as predictors rather than clinical and epidemiological factors. Clinical experience and epidemiological characteristics have been reported as major factors associated with heterogeneity of prognosis after COVID-19 confirmation14. Therefore, the aim of this study was to establish a COVID-19 mortality prediction model using clinical and epidemiological variables nationally collected by Central Disease Control Headquarters.
Results
Baseline characteristics
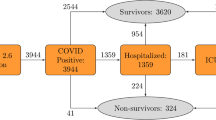
Since the first patient was confirmed with COVID-19 on January 20, 2020, 4049 patients were managed by the government database and released from quarantine or death until April 30, 2020. Among 4049 released patients, the case mortality was 5.51% (223 deaths and 3826 recoveries).
We compared the distribution of patients according to epidemiological and clinical characteristics. We also conducted a logistic regression analysis for mortality outcome by unadjusting (univariable) or adjusting (multivariable) covariates. Results are shown in Table 1. In univariable analysis, age over 40, male sex, runny nose, and headache significantly increased the risk of mortality, while having abnormal changes in consciousness (ACC), diabetes, hypertension, cancer history, dementia, and hospitalization in the intensive care unit was protective. In the multivariable analysis after adjusting for covariates, age over 40 years and having a runny nose remained significant risk factors for mortality. Protective variables remained protective after adjusting for covariates.
Factors associated with mortality from COVID‑19
Table 2 summarizes differences in clinical characteristics for continuous variables and the risk of COVID-19 mortality by 1-unit increase of each clinical variable. Heart rate intensity (OR 1.03, 95% CI 1.02–1.04) and temperature (OR 1.94, 95% CI 1.55–2.43) were associated with an increased risk of COVID-19 mortality. Higher levels of hemoglobin, hematocrit, and lymphocytes were associated with a significantly lower risk of mortality. Based on the exploratory analysis results shown in Tables 1 and 2, a prediction model for the development set was established, as shown in Table 3. The odds ratio (regression coefficient) of mortality risk was determined to produce a risk score.
COVID 19 Mortality = 0.9596*(Age 60–69) + 1.4935*(Age 70–79) + 3.3010*(Age ≥ 80) − 0.7845*(Sex) − 0.8813*(Fever) − 0.9160*(SOB) − 2.9806*(ACC) − 0.6318*(Diabetes) − 1.1150*(Malignancy) − 1.5940*(Dementia) − 2.0619*(Sickbed type) − 0.0767*(hematocrit) − 0.0694*(Lymphocyte).
Performance of prediction model
We applied our risk score to our total set, the development set, and the validation set. Figures 1, 2, 3 show comparison results between the predicted mortality and the actual mortality by risk score stratified by decile. Figure 1 shows the results for the total set of participants. Figure 2 describes results for the development set. Figure 3 shows results for the validation set. The performance of each dataset was evaluated using ROC curves. Results are shown in Fig. 4. Our prediction model showed good performance for both the development set and the validation set, with areas under the curve of 0.9656 and 0.9684, respectively.
Discussion
Our study developed and validated a COVID-19 mortality prediction model based on clinical and epidemiological data of COVID-19 4049 confirmed patients recruited by Korea Centers for Disease Control and Prevention. The high AUC value of 0.9684 indicated the good reliability and performance of our model. The course of clinical symptoms of coronavirus ranges from asymptomatic infection to acute respiratory distress (ARDS) and death. As the period of the COVID-19 global pandemic lasts longer, a shortage of medical resources comes earlier. Therefore, differentiated patient management based on evidence is required. Risk stratification also suggests evidence to allocate resources efficiently when medical resources are limited4. Several previous Korean studies have reviewed the characteristics of mortality cases of COVID-19. The Korean Society of Infectious Diseases and Korea Centers for Disease Control and Prevention has analyzed 54 COVID-19 mortality cases since the first mortality occurred from February 19 to March 10, 2020. The median age of mortality cases was 75.5 years. Of all mortality cases, 61.1% were men. The majority of such patients also had various underlying diseases, such as hypertension, heart disease, diabetes, dementia, and stroke. Another study reported in Korea focused on 20 mortality cases in Gyeongbuk Province and Daegu city, where the second outbreak wave occurred in February based on medical chart review15. Average age of mortality cases was 72 years. Of these mortality cases, 55.1% were women, and 74.5% had an underlying disease. The median length from hospitalization to death was 8 days. Comorbidities such as diabetes, chronic lung disease, and chronic neurologic disease were significant risk factors associated with COVID-19 mortality. Clinical manifestations observed before death were abnormal heart rate intensity, systolic blood pressure, respiratory rate, oxygen saturated by pulse oximetry on room air, and altered mental status16. Although these two studies reported the clinical characteristics of the deceased in detail at the level of descriptive epidemiology, which contributed to the overall understanding of COVID-19 patients, their numbers of cases were relatively small and were not enough for associational inference. One study developed an evidence-based COVID-19 prognostic model for military personnel in Korea17. Although there was a problem of generalization since it was developed for soldiers, age, body temperature, physical activity, history of cardiovascular disease, hypertension, visit to a region with an outbreak, feverishness, dyspnea, lethargy, and symptoms of chills were reported as significant predictors (overall C statistic: 0.963; 95% CI: 0.936–0.99)17.
