Abstract
Thunbergia coccinea Wall. ex D. Don being a rare, ornamental and medicinal plant of India, is needed to propagate for conserving the germplasm and analyzing its phytochemical compounds in the future. A reliable protocol for direct in vitro propagation using nodal shoot meristem of T. coccinea as explant was standardized. The highest number of shoots per explant (22.17 ± 0.54) with maximum shoot length (2.36 ± 0.28) in cm was obtained in Murashige and Skoog (MS) medium supplemented with 9.70 µM of 6-furfurylaminopurine (Kinetin) and 0.053 µM of α-naphthaleneacetic acid (NAA) combination, among all the different plant growth regulators (PGR’s) and concentrations tested. The aforesaid PGR’s combination was optimum for axillary shoot bud induction and multiplication in T. coccinea. The best rooting was observed on the half-strength MS medium fortified with 2.68 µM NAA with the highest number of roots per shoot (3.75 ± 0.12) and maximum length (5.22 ± 0.32) in cm. All the in vitro raised plantlets were acclimatized in sterile sand and soil mixture (1:1) with a survival rate of 70% on earthen pots under greenhouse conditions. PCR-based RAPD (Random Amplified Polymorphic DNA) and ISSR (Inter-Simple Sequence Repeat) molecular markers were employed to determine the genetic homogeneity amongst the plantlets. Twelve (12) RAPD and nine (9) ISSR primers developed a total of 104 and 91 scorable bands, respectively. The band profiles of micropropagated plantlets were monomorphic to the mother, donor in vivo plant, and similarity values varied from 0.9542–1.000. The dendrogram generated through UPGMA (unweighted pair group method with arithmetic mean) showed 99% similarities amongst all tested plants confirming the genetic uniformity of in vitro raised plants.
Similar content being viewed by others
Introduction
Thunbergia coccinea Wall. ex D. Don is a perennial, scarlet red clock vine that belongs to the Acanthaceae family1. There is evidence to become rare of this ornamental plant in North-Eastern parts of India2. The plant is recorded as a rare medicinal plant in a recent report on the impending threats to medicinal plants of the Himalayas region (North-Eastern India) owing to increased demand3. Previous reports have revealed the medicinal importance of the plant as tribal people use the different plant parts to treat fresh wounds, cut, and stomach infections4,5. The roots of T. coccinea are used to treat tongue blisters and skin infection or inflammation. There is evidence of using the root extract of T. coccinea as a health tonic and aphrodisiac in Assam6. Antipyretic, analgesic, anti-inflammatory and antioxidant activities of T. coccinea leaf reflect its medicinal potency7,8. But, the phytochemical constituents of the plant employed in biological activities are still under-explored and the present study might have a great contribution in this discipline. Several phytochemicals like glucosides, phenolic acids and flavonoid compounds have been identified earlier in other species of Thunbergia regarding some pharmacological activities. Thunbergia alata showed anti-inflammatory, antimicrobial, antiviral and immunomodulatory responses due to presence of iridoid glucosides like thunaloside, alatoside, stilbericoside, 6-epi-stilbericoside, thunbergioside and phenolic acids like caffeoylmalic acid, feruloylmalic acid, p-coumaroylmalic acid9,10,11. Thunbergia grandiflora possess two significant iridoid glucosides for example isounedoside having C-10 as a carboxylic acid group and grandifloric acid with a rare 6,7-epoxide functional group. Several reports demonstrated that T. grandiflora also contains some important phytochemical constituents including proanthocyanidin (tannin), apigenin-7-glucronide, luteolin (flavonoids) related to antibacterial, antifungal and antihelmentic activities12,13. Leaves of Thunbergia laurifolia is popular as Thai herbal tea for the high content of glucosides and phenolics including 3'-O-beta-glucopyranosyl stilbericoside, benzyl-2'-O-β-glucopyranosyl glucopyranoside, E-2-hexenyl-β-glucopyranoside, hexanol-β-glucopyranoside, apigenin, apigenin-7-O-β-D-glucopyranoside, chlorogenic acid, 6-C-glucopyranosyl apigenin, 6,8-di-C- glucopyranosyl apigenin14,15,16. In addition, T. laurifolia contains gallic acid, caffeic acid, protocatechuic acid, rosmarinic acid and it exhibits antinociceptive, anti-inflammatory, antidiabetic, antidote, detoxification, antitumor, antioxidant and hepatoprotective activities17,18,19,20,21,22,23,24,25,26. The medicinal importance of Thunbergia indicates that the T. coccinea might have some significant phytochemicals.
There are many reports of efficient in vitro propagation protocols to conserve and multiply the rare, threatened, endangered plants having ornamental and medicinal values27. Being a rare medicinal plant, it is required to propagate on a mass scale. Clonal propagation or micropropagation, an alternative mean of propagation has a significant contribution to cope with insufficiency and further extinction problem facilitating large scale production in a short duration. It can be achieved by direct and indirect organogenesis. Inadequacy of seeds, low germination rate, and unavailability of the fruit of this plant necessitate the establishment of an efficient protocol for in vitro propagation. The previous study was about the indirect regeneration of Thunbergia coccinea through somatic embryogenesis from leaf callus28, while the present effort is to propagate the plant by direct multiple shoot induction from nodal segments under in vitro conditions. Since there is no report on in vitro propagation of the plant via direct shoot multiplication.
