Abstract
Several limitations regarding pulse pressure variation (PPV) use have been reported. Our aim was to describe changes in the PPV operative performance as a predictor of fluid responsiveness during the development of a swine endotoxin shock model and to assess hemodynamic variables associated with PPV changes. A swine porcine endotoxin shock model was established (Escherichia Coli 055:B5 endotoxin) in 7 pigs, and 3 pigs were included in the control group. The endotoxin was infused until the mean arterial pressure (MAP) dropped below 50 mmHg (TH0); then, the model animal was reanimated with fluids and vasopressors. We performed fluid challenges every hour for 6 h. ROC curve analysis and a linear mixed model were performed. The area under the curve of PPV decreased from 0.95 (0.81–1.00) to 0.60 (0.17–1.00) at TH0. Its cutoff increased from 10.5 to 22.00% at TH0. PPV showed an inverse relationship with stroke volume, mean systemic filling pressure, MAP, and systemic vascular resistance (SVR) (p < 0.001, AIC = 111.85). The PPV operative performance as a predictor of fluid responsiveness decreased with the progression of shock. This could lead to an inverse association between PPV and the following variables: MAP and SVR.
Similar content being viewed by others
Introduction
Appropriate fluid therapy is key for the management of critically ill patients in intensive care units or the operating room1,2. The use of intravenous fluid therapy based on physiological variables related to fluid responsiveness has been beneficial in these patients3,4, whereas its use without defined hemodynamic goals has been associated with an increased medical complication rate5.
Dynamic indices related to cardiac preload have been generally used as predictors of fluid responsiveness. A positive response to fluid challenge is usually defined as a 10–15% increase in cardiac output (CO) or stroke volume (SV)6. Among these indices, pulse pressure variation (PPV) has been used as a predictor of fluid responsiveness in mechanically ventilated patients in several clinical settings. However, some limitations have been described7,8.
Although this hemodynamic variable has been studied for 20 years, little is known about its operative performance as a predictor of fluid responsiveness during hypotensive states, such as septic shock. Moreover, Monge et al. showed that PPV is related to effective arterial elastance9, a variable that summarizes the features of arterial vascular load in humans10. Therefore, its operative performance and its cutoff as a predictor of fluid responsiveness could change in hypotensive states.
This study aimed to describe changes in the PPV operative performance and cutoff of PPV as a predictor of fluid responsiveness during the induction of endotoxic shock in a swine model. We also used a statistical model to explore the possible relationship between PPV and other hemodynamic variables that could explicate this decrease in the PPV operative performance.
Materials and methods
Protocol
The study was carried out in accordance with the principles for the care and use of animals in research established by the Guide for the Care and Use of Laboratory Animals (NIH Guide)11, Resolution 008430 of 1993 issued by the Colombian Ministry of Health, and Law 84 of 1989 issued by the Congress of Colombia, the “Estatuto Nacional de Protección de Animales” (National Statute for the Protection of Animals). Additionally, this research was conducted according to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines12.
Preparation of animals
This study was approved by the Bioethics Committee of the Faculty of Veterinary Medicine of Universidad Nacional de Colombia (CB-FMVZ-UN-031-19). The study was conducted at the simulation laboratory of Instituto de Simulacion Medica (INSIMED) from January to December 2019. Ten female pigs (Yorkshire 40 ± 1.0 kg; ± 4 months old) were premedicated using tiletamine-zolazepam (ZOLETIL, Virbac Colombia) at 4.4 mg/kg doses through intramuscular injection. Then, each pig was cannulated through the marginal ear vein, and anesthesia was induced through the inhalation of isoflurane at a 1.5 minimum alveolar concentration (MAC) using an anesthesia mask. Once under general anesthesia, the pig was intubated and mechanically ventilated with a tidal volume of 10 ml/kg and a respiratory rate of 20 breaths/minute. For the maintenance of general anesthesia, isoflurane at doses of 1.5 MAC was used. Additionally, in all animals, the internal jugular vein and the femoral artery were cannulated using a central venous catheter (ARROW CV-17702) and an artery catheter (PiCCO PV2015L20-A). The quality of blood pressure signals was tested using a rapid washout test. All measurements were performed with the animal in a supine position, considering the phlebostatic axis as the zero reference.
Animals were placed on a stationary operating table and thermoregulated using medical blanket warmers, keeping their body temperature at a minimum level of 38 °C. During the development of the model, intravenous fluid was administered using normal saline solution (SS) at an infusion rate of 3 ml/kg/hour.