Machine learning based COVID-19 mortality prediction on Korean population was reported by several studies13,18.
An et al. developed a COVID-19 mortality prediction model using machine learning after recruiting 10,237 COVID-19 confirmed patients and 228 mortality cases between January 20, 2020 and April 16, 202013. This prediction model used various variables, including socioeconomic status linked with National Health Insurance Service. However, specific clinical and epidemiological variables were lacking since that study was focused on the linkage with NHIS data. For mortality prediction, LASSO and linear SVM were used in that study, with AUC values of 0.963 and 0.962, respectively. The most significant factors in the mortality prediction model using LASSO were old age, preexisting DM, and cancer. The most significant factors in random forest were old age, infection route (cluster infection or infection from personal contact), and underlying hypertension13. However, that model could not be immediately applied to the field or clinics due to the lack of specific clinical variables.
Das et al. also aimed to predict mortality among confirmed COVID-19 patients in South Korea using machine learning and deploy the best performing algorithm as an open-source online prediction tool for decision-making. They found that the logistic regression algorithm was the best performer in terms of discrimination18. Oh et al. aimed to investigate whether comorbid musculoskeletal disorders (MSD)s and pain medication use was associated with in-hospital mortality among patients with COVID-19. They found MSDs were not associated with increased in-hospital mortality among patients with COVID-1919. Lee et al. found potential associations between physical activity and risk of infection, severe illness from COVID-19 and COVID-19 mortality using a nationwide cohort from South Korea20.
Previous foreign studies have reported that different clinical experiences can lead to substantial heterogeneity in the prognostic trajectory of COVID-19 confirmed patients spanning from patients who are asymptomatic to those with mild, moderate, and severe disease forms with low survival rates21,22. A COVID-19 mortality prediction model was developed previously by analyzing data from 3841 confirmed patients in New York, USA recruited from March 9 to April 6, 2020 using machine learning21. Sex, age, race, oxygen saturation, COPD, hypertension, and diabetes were found to be significant variables in that model, with AUCs of 0.91 to 0.94. However, blood test results were not included in that model. In that study, the minimum oxygen saturation was emphasized as a central factor in mortality prediction22.
A prediction model was developed after analyzing 53,001 ICU patients requiring mechanical ventilation as well as those diagnosed with pneumonia from the US Medical Information Mart for Intensive Care (MIMIC). When that model was applied to 114 confirmed COVID-19 patients23, the AUCs for 12, 24, 48, and 72 h were reported to be 0.82, 0.81, 0.77, and 0.75, respectively23. Our study probably used the largest data set up to date to predict COVID-19 mortality involving specific clinical features of COVID-19 patients in Korea. The main advantage of our study was that we collected large range of clinical and epidemiological variables at the time when patient was enrolled as a confirmed case. The results were obtained after a certain period of health system encounter or immediately after the diagnosis of COVID-19. Although we merely conducted logistic regression analysis, both the development and validation sets showed high areas under the curve (0.9656 and 0.9684, respectively). although there have been studies with larger sample sizes or extensive data collections, they had difficulty on interpretation on the results due to lack of algorithm.