Different abiotic and biotic factors involved in the in vitro process may develop somaclonal variations in regenerated plantlets, particularly in the callus-mediated plantlets29. Therefore, the genetic homogeneity assessment of in vitro propagated plantlets is of great importance. Genetic fidelity of regenerants is evaluated through a lot of DNA-based molecular markers like Random Amplified Polymorphic DNA (RAPD), Simple Sequence Repeats (SSR), Inter Simple Sequence Repeat (ISSR) and Amplified Fragment Length Polymorphism (AFLP)30,31. RAPD and ISSR are predominant among the various markers for their high reproducibility, reliability, simplicity and cost-effectiveness32. The present study includes the assessment of the genetic stability of in vitro propagated plantlets developed through both direct and indirect organogenesis.
Materials and methods
Materials for micropropagation
The Thunbergia coccinea Wall., a naturally grown plant in the garden of Botany Department, Golapbag, the University of Burdwan (23° 2393ʹ N, 87° 8512ʹ E), West Bengal, India was used as a source of explants. The plant herbarium (Specimen Voucher no. BU/KWS-01) was identified by Kaliyamurthy Karthigeyan, Scientist-E, Central National Herbarium, Botanical Survey of India and deposited at Central National Herbarium, Botanical Survey of India, Howrah, West Bengal. The use of this plant in the present study complies with international, national and/or institutional guidelines. The nodal segments of the plant were used as explants and were surfaced sterilized with 70% (v/v) ethanol for 30 s, followed by washing with 0.1% (v/v) tween 20 solution and then the explants were treated with 0.1% mercuric chloride (HgCl2) solution for 1.0–1.5 min followed by the rinsing with sterile dH2O for three times.
Culture medium and growth conditions
After cutting the ends of the nodal segments, explants were inoculated on the Murashige and Skoog basal media (Hi-media, India) supplemented with 0.44 g−1 CaCl2 (Hi-media, India), 30 gL−1 sucrose (Hi-media, India), 1.5 gL−1 phytagel (Sigma Aldrich, USA), and different concentrations of plant growth regulators (PGRs) like naphthalene acetic acid (NAA) (Sigma Aldrich, USA), 6-benzylaminopurine (BAP) (Sigma Aldrich, USA), 6-furfurylaminopurine (Kin) (Sigma Aldrich, USA), thidiazuron (TDZ) (Sigma Aldrich, USA). The pH of the medium was adjusted to 5.8 ± 0.02 before autoclaving at 121 °C for 15 min33. The cultures were incubated in a plant growth chamber at 25 ± 2 °C temperature with 55% humidity, 16/8 h (light/dark cycle) of photoperiod provided with white fluorescent light of 2000 lx intensity.
PGRs treatments for direct shoot induction establishment and its proliferation
The effect of different concentrations and combinations of PGRs in the fortified MS media was studied on the shoot bud initiation, shoot multiplication and shoot elongation. Treatments with PGRs like BAP of 4.44 µM, 8.88 µM and 13.32 µM, Kin of 4.85 µM, 9.70 µM and 14.55 µM, and TDZ of 4.55 µM and 9.10 µM, in combination with NAA of 0.053 µM, 0.53 µM or individually were tested to establish and standardize the maximum number of shoot multiplication. Each treatment including control was done in triplicates and after 40 d of incubation, the percentage of shoot induction, number of shoots per explants, and shoot length (cm) were recorded.
Rooting
The individual shoot was transferred to the full strength and half-strength MS media supplemented with NAA of 0.53 µM, 2.68 µM and 5.37 µM after cutting off the multiplied shoots. The percentage of root induction and root numbers per shoot were recorded after 40 d.
Acclimatization
Regenerated plantlets were hardened in polythene bags containing sterile soil and sand mixture (1:1) after rinsing the plantlets with sterile water to wash of adhering medium residue and then covered with another polythene bags to maintain high humidity. The plants were kept in the greenhouse at 25 ± 2 °C temperature with 75–85% humidity. After hardening for 15 d, the cover of the polythene bags was removed, and then after 10 d, the plants were transferred to earthen pots filled with garden soil for acclimatization under greenhouse conditions.
