Abstract
Carbon ion radiotherapy (CIRT) has garnered interest for the treatment of locoregional rectal cancer recurrence. No study has compared CIRT and X-ray radiotherapy (XRT) for reirradiation (reRT) in such cases. We analyzed and compared the clinical outcomes such as local control, overall survival, and late toxicity rate between CIRT and XRT, for treating locoregional rectal cancer recurrence. Patients with rectal cancer who received reRT to the pelvis by CIRT or XRT from March 2005 to July 2019 were included. The CIRT treatment schedule was 70.4 Gy (relative biological effectiveness) in 16 fractions. For the XRT group, the median reRT dose was 50 Gy (range 25–62.5 Gy) with a median of 25 fractions (range 3–33). Thirty-five and 31 patients received CIRT and XRT, respectively. Tumour and treatment characteristics such as recurrence location and chemotherapy treatment differed between the two groups. CIRT showed better control of local recurrence (adjusted hazard ratio [HR] 0.17; p = 0.002), better overall survival (HR 0.30; p = 0.004), and lower severe late toxicity rate (HR 0.15; p = 0.015) than XRT. CIRT was effective for treating locoregional rectal cancer recurrence, with high rates of local control and survival, and a low late severe toxicity rate.
Similar content being viewed by others
Introduction
Rectal cancer is one of the most common malignancies worldwide, and its treatment has progressed considerably with a multidisciplinary approach including surgery, chemotherapy, and radiotherapy (RT)1,2. However, despite the advancement in treatment modalities, 5–15% of the patients still develop locoregional recurrences3. The prognosis of such patients who cannot undergo curative R0 surgery is dismal4. Furthermore, uncontrolled locoregional recurrences are found to significantly affect the patients’ quality of life, leading to pelvic pain, bleeding, fistula, and urinary and faecal incontinence5. Thus, adequate curative treatment for locoregional pelvic recurrence is imperative.
In patients with rectal cancer with locoregional recurrence, surgery with negative margins is the preferred first-line treatment option6. However, many patients are ineligible for surgery due to the extent and location of tumour recurrence, or the poor general condition of the patient. A small proportion of patients are eligible for R0 resection, and other extensive resections including techniques like sacropelvic resection. But, these techniques might pose chances of functional compromise6,7. In such cases, RT can be considered as a feasible treatment option to improve local control and provide palliative relief8. However, since the majority of the recurrences are within the initial RT field, many cases will be reirradiation (reRT) cases3. Although the progress in RT techniques allows for higher doses to be administered at the site of the tumour and lower doses nearby, deciding on an effective radiation dose for a recurrence is challenging, considering the extent and location of recurrence at a site, especially when it is close to the bowel and bladder. Moreover, due to the RT dose accumulated from the initial administration, it is very challenging to administer reRT to achieve both an adequate radiation dose for local control and minimise toxicity, simultaneously.
Carbon ion radiotherapy (CIRT) has emerged as a subject of interest for the treatment of locoregional recurrence of rectal cancer. When compared with X-ray radiotherapy (XRT), CIRT has physical and biological advantages due to its unique characteristic of higher linear energy transfer9,10. Another distinct characteristic—known as the Bragg peak—allows a much reduced radiation dose to the nearby structures11. Thus, it can be hypothesised that CIRT might provide better local control and offer less toxicity when compared with XRT. Currently, there are a few studies from Japan and Germany reporting the efficacy of CIRT for reRT in treating locoregional recurrence of rectal cancer12,13,14,15. However, there are limited prospective studies, and only some retrospective studies on XRT for reRT, all showing varying median survival and toxicity rates16. Moreover, no study has yet compared CIRT and XRT for reRT in the treatment of locoregional recurrence of rectal cancer. Patients who receive XRT are usually more heterogenous than those who receive CIRT and this can be a limitation in comparing treatment outcomes. However, despite the possible limitations, the comparison of the two treatment methods can give important clues for choosing treatment modalities in rectal cancer patients with locoregional recurrences. Thus, the aim of this study was to analyze and compare clinical outcomes such as local control, survival, and late toxicity events in patients who received CIRT or XRT for locoregional recurrence of rectal cancer.