Moreover, our model has the advantage of being able to easily interpret factors associated with the high mortality rate of individuals according to the detailed algorithm shown in the model. In that context, our model has high practical value for risk stratification in the clinical field.
The main limitation of our study was the issue of validation. Although our dataset was relatively large and involved specific clinical features, we merely conducted internal validation due to the lack of a dataset that had similar sizes and variables in Korea. Thus, the possibility of overestimation exists, which requires cautious interpretation of our results.
However, in terms of Personal Information Protection issues, current COVID-19 mortality data in Korea is merely collected and managed by the Government agency called Korean Disease Control and Prevention Agency (KDCA). Thus, no other dataset was available in Korea rather than the KDCA dataset. Thus, an external validation study using data from COVID-19 patients that occurred afterwards is required in the future.
Subjects and methods
Study population
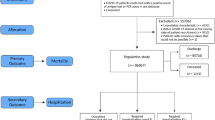
Our study was based on the dataset established by Korean Disease Control and Prevention Agency Central Disease Countermeasure Headquarters. Individual-level data for 4049 COVID-19 patients whose quarantine release was confirmed among patients infected between January 20, 2020 and April 30, 2020 were collected. Complete nationwide inpatient and outpatient data of patients who visited any medical institution with a confirmed diagnosis of COVID-19 during the study period were obtained. The definition of COVID-19 confirmation was determined by positive PCR-based clinical laboratory testing for SARS-CoV-2. Personal information deidentification measures were applied in accordance with governmental guidelines for nonidentification measures and proceeded in accordance with adequacy evaluation.
Risk factor measurement
The collected data used in our study included 41 variables categorized into seven subtypes as follows: (1) basic data (age, sex, death/quarantine released, length of stay between infection and death/quarantine released, pregnancy), (2) body index (height, weight), (3) initial examination finding (systolic/diastolic blood pressure, heart rate, body temperature), (4) clinical findings at hospitalization (history of fever, cough, sputum production, sore throat, runny nose/rhinorrhea, muscle aches/myalgia, fatigue/malaise, shortness of breath/dyspnea, headache, altered consciousness/confusion, vomiting/nausea, diarrhea), (5) comorbidity and past history (diabetes, hypertension, heart failure, chronic heart condition, asthma, chronic obstructive pulmonary disease, chronic kidney failure, cancer, chlimitaronic hepatic disease, rheumatism, autoimmune disease, dementia), (6) sickbed type and clinical severity, and (7) complete blood cell count. Each variable was either self-reported or recorded by professional health care providers. Mortality was defined when a patient with COVID-19 died during their encounter with the health system during the study period (January 1, 2020 ~ April 30, 2020). The data usage and study design of our study were approved by the Institutional Review Board of Ewha Womans University Seoul Hospital, and informed consent was obtained from each subject (SEUMC 2020-09-009). All methods were performed in accordance with relevant guidelines and regulations.
Statistical analysis
Risk scores for our COVID-19 mortality prediction model were developed by logistic regression analysis. We stratified our data into two groups: 60% random sampling (development set data) for model development and the remaining 40% (test data set) for internal validation. All analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
References
Mahase, E. Coronavirus covid-19 has killed more people than SARS and MERS combined, despite lower case fatality rate. BMJ 368, 641 (2020).
Ranney, M. L., Griffeth, V. & Jha, A. K. Critical supply shortages—the need for ventilators and personal protective equipment during the Covid-19 pandemic. N. Engl. J. Med. 382, e41 (2020).
Wu, J. T. et al. Estimating clinical severity of COVID-19 from the transmission dynamics in Wuhan, China. Nat. Med. 26, 506–510 (2020).
Centers for Disease Control and Prevention. People Who Are at Higher Risk for Severe Illness, https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-at-higher-risk.html (2021).
Wynants, L. et al. Prediction models for diagnosis and prognosis of covid-19: Systematic review and critical appraisal. BMJ 369, 2 (2020).
Caramelo, F., Ferreira, N. & Oliveiros, B. Estimation of risk factors for COVID-19 mortality-preliminary results. MedRxiv 25, 2 (2020).