Assessment of genetic homogeneity by RAPD and ISSR
Leaves from selected in vitro plantlets and mother plant (in vivo T. coccinea) were used for the extraction of genomic DNA by the cetyltrimethylammonium bromide (CTAB) method34. Quantification of DNA was accomplished by analyzing the DNA on 1% agarose gel using diluted uncut λ (lambda) DNA as a standard. Finally, all the genomic DNA samples were diluted to a final concentration of 40 ng/µl with 1X TE buffer (10 mM Tris–HCl; pH 8.0; 1 mM EDTA). DNA samples were stored at − 20 °C for further use. A set of twelve (12) RAPD primers and a set of nine (9) ISSR primers have been used to evaluate the polymorphism among the in vitro grown plantlets including callus-mediated plantlets and mother plants. PCR amplification reactions were carried out in a 20 µl cocktail containing 40 ng of genomic DNA template, 1X buffer, 1 µl of Taq DNA polymerase, 1.5 mM MgCl2, 2.5 mM dNTPs, 10 mg/ml BSA and 10 pmol primers. The PCR amplification protocol was programmed in a thermal cycler (Applied Biosystems Corp., USA) for reaction steps of an initial denaturation at 94 °C for 5 min, 38 cycles of 94 °C for 30 s, primer annealing 30–55 °C for 30 s, extension at 72 °C for 1 min, 30 s final extension step of 72 °C for 5 min. The PCR products were analyzed on 1.8% agarose gel along with 1000 bp molecular weight marker for RAPD and photographed under UV transilluminator using Bio-Rad documentation gel system (Bio-Rad Laboratories Inc., USA). The banding patterns generated by RAPD and ISSR analysis were scored to determine the genetic variance among tested samples. The data matrix was prepared based on the presence and absence of amplified fragments as 1 and 0, respectively. Jaccard’s coefficient was used to estimate the genetic similarity and the similarity matrix was used in the cluster analysis which was performed with NTSYSpc 2.2 software using unweighted pair group method with arithmetic mean (UPGMA)35,36.
Statistical analysis
Data from all experiments were analyzed with SPSS 26.0 version software package (SPSS Inc., USA) to measure the mean using one-way ANOVA. Duncan’s Multiple Range Test was carried out to compare and determine the significant difference in means at 5% probability level (P ≤ 0.05)37.
Results and discussion
Effect of cytokinins on shoot bud induction and shoot bud multiplication
Several reports on in vitro micropropagation of medicinal plant species such as Momordica dioica, Passiflora foetida, Salvadora persica, etc. suggested the use of nodal explants (nodal meristem) for the presence of cytokinins at the nodal region resulting in activation of axillary buds38,39. In the present study, nodal segments of the plant were inoculated into the shoot induction media following sterilization and then growth was initiated after 10 d. The axillary buds had appeared after 10–20 d of inoculation on the shoot induction media. Nodal explants on MS medium without any cytokinin showed 10% response for axillary bud induction. The highest frequency of shoot bud induction (83%) was noticed on BAP of 8.88 µM as well as Kin of 9.70 µM in combination with 0.053 µM NAA as mentioned in Table 1. Though, both BAP and Kin showed their effectiveness in the induction of lateral shoot buds (Fig. 1a,b), but inevitable basal callus was induced in the treatments containing BAP alone and in combinations with NAA (Fig. 1c,d). TDZ alone and in combination with Kin showed no significant difference in the frequency of shoot bud induction after 10 d of inoculation (Table 1). The rate of shoot proliferation was increased with increasing concentration of BAP and Kin beyond the optimum level it declined in T. coccinea. The result was substantiated by the early report claiming that the rate of shoot proliferation from nodal meristem is increased with increasing concentrations of cytokinins to some extent39.
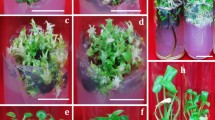
Effect of BAP, Kin, TDZ and NAA on shoot induction and shoot multiplication from nodal meristem of T. coccinea. (a) Axillary shoot buds induced on NAA 0.053 µM + Kin 9.70 µM after 10 d of inoculation; (b,c) Axillary shoot buds induced on NAA 0.053 µM + BAP 8.88 µM with basal callus after 10 d; (d) Proliferated shoots with a basal callus on BAP with NAA after 40 d of inoculation; (e) Proliferated shoots on Kin 9.70 µM with NAA 0.053 µM; (f) Shoots with callus on TDZ 4.55 µM along with Kin 4.85 µM.
The intervening callus induced on shoot multiplication media containing BAP and NAA decreased the number of shoots and shoot length in Vigna radiate40. In line with the aforementioned report, the short and reduced number of shoots per explants of T. coccinea was produced with BAP along with NAA in presence of intervening callus as depicted in Fig. 1d and BAP alone was also insignificant to shoot multiplication as shown in Fig. 1c. Whereas, the effect of Kin along with NAA was better in shoot multiplication and elongation of T. coccinea than BAP accompanying NAA. Among all the treatments, Kin gave rise to the highest number of shoots per explant i.e., 22.17 ± 0.54 (Table 2 and Fig. 1e) and the significantly highest shoot length was achieved in these combinations of PGRs (Fig. 2a). The shoot length on MS medium fortified with Kin 9.70 µM and NAA 0.053 µM was 2.36 ± 0.28. The shoot numbers were decreased with the increased concentration of NAA along with Kin. Adugna et al. reported a similar effect of kinetin along with a low concentration of NAA on shoot multiplication of Moringa stenopetala41. It was observed that BAP 8.88 µM combined with NAA 0.053 µM produced the second maximum mean number of shoots per explants (15.30 ± 0.33). The mean length of shoot was significantly different i.e., 1.55 ± 0.24 on BAP 8.88 µM and NAA 0.053 µM combination, but 1.5 times less than shoot length on Kin 9.70 µM and NAA 0.053 µM as reflected in Table 2.