Methods
Patients and treatment
Patients who received CIRT in Japan and those who received XRT in Korea were included in this study. Medical records of patients with rectal cancer who received reRT to the pelvis with a curative intent from March 2005 to July 2019 were retrospectively reviewed. Only patients who received preoperative and postoperative RT previously as part of the initial curative treatment were included. Patients who did not receive reRT to the pelvis, those who had distant metastasis, and those who received reRT with a palliative intent were excluded. Patients with recurrence involving the anastomosis were also excluded since anastomotic recurrence was a contraindication for CIRT. Since this study included patients from two different institutions, it was approved by the Institutional Review Board of Severance hospital (IRB no. 420191320) and that of NIRS (20-013 [National Institutes for Quantum Science and Technology Certified Review Board]). Informed consent from participants was waived by the Institutional Review Board of Severance hospital and the National Institutes for Quantum Science and Technology Certified Review Board due to the retrospective nature of the study. All methods were performed in accordance with the Declaration of Helsinki.
The treatment schedule for CIRT was 70.4 Gy (relative biological effectiveness [RBE]) in 16 fractions, which is 101.38 Gy in biological effective dose with an alpha/beta ratio of 10 (BED10), without concurrent chemotherapy. CIRT was administered daily, 4 days a week from Tuesday through Friday. A median of 3 ports (range 2–5 ports) was used for treatment. The clinical target volume (CTV) was defined as gross tumour volume (GTV) with a 5-mm margin, and dose constraints of D2cc for the bowel and bladder were 44 Gy (RBE) and 50 Gy (RBE) in 16 fractions, respectively. In patients who had recurrence close to the bowel or bladder (< 3 mm), Goretex soft tissue patch or biomaterials such as muscle, mesentery, or omentum were inserted as spacers between the tumour and the bowel or bladder.
For XRT, imaging studies were used for clinical diagnoses of recurrence; pathologic diagnosis was not mandatory. Planning target volume (PTV) was defined as GTV of the recurred tumour plus a 0.5–3-cm margin, depending on the physician’s preference. For cases in which the organs-at-risk (OAR) were in close vicinity, the margins were generally 0.5–1-cm, or further reduced. Dose constraints for OARs were the first priority. Both hypofractionation and conventional fractionation were used based on the physician’s preferences. Nine patients (29%) received XRT via 3D conformal RT and the rest received intensity-modulated RT or RT via CyberKnife. The median reRT dose was 50 Gy (range 25–62.5 Gy) with a median fraction number of 25 (range 3–33), which corresponds to a BED10 of 60 Gy, and 68% of the patients received concurrent chemotherapy during reRT with regimens such as capecitabine, 5-fluorouracil/leucovorin, and 5-fluorouracil/leucovorin/oxaliplatin.
Follow-up
Patients who received CIRT were evaluated by imaging studies within 1 month of CIRT and were followed-up every 1 or 2 months for 6 months, and every 3–6 months thereafter. For the XRT group, follow-up evaluation was performed at 1 and 3 months after reRT and routinely thereafter. Acute and late toxicity events in both groups were retrospectively reviewed and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. The primary endpoint was local failure (LF), and secondary endpoints were overall survival (OS) and severe late toxicity rate (SLTR). Local failure was defined as tumour recurrence or progression within the CTV or PTV after treatment. Severe toxicity events were defined as toxicity events of grade 3 or higher.
Statistical analysis
Clinical factors between the two groups were analyzed by χ2 and Fisher’s exact tests. The cumulative probabilities of LF, OS, and SLTR were calculated using the Kaplan–Meier method and compared using the log rank test. All LF, OS, and SLTR were defined as time from reRT until the corresponding events or the date of last follow-up. Cox regression analysis was used for univariate and multivariate analyses. For multivariate analysis, the backwards elimination method including all variables was used. All analyses were performed using the SPSS version 23.0 (IBM Inc., Armonk, NY, USA).