Lu, J. et al. ACP risk grade: A simple mortality index for patients with confirmed or suspected severe acute respiratory syndrome coronavirus 2 disease (COVID-19) during the early stage of outbreak in Wuhan, China. medRxiv https://doi.org/10.1101/2020.02.20.20025510 (2020).
Shi, Y. et al. Host susceptibility to severe COVID-19 and establishment of a host risk score: Findings of 487 cases outside Wuhan. Crit. Care 241, 1–4 (2020).
Xie, J. et al. Development and external validation of a prognostic multivariable model on admission for hospitalized patients with COVID-19. MedRxiv 2, 2 (2020).
Yan, L. et al. Prediction of criticality in patients with severe Covid-19 infection using three clinical features: a machine learning-based prognostic model with clinical data in Wuhan. MedRxiv 2, 2 (2020).
Barda, N. et al. Developing a COVID-19 mortality risk prediction model when individual-level data are not available. Nat. Commun. 11(1), 1–9 (2020).
Kim, J. Y. et al. The first case of 2019 novel coronavirus pneumonia imported into Korea from Wuhan, China: Implication for infection prevention and control measures. J. Korean Med. Sci. 35(5), e61 (2020).
An, C. et al. Machine learning prediction for mortality of patients diagnosed with COVID-19: a nationwide Korean cohort study. Sci. Rep. 10(1), 1–11 (2020).
Park, S. et al. Analysis on 54 mortality cases of coronavirus disease 2019 in the Republic of Korea from January 19 to March 10, 2020. J. Korean Med. Sci 35, e132 (2020).
Lee, J. Y. et al. Risk factors for mortality and respiratory support in elderly patients hospitalized with COVID-19 in Korea. J. Korean Med. Sci. 35, 23 (2020).
Guan, W. J. et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 382, 1708–1720 (2020).
Heo, J. et al. COVID-19 outcome prediction and monitoring solution for military hospitals in South Korea: Development and evaluation of an application. J. Med. Internet Res. 22(11), e22131 (2020).
Das, A. K., Mishra, S. & Saraswathy, G. S. Predicting CoVID-19 community mortality risk using machine learning and development of an online prognostic tool. PeerJ 8, e10083 (2020).
Oh, T. K., Song, I., Lee, J., Eom, W. & Jeon, Y. T. Musculoskeletal disorders, pain medication, and in-hospital mortality among patients with COVID-19 in South Korea: A Population-Based Cohort Study. Int. J. Environ. Res. Public Health 18(13), 6804 (2021).
Lee, S. W. et al. Physical activity and the risk of SARS-CoV-2 infection, severe COVID-19 illness and COVID-19 related mortality in South Korea: A nationwide cohort study. Br. J. Sports Med. 2, 2 (2021).
Richardson, S. et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 323, 2052–2059 (2020).
Yadaw, A. S. et al. Clinical features of COVID-19 mortality: Development and validation of a clinical prediction model. Lancet Digital Health 2(10), e516–e525 (2020).
Ryan, L. et al. Mortality prediction model for the triage of COVID-19, pneumonia, and mechanically ventilated ICU patients: A retrospective study. Ann. Med. Surg. 59, 207–216 (2020).
Acknowledgements
This study was supported by the Korea Disease Control and Prevention Agency, KDCA, by establishing a dataset named “Clinical epidemiology information of COVID-19 confirmed patients”. This study was supported by the 2020 Korea Medical Institute Research and Development Project fund. This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI21C1243).
Author information
Authors and Affiliations
Contributions
Y.J. planned the study, performed all statistical analyses, and wrote the paper. Y.J.K. supported the data analysis and contributed to revising the paper. J.O., Y.K., E.H.H. and I.J. helped plan the study and revise the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jee, Y., Kim, YJ., Oh, J. et al. A COVID-19 mortality prediction model for Korean patients using nationwide Korean disease control and prevention agency database. Sci Rep 12, 3311 (2022). https://doi.org/10.1038/s41598-022-07051-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-07051-4
This article is cited by
-
Machine learning-based mortality prediction models for smoker COVID-19 patients
BMC Medical Informatics and Decision Making (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.