Shoot multiplication, rooting, and acclimatization (a) Shoots on Kin 9.70 µM with NAA 0.053 µM; (b) Rooting of regenerated shootlets on half-strength MS medium supplemented with NAA 2.68 µM; (c,d) In vitro plantlets; (e) Hardening of plantlets in sand and soil mixture (1:1) covered with polythene bags. (f) Acclimatized plants on earthen pot soil in the greenhouse.
There was no significant difference in the mean of shoot number with treatments like BAP, Kin and TDZ alone for shoot multiplication and elongation (Table 2). In the present study, it was found that NAA of 0.53 µM along with BAP induced profuse callus (Fig. 1d) and it retarded the growth of shoots. Therefore, a high cytokinin to very low auxin ratio was optimum for shoot multiplication. Cytokinins have a great contribution to plant tissue culture for its cell differentiation and cell elongation activities. Shoot proliferation is the major function of cytokinin, but the synergistic balance between the cytokinin and auxin regulates the consistent and successful growth of shoots42. According to Song et al. (2010), a combination of cytokinin and an auxin is often used to achieve high ratios of shoot induction43. Therefore, the effect of BAP and Kin were tested in combination with a low concentration of NAA in the present experiment. As the early reports suggested the greater shoot proliferation in TDZ at low concentration alone and in combination with cytokinin44, shoot proliferation and elongation were also studied with TDZ in the T. coccinea. But there was no improvement in shoot multiplication rather it induced callus with hyperhydric shoots (Fig. 1f). Some reports about the effect of TDZ on species like Cercis canadensis, Vitis rotundifolia and Pyrus pyrifolia corroborated the present findings45. Varied translocation rates of cytokinins to the responsive regions, their differential uptake, varied effect on metabolic processes and ability to change the level of endogenous cytokinins may influence the response with different cytokinins and different explants during in vitro propagation46.
Rooting
In vitro regenerated shootlets showed no response in MS medium without any growth regulators. The regenerated shootlets were inoculated on half and full-strength MS medium supplemented with NAA (0.26–5.37 µM) to induce roots. The best result for rooting was recorded in a half-strength MS medium containing 2.68 µM NAA after 40 d of inoculation (Fig. 2b) and a maximum of 3.75 ± 0.12 roots per shoot with 5.22 ± 0.32 cm root length was induced on the medium (Table 3). Earlier researchers established the beneficial effect of reducing the concentration of MS basal medium on in vitro rooting in Quercus sobur L., Solanum trilobatum and Wrightia tomentosa47,48,49. Half strength MS basal medium suited the best for in vitro rooting in regenerated T. coccinea shootlets. Many reports found the effectiveness of NAA on rooting in plant species like Iris sanguinea, Scaevola serica and Withania somnifera which is consistent with the present outcome of T. coccinea50,51,52.
Acclimatization
The acclimatization of in vitro regenerated plantlets was a difficult step of the micropropagation protocol establishment of their susceptibility to fungal diseases53. In the present study, the healthy rooted plantlets (Fig. 2c,d) were transferred to sterile soil and sand mixture (1:1) followed by rinsing thoroughly with sterile water. A similar combination of 1:1 ratio of compost and soil was followed for successful hardening of Morus spp.54. The plantlets must be watered and covered within polythene sheets to maintain high humidity (Fig. 2e). A diluted carbendazim solution was sprayed to prevent fungal infection and to increase its tolerance to environmental stresses. The polythene sheets were pricked for proper ventilation and after 15 d, the polythene covers were removed to increase their survival rate in the environmental conditions followed by transferring those plantlets to the greenhouse (Fig. 2f). The survival rate for the regenerated plantlets was increased to 70% from the previous study of T. coccinea28.
Genetic homogeneity analysis with RAPD and ISSR
It is necessary to assess the genetic stability among the in vitro raised plantlets and mother plants (in vivo plant) for the establishment of a micropropagation protocol. Tables 4 and 5 revealed the results of 4 different samples of T. coccinea represented as TC1 (mother plant), TC2 (in vitro raised direct regenerants), TC3 (in vitro raised direct regenerants), and TC4 (callus mediated plants). In the present study, a previously reported callus-derived T. coccinea regenerant sample (TC4) was also assessed with the mother and direct propagated plants to check their genetic variability using RAPD and ISSR markers. Authors adopted the two PCR-based RAPD and ISSR analyses amongst the various molecular techniques due to their ease of use, cost and time effectiveness.
RAPD analysis
12 RAPD primers generated a total of 104 distinct and scorable bands with an average of 8.6 bands per primer with sizes ranging from 100–1200 bp. All the bands of in vitro raised plants were monomorphic to the mother plant (TC1) with the RAPD primers except OPA18 and OPC14 which displayed only 3 polymorphic bands in TC3 and TC4 (Table 4). Monomorphism among all the regenerants and mother plants with the RAPD primers such as OPC5 and OPA15 was visualized to confirm the genetic uniformity and stability of the regenerants of T. coccinea (Fig. 3).