Measurements
A monitor with the pulse contour cardiac output system (TFT Mindray BeneVision N22 Patient Monitor) was used. CO was calculated as the average of three thermodilution boluses (20 ml of < 8 °C SS through the jugular venous catheter). SV was calculated as CO/heart rate (HR). Systemic vascular resistance (SVR) and PPV were automatically calculated by the PiCCO system. Finally, MSFP was estimated using the method of Parkin13: MSFP = 0.96 (CVP) + 0.04 (MAP) + 0.5 (CO).
Experimental protocol
After completion of the surgical procedures, animals were allowed to stabilize MAP (variation < 10%) at least for 10 min. After hemodynamic stabilization, animals were assigned to the control group (3 animals) or the endotoxin group (7 animals).
Animals in the endotoxin group received a continuous infusion of endotoxin (LPS E. Coli 055: B5, Sigma, St. Louis, MO) through the central venous catheter at an infusion rate of 7 μg/kg/h that was increased every 10 min (7, 14, and 20 μg/kg/h) until reaching 20 μg/kg/h 14. The infusion of endotoxin ended when an MAP < 50 mm Hg for at least 10 min was achieved. Pigs in the control group were not administered endotoxin.
When an MAP < 50 mm Hg was reached in the endotoxin group a fluid load was administered at a dose of 20 ml/kg for 20–30 min through the central venous catheter. In the control group, a similar fluid load was administered at 3 h of observation. Then, a noradrenaline infusion at a dose of 0.05 mcg/kg/min was started after the fluid load, and the infusion rate was increased 0.05 mcg/kg/min every 5 min until an MAP of 65 mmHg was reached. Noradrenaline was not administered in the control group. After completing the protocol, all animals were euthanized by a certified veterinarian with a bolus of pentobarbital+diphenylhydantoin at 100 mg/kg (EUTHANEX), in accordance with the international criteria for this procedure established by the American Veterinary Medical Association (AVMA).
Fluid challenges and times of measurements
Time zero (T0) was defined in the endotoxin group and the control group as the time immediately after hemodynamic stabilization. TH0 was defined in the endotoxin group as the time when an MAP < 50 mm Hg was reached. In the control group, this time was set at 3 h of observation. This time was selected in the control group because it was the median time required to reach an MAP < 50 mm Hg for at least 10 min during the pre-experimental standardization phase of the endotoxin models.
A total of 6 fluid challenges were performed in each group. The first three fluid challenges were performed every hour starting at T0 (T0, T1, and T2). Afterward, three fluid challenges were performed every hour starting at TH0 (TH0, TH1, and TH2). Hemodynamic measurements were performed each time. This approach allowed us to assure that both groups had the same amount of fluid administered and that the variables could be compared.
All fluid challenges consisted of 4 ml/kg SS IV infused for 5 min through a central venous catheter. This is a standardized approach to perform a fluid challenge in humans15. Animals in which a fluid challenge induced an increase in CO > 10% were defined as fluid responders6 6. CO was measured by transpulmonary thermodilution in all cases. The study protocol is depicted in Fig. 1.
Statistical analysis
Data are presented using medians and interquartile ranges. Receiver operating characteristic (ROC) curve analysis was conducted for each fluid challenge to assess changes in the PPV operative performance over time in the endotoxin group. Its 95% confidence intervals (95% CI) were calculated by the bootstrap method. The cutoff was calculated by maximizing the sum of sensitivity and specificity16.
Two-way analysis of variance (ANOVA) was conducted to assess differences between both groups. Additionally, one-way ANOVA was performed to determine changes in the variables over time in the endotoxin group. Post hoc analysis was carried out using the Bonferroni correction for multiple comparisons. Categorical variables were evaluated by the Chi-square test in each fluid challenge.
A linear mixed model was performed to determine the associations between PPV and SVR, MAP, SV, baseline PPV, MSFP, and the endotoxin or the control group. Time was used as the random effect, the variables in the model were assessed by the restricted maximum likelihood (REML), and the contribution on each variable was quantified using the estimated value and its standard deviation. Models were compared using the Akaike information criterion (AIC), the Bayesian information criterion (BIC), and the REML. The models with the lowest AIC and BIC were considered the best models.
Data were analyzed using R statistical software17,18. A p value < 0.05 was considered statistically significant for all analyses.