Results
Totally, 35 patients who received CIRT (CIRT group) and 31 patients who received XRT (XRT group) were analyzed. The characteristics of patients and tumours as well as details regarding treatments are shown in Table 1. Patient characteristics such as sex, age, and initial tumour stage showed no statistically significant differences between the groups. In the CIRT group, approximately half of the patients experienced recurrence in the presacral area (51%), whereas the major location of the recurred lesion was non-presacral, regional, or nodal (71%) in the XRT group. The median size of the recurred tumour was 2.5 cm and 3.0 cm in the CIRT and XRT groups, respectively. A higher number of patients in the XRT group (74%) received chemotherapy before or after reRT compared with those in the CIRT group (40%). No patients in the CIRT group received concurrent chemotherapy, while 68% of the patients in the XRT group received concurrent chemotherapy during reRT. All patients in the CIRT group received CIRT without any surgery before or after RT, while 30% of the patients in the XRT group (n = 11) underwent surgery before or after RT.
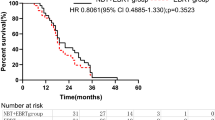
The median follow-up period was 45.7 months (range 7.0–148.4 months) and 22.8 months (range 7.2–148.4 months) in the CIRT and XRT groups, respectively (p = 0.966). One-year LF rates were 6.1% and 10.7% and 3-year LF rates were 12.7% and 56.3% in the CIRT and XRT groups, respectively (Fig. 1, p = 0.010). A total of 7 patients experienced LF in the CIRT group and 6 patients received a second CIRT with the same dose-fractionation schedule. In the XRT group, a total of 11 patients experienced LF. When other factors were adjusted, receiving CIRT compared with XRT was a statistically significant favourable factor for LF (Table 2, adjusted hazard ratio [HR] 0.17; 95% confidence interval [CI] 0.05–0.51; p = 0.002). One-year OS rates were 97.0% and 88.9% and 3-year OS rates were 86.4% and 54.5%, in the CIRT and XRT groups, respectively. (Supplementary Fig. 1, p = 0.005). While median survival in the CIRT group was not achieved, the median survival was 36.9 months in the XRT group. Multivariate analysis showed that an increase in tumour size per millimetre was a statistically significant unfavourable factor (HR 1.04; CI 1.02–1.06; p < 0.001), while CIRT compared with XRT was a significant favourable factor (HR 0.30; CI 0.13–0.68; p = 0.004) for OS (Supplementary Table 1).
Acute toxicity was similar between the groups. Acute gastrointestinal (GI) toxicity of grade 2 or higher was seen in 1 (3%) and 4 patients (13%) in the CIRT and XRT groups, respectively. Acute genitourinary (GU) toxicity of grade 2 or higher was seen in 3 (9%) and 2 (6%) patients in the CIRT and XRT groups, respectively. As shown in Table 3, severe late GI toxicity events were seen in 2 patients in the CIRT group, with a median time to event of 12 months (range 5.8–18.2 months), compared with 6 patients in the XRT group with a median time to event of 8.6 months (range 4.3–30.0 months). No events of severe late GU toxicity were observed in the CIRT group, while they occurred in 4 patients in the XRT group, with a median time to event of 12.2 months (range 6.9–14.7 months).
When seen in detail, the location of the recurred tumour in patients who experienced severe late GI toxicity was presacral (n = 2, 6%) in the CIRT group while that in the XRT group was both presacral and non-presacral (Supplementary Table 2).