RAPD profiles generated by PCR amplification with primer (a) OPC 5 and (b) OPA 15. Lane M: Molecular marker (100–1500 bp); Lane TC 1: In vivo mother plant; Lane TC2-3: In vitro propagated plantlets; TC 4: Callus-derived plantlets. (The full-length gels/blots are presented in Supplementary Fig. 1a,b).
ISSR analysis
In the case of ISSR analysis, 9 ISSR primers developed a total of 91 distinct and scorable bands with an average of 10.1 bands per primer ranging in size from 100–1800 bp (Table 5). Monomorphism among the mother plant (TC1) and three in vitro regenerants of T. coccinea were detected by the 7 ISSR primers (Fig. 4). Three polymorphic bands were observed in only TC4 regenerants with two UBC primers like UBC 820 and UBC 846.
Dendrogram
The similarity indices were estimated from the combined data of RAPD and ISSR using Jaccard’s similarity coefficient between the in vitro raised plants and their mother plant ranging from 0.9542–1.000. The UPGMA analysis grouped all 4 genotypes into two major groups at a similarity coefficient of 0.9542 indicating the low genetic variations among the mother plant and in vitro regenerants. One major group includes the mother plant and two in vitro direct propagated plants through axillary bud proliferation and the other group includes callus mediated plant (Fig. 4). TC1 and TC2 plants showed maximum genetic similarity between them with a similarity coefficient of 1.000, while TC1 and TC2 showed genetic similarity with TC3 and TC4 with a similarity coefficient of 0.9922 and 0.9615 respectively. But, the similarity coefficient between TC3 and TC4 was 0.9542 (Table 6). Jaccard’s similarity indices measure the genetic distance between the tested samples. TC1, TC2 and TC3 were pretty closed to each other, whereas, the distances of TC4 to TC1, TC2 and TC3 were 0.04. Therefore, the UPGMA analysis confirmed the genetic stability and uniformity amongst the mother plant and in vitro propagated plants with a very low percentage (1%) of variation as indicated in Fig. 5. Overall, all the in vitro raised plants of T. coccinea including callus mediated plants were genetically stable. Naturally occurring variations including environmental factors, accumulation of mutation by factors like duration of treatment, in vitro stress, auxin to cytokinin ratio (hormonal balance), added biochemicals, nutritional conditions, all of which played a vital role in the development of small genetic variation55. On contrary to the reports of no genetic variation among the micropropagated plant and mother plant in Asparagus officinalis, Chlorophytum arundinaceum, Simmondsia chinensis56,57,58, there are some reports of somaclonal variation in Codonopsis lanceolata Benth et Hook, Dactyospermum ovalifolium Wight, Spilanthes calva, Jatropha curcas developed during in vitro micropropagation29,59,60. As the callus incurred genetic variation in the callus-mediated plants, the protocol of micropropagation through direct shoot proliferation for the T. coccinea demonstrated by the author in the present study was established successfully. The axillary shoot proliferation minimizes the chance of variability in the in vitro plants, consistent with the previous reports38,61,62.
Conclusion
In summary, this work establishes an efficient protocol for the micropropagation from the nodal shoot meristem of Thunbergia coccinea through axillary bud multiplication in contrast to the reported study of the authors was about the callus induction and indirect regeneration of T. coccinea. Maximum shoot induction and shoot multiplication was achieved on MS medium containing 9.70 µM of Kin along with 0.053 µM of NAA for direct regeneration of the plant. The highest number of roots with maximum length was observed on half strength MS medium supplemented with 2.68 µM of NAA. The experimental findings of genetic homogeneity testing through RAPD and ISSR markers among the mother plant and all in vitro raised plants strongly suggest that the risk of genetic instability can be reduced with direct axillary shoot proliferation. Hence, this protocol may be useful for the commercial multiplication of T. coccinea. Moreover, the findings will play a significant role to meet the demand of this plant and it will also provide support to the researcher for phytochemical analysis.
References
Sultana, K. W., Chatterjee, S., Roy, A. & Chandra, I. Ethnopharmacological and phytochemical review on Thunbergia Retz. (Montin.) Species. Med. Aromat. Plants 4, 1–6 (2015).
Mir, A. H., Upadhaya, K. & Choudhury, H. Diversity of endemic and threatened ethnomedicinal plant species in Meghalaya, North-East India. Int. Res. J. Environ. Sci. 3, 64–78 (2014).
Ray, D. S. & Saini, M. K. Impending threats to the plants with medicinal value in the Eastern Himalayas Region: an analysis on the alternatives to its non-availability. Phytomed. Plus 2, 100151 (2021).
Borah, P. Various ethno-medicinal plants used for treatment of reproductive health problems by morans of Tinsukia district, Assam. Int. J. New Technol. Res. 3, 38–40 (2017).