Results
An MAP < 50 mmHg was reached between 2 and 3 h after the start of endotoxin infusion. The changes in the hemodynamic variables are shown in Table 1 and Fig. 2. Systolic arterial pressure (SAP), diastolic arterial pressure (DAP), and MAP decreased, and their levels were lower in the endotoxin group. From T2 to TH1, SAP, DAP, and MAP were lower in the endotoxin group than in the control group (p < 0.05) (Table 1 and Fig. 2). Additionally, in the endotoxin group, these variables decreased over time (p < 0.05). SVR showed similar behavior when measured at TH0 and TH1 (p < 0.05) (Fig. 2D). The changes in the remaining variables are shown in Table 1.
Changes of hemodynamic variables for 6 h of observation. (A) changes of pulse pressure variation (PPV); (B) changes of heart rate (HR); (C) changes of mean arterial pressure (MAP); (D) changes of systemic vascular resistance (SVR); (E) changes of cardiac output (CO). += p < 0.05, ++ = p < 0.01, +++ = p < 0.001 to comparison between Control and Endotoxin Group. *= p < 0.05, **= p < 0.01, ***= p < 0.001 to comparison between each time and T0 time on Endotoxin group.
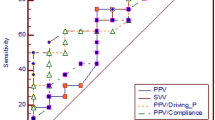
PPV operative performance in each fluid challenge
The PPV was 11.0% (8.00–15.00%) at T0 and increased to 23.5% at TH0 (18.0–26.5%) in the endotoxin group (Table 1, Fig. 2A).
A decrease in the PPV operative performance as a predictor of fluid responsiveness was observed, as the AUC decreased from 0.95 at T0 to 0.60 at TH0. Moreover, its cutoff increased from 10.5 at T0 to 22.0% at TH0 (see Table 2). When noradrenaline infusion was initiated, the PPV operative performance increased to 0.75 at TH2.
Association between PPV and other hemodynamic variables
The PPV model showed heteroscedasticity; therefore, it was necessary to transform PPV to the logarithm of PPV. The logarithm of PPV, which was obtained using a linear mixed model, showed a positive relationship with the baseline PPV; it was also higher in the endotoxin group. PPV showed an inverse relationship with SV, SVR, MAP, and MSFP. The analyses of all variables were statistically significant (p < 0,0001) (Additional file 1, Table S1). The AIC was 111.85, the BIC was 139.12, and the REML was -46.92. Additionally, the model showed homoscedasticity and normality of errors; therefore, its use was appropriate (Additional file 2, Figure S1).
The logarithm of PPV used in the present study was calculated as follows:
Discussion
This study observed a decrease in the PPV operative performance and an increase in the cutoff as a predictor in the endotoxin group when an MAP < 50 mmHg was achieved. Moreover, when noradrenaline infusion was initiated, the PPV operative performance improved. These changes could be related to the inverse association between PPV, and SVR and MAP.
These findings should be considered when a fluid challenge will be performed in critically ill patients with hypotensive states, and PPV will be used as a predictor of fluid responsiveness. Some studies have reported several limitations of PPV as a predictor of fluid responsiveness7,8; however, we did not find studies that reported changes in the PPV operative performance in critically ill patients with low arterial pressure. We suggest that these findings can be explained by the relationship among MSFP, SVR, stressed volume, and unstressed volume. MSFP is one of the determinants of venous return, and this is determined by the relationship between the stressed/unstressed volume (30/70%)19 and SVR. In clinical conditions when SVR is low, MSFP decreases20, and the relationship between the stressed/unstressed volume changes, leading to a clinical condition of relative hypovolemia. PPV will be affected by MSFP; a decrease in MSFP would decrease the driving pressure for venous return, leading to a decrease in preload and SV. This clinical condition may not show a fluid response despite a high PPV value because the fluid load leads to an increase in the unstressed volume preventing an increase in MCFP, and hence an increase in venous return and SV21. Therefore, a high PPV does not mean that there is a high likelihood of fluid response in this setting. Moreover, when MAP increased due to noradrenaline infusion, the operative performance improved, hence confirming the relationship between the PPV operative performance and SVR described above.
We believe that these changes do not happen with stroke volume variation (SVV); since SVV is related to ventricular elastance9. Meanwhile, PPV is related to load arterial variables. Therefore, SVV should be a better predictor of fluid challenge in this setting. Since SVV is a central parameter and PPV is a peripheric parameter. Indeed, their PPV/SVV ratio (dynamic arterial elastance) could describe the relationship between the ventricular system and arterial system (ventricular-arterial coupling)9.