In the CIRT group, 1 out of 2 patients who experienced severe late GI toxicities received bevacizumab after CIRT. In the XRT group, 3 out of 6 patients who experienced severe late GI toxicities received bevacizumab prior to XRT. In addition, out of 6 patients who experienced severe late GI toxicity in the XRT group, 5 patients (83%) received chemotherapy before or after reRT. All 4 patients who experienced severe late GU toxicity in the XRT group received chemotherapy before or after reRT. Patients in the XRT group who experienced severe late GI or GU toxicity showed a shorter median period from initial RT to reRT. Skin toxicity was seen in 4 patients (11%) in the CIRT group, in the form of skin ulcers and skin tumour fistula, and in 2 patients (6%) in the XRT group, in the form of skin reaction and postoperative skin dehiscence. For analysis of SLTR, severe late GI and GU toxicity events were included. The 1-year and 3-year SLTRs were 2.9% and 6.3%, respectively, in the CIRT group, and 20.9% and 37.6% in the XRT group, respectively (Fig. 2, p = 0.005).
After adjusting for other factors, CIRT compared with XRT was a significant favourable factor for less severe late toxicity (HR 0.15; CI 0.30–0.69; p = 0.015) (Table 4).
Discussion
Treatment of locoregional recurrence of rectal cancer in the pelvis remains a challenge for clinicians. Preoperative RT is frequently considered as the initial treatment strategy; thus, the majority of the locoregional recurrences are cases that require reRT3. In this study, we showed the efficacy of CIRT as a treatment modality for locoregional recurrence of rectal cancer. CIRT was found to be preferable for local control, preventing severe late toxicity, and providing better OS when compared with XRT.
Previously, a limited number of prospective trials and some retrospective studies about reRT using XRT in locoregional recurrence of rectal cancer have been reported8,17. The rates of local control, survival, and late toxicity varied widely in these studies given the heterogeneity of the patients. In these previous studies, the overall rates of local control generally ranged from 25 to 70%. Patients who did not undergo any surgery had a median survival of 10–17 months, while those who underwent resection had a median survival of 22–60 months8,17. In our study, the XRT group included a mix of patients who, whether they underwent resection of the recurred tumour or not, had a median survival of 36.9 months. One-year and 3-year rates of LF were 10.7% and 56.3%, respectively, which are within the range reported by previous studies8,17. Late toxicity has been inadequately reported in previous studies, and the rates of severe late toxicity vary widely, ranging from 12 to 39%16,17,18. Late toxicity events usually include small bowel obstruction, urinary obstruction, hydronephrosis, and fistula formation. In patients receiving reRT, it is difficult to clearly determine if the late toxicity events are caused by the treatment or by the recurrence of the tumour; hence, assessment of late severe toxicity is challenging. In this study, the rate of severe late toxicity events in the XRT group was similar to that reported in previous studies.
Considering that clinical outcomes of the XRT group in this study were in line with those reported in previous studies, the clinical outcomes of the CIRT group are very encouraging. Despite differences in some recurred tumour or treatment characteristics, patients in the CIRT group showed excellent results in terms of local control, OS, and fewer late toxicity events. CIRT physical properties of dose deposition allowed the delivery of a higher dose to the target volume. When the median prescribed doses were converted to BED10 for comparison, the median BED10 for the CIRT group was 101.4 Gy, whereas it was 60 Gy in the XRT group. This might have led to better local control in the CIRT group compared with that in the XRT group, since higher doses could be related to higher local control19. Moreover, previous studies reported that rectal adenocarcinoma and its recurrence show significantly increased hypoxia, which leads to radio-resistance20,21. In such cases, the unique characteristic of CIRT, which is a high linear energy transfer, provides a superior biological effect by causing direct double strand breaks9. Consequently, effective local control of the recurred tumour by CIRT may have led to an increased OS by reducing distant metastases and overall progression22. It is notable that the patients in the CIRT group showed excellent local control and OS even without resection of the tumour, as R0 resection is generally considered as the most important factor for survival. Since only a limited number of patients can undergo curative resection, CIRT may act as an effective strategy for treating locoregional recurrence of rectal cancer. In addition, most patients with LF after CIRT were treated with reRT using CIRT again.