Taid, T. C., Rajkhowa, R. C. & Kalita, J. C. A study on the medicinal plants used by the local traditional healers of Dhemaji district, Assam, India for curing reproductive health related disorders. Adv. Appl. Sci. Res. 5, 296–301 (2014).
Kar, A., Goswami, N. & Saharia, D. Distribution and traditional uses of Thunbergia Retzius (Acanthaceae) in Assam, India. Pleione 7, 325–332 (2013).
Victoria, S. H. et al. Study of analgesic, antipyretic and anti-inflammatory activities of the leaves of Thunbergia coccinea Wall. Int. Multidiscip. Res. J. 2, 83–88 (2012).
Victoria, S. H. Antioxidant activities of the leaves of Thunbergia coccinea Wall. Int. J. Phytopharma 5, 441–444 (2014).
Cho, Y. C., Kim, Y. R., Kim, B. R., Bach, T. T. & Cho, S. Thunbergia alata inhibits inflammatory responses through the inactivation of ERK and STAT3 in macrophages. Int. J. Mol. Med. 38, 1596–1604 (2016).
Damtoft, S., Frederiksen, L. & Jensen, S. Alatoside and thunaloside, two iridoid glucosides from Thunbergia alata. Phytochemistry 35, 1259–1261 (1994).
Jansen, P. & Mendes, O. Plantamedicinais, Seu uso traditional em Mocambique. Gabinete de Estudos de Medicina Tradicional. Minist. da Saude Maputo Mocambique 1, 216 (1983).
Kabir, M. S. H. et al. Evaluation of total condensed tannin content and anthelmintic activities of organic extracts of four Bangladeshi plants on Tubifex tubifex worm using in vitro method. Int. J. Pharm. 5, 903–910 (2015).
Subramanian, S. & Nair, A. Flavonoid of Thunbergia grandiflora and Asystia travancorica. Curr. Sci. 40, 404 (1971).
Kanchanapoom, T., Kasai, R. & Yamasaki, K. Iridoid glucosides from Thunbergia laurifolia. Phytochemistry 60, 769–771 (2002).
Purnima, M. & Gupta, P. Colouring matters from the flowers of T. laurifolia. J. Indian Chem. Soc. 55, 622–623 (1978).
Oonsivilai, R., Cheng, C., Bomser, J., Ferruzzi, M. G. & Ningsanond, S. Phytochemical profiling and phase II enzyme-inducing properties of Thunbergia laurifolia Lindl. (RC) extracts. J. Ethnopharmacol. 114, 300–306 (2007).
Boonyarikpunchai, W., Sukrong, S. & Towiwat, P. Antinociceptive and anti-inflammatory effects of rosmarinic acid isolated from Thunbergia laurifolia Lindl. Pharmacol. Biochem. Behav. 124, 67–73 (2014).
Aritajat, S., Wutteerapol, S. & Saenphet, K. Anti-diabetic effect of Thunbergia laurifolia Linn aqueous extract. Southeast Asian J. Trop. Med. Public Heal. 35(Suppl 2), 53–58 (2004).
Tejasen, P. & Thongtharb, C. Experimental using Thunbergia laurifolia as antidote for insecticide poisoning. Chiang Mai Med. Bull. 19, 105–114 (1990).
Thongsaard, W., Marsden, C. A., Morris, P., Prior, M. & Shah, Y. B. Effect of Thunbergia laurifolia, a Thai natural product used to treat drug addiction, on cerebral activity detected by functional magnetic resonance imaging in the rat. Psychopharmacology 180, 752–760 (2005).
Cowawintaweewat, S., Somroop, S., Khantisitthiporn, O. & Pootong, A. The study of anti-tetrodotoxin effect of Thunbergia laurifolia Linn. crude Extract. J. Med. Technol. Assoc. Thail. 39, 2 (2011).
Phyu, M. P. & Tangpong, J. Protective effect of Thunbergia laurifolia (Linn.) on lead induced acetylcholinesterase dysfunction and cognitive impairment in mice. Biomed. Res. Int. 2013, 1–6 (2013).
Jetawattana, S., Boonsirichai, K., Charoen, S. & Martin, S. M. Radical intermediate generation and cell cycle arrest by an aqueous extract of Thunbergia laurifolia Linn. in human breast cancer cells. Asian Pacific J. Cancer Prev. 16, 4357–4361 (2015).
Chan, E. W. C. & Lim, Y. Y. Antioxidant activity of Thunbergia laurifolia tea. J. Trop. For. Sci. 18, 130–136 (2006).
Pramyothin, P., Chirdchupunsare, H., Rungsipipat, A. & Chaichantipyuth, C. Hepatoprotective activity of Thunbergia laurifolia Linn extract in rats treated with ethanol: In vitro and in vivo studies. J. Ethnopharmacol. 102, 408–411 (2005).
Suwanchaikasem, P., Chaichantipyuth, C. & Sukrong, S. Antioxidant-guided isolation of rosmarinic acid, a major constituent from Thunbergia laurifolia, and its use as a bioactive marker for standardization. Chiang Mai J. Sci. 41, 117–127 (2014).