Our findings also showed an increase in the cutoff when an MAP < 50 mmHg was reached. Several cutoffs have been reported in the literature in different clinical settings; however, we are not aware of studies that reported an increase in the cutoff in patients with low arterial pressure. This parameter should be taken into account since it increases the amount of fluid used for reanimation, increasing the risk of fluid overload.
Other findings from our study included an inverse relationship between PPV and load arterial variables, such as SVR and MAP. Some studies support this relationship. Monge et al. showed an association between PPV and effective arterial elastance (Ea)9, a variable that summarizes the features of arterial vascular load in humans10. Other studies described a decrease in PPV after alpha-agonist infusion in experimental models of hemorrhagic shock, which could suggest a relationship between PPV and load arterial variables22,23. We suggest that this relationship could explain the low PPV operative performance when low arterial pressure was achieved.
Finally, we suggest that these findings should not be considered a limitation of PPV; instead, PPV should be considered a variable of preload dependency or a variable that allows us to recognize changes in the relationship of the stressed/unstressed volume, and the absence of changes related to a fluid challenge should alert us of the need for increased SVR. Thus, the risk of fluid overload will decrease.
The present study had some limitations. First, the evolution of PPV observed in our study may have been caused by increased vascular permeability, resulting in a decreased stressed blood volume, or by splenic red cell sequestration, as has been widely reported24,25,26,27. Second, we used the MSFP formula proposed by Parkin and Leaning13 instead of using an invasive measure of this variable. Nevertheless, this formula has yielded good results over time in swine models26,28. Moreover, the MSFP values found in the present study are similar to those reported in studies conducted in humans29. Third, our findings should not be extrapolated to all clinical settings since our model of endotoxin shock was characterized by low SVR with a compensatory increase in CO, such as early septic shock. Finally, Ea was not included in our regression model since this is calculated as MAP/SV or PP/SV; and, could have collinearity among Ea and MAP or SV. New studies are needed that allow the determination of the causes of the worsening of the PPV operative performance in this setting, assessing stressed/unstressed volume, MSFP, and venous return.
In conclusion, the PPV operative performance decreased, and the cutoff increased when MAP was < 50 mmHg. We also found an inverse association between PPV and load arterial variables, such MAP and SVR, that could be related to changes in the PPV operative performance.
References
Venn, R. et al. Randomized controlled trial to investigate influence of the Fluid challenge on duration of hospital stay and perioperative morbidity in patients with hip fractures. Br. J. Anaesth. 88(1), 65–71 (2002).
Yogendran, S., Asokumar, B. C. D., Cheng, H. & Chung, F. A Prospective randomized double-blinded study of the effect of intravenous fluid therapy on adverse outcomes on outpatient surgery. Anesth. Analg. 80, 682–6 (1995).
Lee, J. et al. Association between fluid balance and survival in critically ill patients. J. Internal Med. https://doi.org/10.1111/joim.12274 (2014).
Sirvent, J.-M., Ferri, C., Baró, A., Murcia, C. & Lorencio, C. Fluid balance in sepsis and septic shock as a determining factor of mortality. Am. J. Emerg. Med. 33(2), 186–189 (2015).
Kelm, D. J. et al. Fluid overload in patients with severe sepsis and septic shock treated with early goal-directed therapy is associated with increased acute need for fluid-related medical interventions and hospital death. Shock 43(1), 68–73 (2015).
Alvarado Sánchez, J. I., Amaya Zúñiga, W. F. & Monge García, M. I. Predictors to intravenous fluid responsiveness. J. Intens. Care Med. 33(4), 227–240 (2018).
Alvarado Sánchez, J. I. et al. Predictors of fluid responsiveness in critically ill patients mechanically ventilated at low tidal volumes: Systematic review and meta-analysis. Ann. Intens. Care https://doi.org/10.1186/s13613-021-00817-5 (2021).
Teboul, J. L., Monnet, X., Chemla, D. & Michard, F. Arterial pulse pressure variation with mechanical ventilation. Am. J. Respir. Crit. Care Med. 199(1), 22–31 (2019).
Monge García, M. I. et al. Dynamic arterial elastance as a ventriculo-arterial coupling index: An experimental animal study. Front. Physiol. 11(April), 1–16 (2020).
Kelly, R. P. et al. Effective arterial elastance as index of arterial vascular load in humans. Circulation 86(2), 513–521 (1992).
National Research Council (US) Committee for the update of the guide for the care and use of laboratory animals. 8th ed. Guide for the Care and Use of Laboratory Animals. Washington (DC): National Academies Press (US). 2011. 8th ed.