CIRT showed low rates of severe late toxicity. Another unique characteristic of CIRT is the Bragg peak, which allows lesser radiation to the organs at risk, thereby lowering the late toxicity rates10. When compared with the CIRT group, a higher proportion of patients in the XRT group received pre- or post-RT chemotherapy. Chemotherapy can also be associated with higher rates of toxicity23. Furthermore, more aggressive measures to protect OARs, such as the spacer insertion, were taken in the CIRT group. Thus, while the low rates of late toxicity in the CIRT group can be accepted, direct comparison of the severe late toxicity rates between the CIRT and XRT groups has its limitations.
Due to the advantage of CIRT in the locoregional recurrence of rectal cancer, favorable results from a prospective observational study (GUNMA 0801) has been reported and further studies on CIRT in the treatment of recurrent rectal cancer are ongoing, including the HIMAT1351 and PANDORA01 trials15,24,25. Nevertheless, despite the clinical advantages, the cost of CIRT can be a concern for the patients. In Japan, a cost-effectiveness study showed that CIRT can be potentially cost-effective compared with the multimodality treatment26. Further studies about CIRT in locoregional recurrence of rectal cancer are anticipated.
The limitations of this study are mainly due to its retrospective nature. Two groups from two different institutions in two different countries were included in this study, thus increasing the heterogeneity in patient, tumour, and treatment characteristics. A propensity score matching could have reduced the heterogeneity; however, this was not possible due to the less number of patients. We focused on comparing clinical results in patients treated with CIRT and XRT with curative intent. Moreover, late toxicity events were seen retrospectively, which might have affected the results. However, this study is unique in that it is the only study comparing the efficacy of CIRT and XRT in locoregional recurrence of rectal cancer.
Conclusion
In conclusion, reRT with CIRT is an effective treatment modality for locoregional recurrence of rectal cancer, showing high rates of local control and survival and low rates of late toxicity, compared with XRT. CIRT may be an encouraging strategy in these patients.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
van den Brink, M. et al. Clinical nature and prognosis of locally recurrent rectal cancer after total mesorectal excision with or without preoperative radiotherapy. J. Clin. Oncol. 22, 3958–3964 (2004).
Yu, T.-K. et al. Patterns of locoregional recurrence after surgery and radiotherapy or chemoradiation for rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 71, 1175–1180 (2008).
Wanebo, H. J. et al. Pelvic resection of recurrent rectal cancer. Dis. Colon. Rectum 42, 1438–1448 (1999).
Camilleri-Brennan, J. & Steele, R. The impact of recurrent rectal cancer on quality of life. Eur. J. Surg. Oncol. 27, 349–353 (2001).
Warrier, S. K., Heriot, A. G. & Lynch, A. C. Surgery for locally recurrent rectal cancer: Tips, tricks, and pitfalls. Clin. Colon. Rectal Surg. 29, 114–122 (2016).
Mirnezami, A. & Sagar, P. Surgery for recurrent rectal cancer: Technical notes and management of complications. Tech. Coloproctol. 14, 209–216 (2010).
Tao, R. et al. Pelvic reirradiation for the treatment of locally recurrent rectal cancer. Curr. Colorectal Cancer Rep. 13, 175–182 (2017).
Kanai, T. et al. Biophysical characteristics of HIMAC clinical irradiation system for heavy-ion radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 44, 201–210 (1999).
Cui, X. et al. Effects of carbon ion beam on putative colon cancer stem cells and its comparison with X-rays. Cancer Res. 71, 3676–3687 (2011).
Allen, C., Borak, T. B., Tsujii, H. & Nickoloff, J. A. Heavy charged particle radiobiology: Using enhanced biological effectiveness and improved beam focusing to advance cancer therapy. Mutat. Res. Fundam. Mol. Mech. Mutagen. 711, 150–157 (2011).