Cui, Y., Deng, Y., Keyuan, Z., Hu, X. & Zhu, M. An efficient micropropagation protocol for an endangered ornamental tree species (Magnolia sirindhorniae Noot. & Chalermglin) and assessment of genetic uniformity through DNA markers. Sci. Rep. 9, 9634–9644. https://doi.org/10.1038/s41598-019-46050-w (2019).
Sultana, K. W., Chandra, I. & Roy, A. Callus induction and indirect regeneration of Thunbergia coccinea Wall. Plant Physiol. Rep. 25, 58–64. https://doi.org/10.1007/s40502-020-00501-z (2020).
El-sayed, M., Aly, U. I., Mohamed, M. S. & Rady, M. R. In vitro regeneration and molecular characterization of Jatropha curcas plant. Bull. Natl. Res. Cent. 44, 70 (2020).
Singh, L., Nailwal, T. K. & Tewari, L. An in vitro approach for the conservation of Meizotropis pellita: An endangered and endemic plant. Am. J. plant Sci. 2, 1233–1240 (2013).
El-mahdy, M. T. & Youssef, M. Genetic homogeneity and high shoot proliferation in banana (Musa acuminata Colla) by altering medium thiamine level and sugar type. In Vitro Cell Dev. Biol. Plant 55, 668–677 (2019).
Cui, C. et al. Determination of genetic diversity among Saccharina germplasm using ISSR and RAPD markers. Comptes Rendus Biol. 340, 76–86 (2017).
Murashigue, T. & Skoog, F. A revised medium for rapid groth and bioassays with tobaco tissue cultures. Physiol. Planta. 15, 473–497. https://doi.org/10.1111/j.13993054.1962.tb08052.x (1962).
Doyle, J. & Doyle, J. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 19, 11–15 (1987).
Paul, J. Nouvelles recherches sur la distribution florale. Bull. de la Soc. vaudoise des Sci Nat. 44, 223–270 (1908).
Rohlf, F. J. NTSyS-p.c. Numerical Taxonomy and Multivariate Analysis System (Version 2.0). Exeter Software Publishers Ltd., Setauket., (1998).
Duncan, D. B. Multiple range and multiple F tests. Biometrics 11, 1–41 (1955).
Phulwaria, M., Rai, M. K. & Shekhawat, N. S. An improved micropropagation of Arnebia hispidissima (Lehm.) DC and assessment of genetic fidelity of micropropagated plants using DNA-based molecular markers. Appl. Biochem. Biotechnol. 170, 1163–1173. https://doi.org/10.1007/s12010-013-0266-3 (2013).
Shekhawat, M. S., Kannan, N., Manokari, M. & Ravindran, C. P. In vitro regeneration of shoots and ex vitro rooting of an important medicinal plant Passiflora foetida L. through nodal segment cultures. J. Genet. Eng. Biotechnol. 13, 209–214 (2015).
Gulati, A. & Jaiwal, P. K. In vitro induction of multiple shoots and plant regeneration from shoot tips of mung bean [Vigna radiata (L.) Wilczek]. Plant Cell Tiss. Organ Cult. 29, 199–205 (1992).
Adugna, A. Y., Feyissa, T. & Tasew, F. S. Optimization of growth regulators on in vitro propagation of Moringa stenopetala from shoot explants. BMC Biotechnol. 20, 1–10. https://doi.org/10.21203/rs.3.rs-31317/v1 (2020).
Gaspar, T. et al. Plant hormone and plant growth regulators in plant tissue culture. In Vitro Cell. Dev. Biol. Plant 1, 272–289 (1996).
Song, J. Y., Sivanesan, I., An, C. G. & Jeong, B. R. Adventitious shoot regeneration from leaf explants of miniature paprika (Capsicum annuum) ‘Hivita Red’ and ‘Hivita Yellow’. Afric. J. Biotechnol. 9, 2768–2773 (2010).
Huetteman, C. A. & Preece, J. E. Thidiazuron: A potent cytokinin for woody plant tissue culture. Plant Cell Tiss. Organ Cult. 33, 105–119 (1993).
Kadota, M. Effects of cytokinin types and their concentrations on shoot proliferation and hyperhydricity in in vitro pear cultivar shoots. Plant Cell Tiss. Organ Cult. 72, 261–265 (2003).
Rathore, M. S. et al. Shoot regeneration from leaf explants of Withania coagulans (Stocks) Dunal and genetic stability evaluation of regenerates with RAPD and ISSR markers. South Afric. J. Bot. 102, 12–17 (2016).
Manzanera, J. A. & Pardos, J. A. Micropropagation of juvenile and adult Quercus suber L. Plant Cell Tiss. Organ Cult. 21, 1–8 (1990).
Purohit, S. D., Kukda, G. & Sharma, P. In vitro propagation of an adult tree Wrightia tomentosa through enhanced axillary branching. Plant Sci. 103, 67–72 (1994).
Pendli, S. et al. High frequency in vitro plantlet regeneration in Solanum trilobatum L, an important ethno-medicinal plant and confirmation of genetic fidelity of R1 plantlets by using ISSR and RAPD markers. Vegetos 32(4), 508–520 (2019).