Reynolds, P. et al. Shock supports the use of animal research reporting guidelines. Shock. 38, 1–3 (2012).
Parkin, W. G. & Leaning, M. S. Therapeutic control of the circulation. J. Clin. Monit. Comput. 22(October), 391–400 (2008).
Hatib, F., Jansen, J. R. C. & Pinsky, M. R. Peripheral vascular decoupling in porcine endotoxic shock. J. Appl. Physiol. 111, 853–860 (2011).
Cecconi, M., Parsons, A. K. & Rhodes, A. What is a fluid challenge?. Curr. Opin Crit. Care. 17(3), 290–295 (2011).
Kaivanto, K. Maximization of the sum of sensitivity and specificity as a diagnostic cutpoint criterion. J. Clin. Epidemiol. 61(5), 517–518 (2008).
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D., R core team (2021). nlme: Linear and nonlinear mixed effects models. R package version 3.1–153. https://CRANR-project.org/package=nlme.
Robin, X. et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinf. 12, 77 (2011).
Gelman, S., Warner, D. S. & Warner, M. A. Venous function and central venous pressure. Anesthesiology 108(4), 735–748 (2008).
Persichini, R. et al. Effects of norepinephrine on mean systemic pressure and venous return in human septic shock. Crit. Care Med. 40(12), 3146–3153 (2012).
Gelman, S. & Bigatello, L. The physiologic basis for goal-directed hemodynamic and fluid therapy: The pivotal role of the venous circulation. Can J Anesth. 65(3), 294–308. https://doi.org/10.1007/s12630-017-1045-3 (2018).
Nouira, S. et al. Effects of norepinephrine on static and dynamic preload indicators in experimental hemorrhagic shock. Crit. Care Med. 33, 2339–2343 (2005).
Bouchacourt, J. P., Riva, J. A. & Grignola, J. C. The increase of vasomotor tone avoids the ability of the dynamic preload indicators to estimate fluid responsiveness. BMC Anesth. https://doi.org/10.1186/1471-2253-13-41 (2013).
Sakai, I., Ishihara, H., Iwakawa, T., Suzuki, A. & Matsuki, A. Ratio of indocyanine green and glucose dilutions detects capillary protein leakage following endotoxin injection in dogs. Br. J. Anaesth. 81(2), 193–197 (1998).
van Eijk, L. T. G. J. et al. Microvascular permeability during experimental human endotoxemia: An open intervention study. Crit. Care 9(2), R157-64 (2005).
Sánchez, M. et al. Comparison of fluid compartments and fluid responsiveness in septic and non-septic patients. Anaesth. Intens. Care. 39(6), 1022–1029 (2011).
Chien, S. et al. Blood volume and its distribution in endotoxin shock. Am. J. Physiol. 210(6), 1411–1418 (1966).
Werner-Moller, P., Sondergaard, S., Jakob, S. M., Takala, J. & Berger, D. Effect of volume status on the estimation of mean systemic filling pressure. J. Appl. Physiol. 123, 1503–13 (2019).
Repessé, X. et al. Value and determinants of the mean systemic filling pressure in critically ill patients. Am. J. Physiol. Hear Circ. Physiol. 309(5), H1003–H1007 (2015).
Acknowledgements
To MINDRAY Colombia and BIMEDCO-GEMEDCO Colombia for the provision of the cardiac monitoring equipment and devices used in the study. Also, to Instituto de Simulación Médica (INSIMED) for allowing us to use their simulation laboratory for conducting the study.
Funding
They received financial support by Universidad Nacional de Colombia and Fundación Universitaria de Ciencias de la Salud. Also, the author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: M. Ignacio Monge García is a consultant for Edwards Lifesciences.
Author information
Authors and Affiliations
Contributions
Design: all authors. Experimental protocol: J.I.A.S., J.D.C.R., J.J.D.F., G.A.R.N., L.E.C.M. Acquisition of data: J.I.A.S., J.D.C.R. Statistical analysis: J.I.A.S. Interpretation of data: all authors. Wrote the manuscript: all authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
M. Ignacio Monge García is a consultant for Edwards Lifesciences.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alvarado Sánchez, J.I., Caicedo Ruiz, J.D., Diaztagle Fernández, J.J. et al. Changes of operative performance of pulse pressure variation as a predictor of fluid responsiveness in endotoxin shock. Sci Rep 12, 2590 (2022). https://doi.org/10.1038/s41598-022-06488-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-06488-x
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.