Yamada, S. et al. Carbon-ion radiation therapy for pelvic recurrence of rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 96, 93–101 (2016).
Shinoto, M. et al. Carbon-ion radiotherapy for locally recurrent rectal cancer: Japan carbon-ion radiation oncology study group (J-CROS) study 1404 rectum. Radiother. Oncol. 132, 236–240 (2019).
Habermehl, D. et al. Reirradiation using carbon ions in patients with locally recurrent rectal cancer at HIT: First results. Ann. Surg. Oncol. 22, 2068–2074 (2015).
Shiba, S. et al. Prospective observational study of high-dose carbon-ion radiotherapy for pelvic recurrence of rectal cancer (GUNMA 0801). Front. Oncol. 9, 702 (2019).
Guren, M. G. et al. Reirradiation of locally recurrent rectal cancer: A systematic review. Radiother. Oncol. 113, 151–157 (2014).
Owens, R. & Muirhead, R. External beam re-irradiation in rectal cancer. Clin. Oncol. 30, 116–123 (2018).
Valentini, V. et al. Preoperative hyperfractionated chemoradiation for locally recurrent rectal cancer in patients previously irradiated to the pelvis: A multicentric phase II study. Int. J. Radiat. Oncol. Biol. Phys. 64, 1129–1139 (2006).
Chung, S. Y. et al. Treatment outcomes of re-irradiation in locoregionally recurrent rectal cancer and clinical significance of proper patient selection. Front. Oncol. 9, 529 (2019).
Höckel, M. et al. Tumor hypoxia in pelvic recurrences of cervical cancer. Int. J. Cancer 79, 365–369 (1998).
Wendling, P., Manz, R., Thews, G. & Vaupel, P. Oxygen Transport to Tissue—VI 293–300 (Springer, 1984).
Bernstein, T., Endreseth, B., Romundstad, P., Wibe, A. & Registry, N. C. C. Improved local control of rectal cancer reduces distant metastases. Colorectal Dis. 14, e668–e678 (2012).
Joye, I. & Haustermans, K. Early Gastrointestinal Cancers II: Rectal Cancer 189–201 (Springer, 2014).
Combs, S. E. et al. Phase I/II trial evaluating carbon ion radiotherapy for the treatment of recurrent rectal cancer: The PANDORA-01 trial. BMC Cancer 12, 1–9 (2012).
Venkatesulu, B. P., Giridhar, P., Malouf, T. D., Trifletti, D. M. & Krishnan, S. A systematic review of the role of carbon ion radiation therapy in recurrent rectal cancer. Acta Oncol. 20, 1–6 (2020).
Mobaraki, A., Ohno, T., Yamada, S., Sakurai, H. & Nakano, T. Cost-effectiveness of carbon ion radiation therapy for locally recurrent rectal cancer. Cancer Sci. 101, 1834–1839 (2010).
Funding
This article was funded by Faculty research Grant of Yonsei University College of Medicine (4-2019-1320), Korea Medical Device Development Fund grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health and Welfare, the Ministry of Food and Drug Safety) (202012E0102) and National Research Foundation of Korea (NRF) grant funded by the Korea government (MIST) (2021R1F1A1055641).
Author information
Authors and Affiliations
Contributions
The authors confirm contribution to the paper as follows: study conception and design: B.S.M., H.T., S.Y., W.S.K.; data collection: S.Y.C., H.T., J.H.K., J.S.C., B.S.M.; analysis and interpretation of results: S.Y.C., H.T., H.T., S.Y., W.S.K.; draft manuscript preparation: S.Y.C., H.T.. All authors reviewed the results and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chung, S.Y., Takiyama, H., Kang, J.H. et al. Comparison of clinical outcomes between carbon ion radiotherapy and X-ray radiotherapy for reirradiation in locoregional recurrence of rectal cancer. Sci Rep 12, 1845 (2022). https://doi.org/10.1038/s41598-022-05809-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-05809-4
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.