Wang, L. et al. Establishment of an efficient in vitro propagation system for Iris sanguinea. Sci. Rep. 8, 1–10 (2018).
Liang, H. et al. Shoot organogenesis and somatic embryogenesis from leaf and root explants of Scaevola sericea. Sci. Rep. 10, 1–11 (2020).
Fatima, N. & Anis, M. Role of growth regulators on in vitro regeneration and histological analysis in Indian ginseng (Withania somnifera L.) Dunal. Physiol. Mol. Biol. Plants 18, 59–67 (2012).
Timofeeva, S. N., Elkonin, L. A. & Tyrnov, V. S. Micropropagation of Laburnum anagyroides Medic through axillary shoot regeneration. In Vitro Cell. Dev. Biol. Plant 50, 561–567 (2014).
Rohela, G. K. et al. In vitro clonal propagation of PPR-1, a superior temperate mulberry variety. Ind. J. Biotechnol. 17(4), 619–625 (2018).
Devarumath, G. R. M. & Marimuthu, R. S. RAPD, ISSR and RFLP fingerprints as useful markers to evaluate genetic integrity of micropropagated plants of three diploid and triploid elite tea clones representing Camellia sinensis (China type) and C. assamica ssp. assamica (Assam-India type). Plant Cell Rep. 21, 166–173. https://doi.org/10.1007/s00299-002-0496-2 (2002).
Pablo, J., Williams, R., Lucila, E. & Estacio, P. D. Assessment of somaclonal variation in asparagus by RAPD fingerprinting and cytogenetic analyses. Sci. Horticult. 90, 19–29 (2001).
Bamotra, S. K. L. S., Dhar, R. S. & Dhar, A. K. Rapid plant regeneration and analysis of genetic fidelity of in vitro derived plants of Chlorophytum arundinaceum Baker—an endangered medicinal herb. Plant Cell Rep. 25, 499–506. https://doi.org/10.1007/s00299-005-0103-4 (2006).
Kumar, S., Mangal, M., Dhawan, M. & Singh, N. Assessment of genetic fidelity of micropropagated plants of Simmondsia chinensis (Link) Schneider using RAPD and ISSR markers. Acta Physiol. Planta. 33, 2541–2545. https://doi.org/10.1007/s11738-011-0767-z (2011).
Gong, W. L. et al. Genomic instability in phenotypically normal regenerants of medicinal plant Codonopsis lanceolata Benth. et Hook. F., as revealed by ISSR and RAPD markers. Plant Cell Rep. 25, 896–906. https://doi.org/10.1007/s00299-006-0131-8 (2006).
Razaq, M., Heikrujam, M., Chetri, S. K. & Agrawal, V. In vitro clonal propagation and genetic fidelity of the regenerants of Spilanthes calva DC. using RAPD and ISSR marker. Physiol. Mol. Bio. Plants 19, 251–260 (2013).
Saha, S., Roy, S., Sengupta, C. & Ghosh, P. D. Micropropagation and analysis of genetic stability in regenerated plantlets of Ocimum canun Sims. Ind. J. Plant physiol. 19, 174–183 (2014).
Ahmed, R. & Anis, M. In vitro clonal propagation and evaluation of genetic fidelity using RAPD and ISSR marker in micropropagated plants of Cassia alata L.: A potential medicinal plant. Agrofor. Syst. 91, 637–647 (2017).
Acknowledgements
The authors would like to acknowledge the Department of Biotechnology, The University of Burdwan for providing instrumental assistance. They are also grateful to the University Grant Commission, Government of India for financial assistance.
Funding
Maulana Azad National Fellowship award no. MANF-2017-18-WES-82383 provided by University Grant Commission, Govt. of India.
Author information
Authors and Affiliations
Contributions
K.W.S. and I.C. designed the experiments. K.W.S. and S.D. conducted the experiments and analyzed the result statistically. K.W.S. and S.D. prepared the manuscript. A.R. has contributed substantially to carry out the experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sultana, K.W., Das, S., Chandra, I. et al. Efficient micropropagation of Thunbergia coccinea Wall. and genetic homogeneity assessment through RAPD and ISSR markers. Sci Rep 12, 1683 (2022). https://doi.org/10.1038/s41598-022-05787-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-05787-7
This article is cited by
-
In vitro adventitious regeneration and plantlet transplantation of Atractylodes chinensis (DC.) Koidz., a valuable medicinal plant
Plant Cell, Tissue and Organ Culture (PCTOC) (2023)
-
Silicon Nanoparticles Moderated Morphometric Deficiencies by Improving Micro-Morpho-Structural Traits in Thunbergia erecta (Benth.) T. Anderson
Silicon (2023)
-
In vitro regeneration and its histological characteristics of Dioscorea nipponica Makino
Scientific Reports (2022)
-
Critical factors governing the efficient direct organogenesis in green-fleshed kiwifruit (Actinidia deliciosa) [A. Chev.] var. deliciosa
In Vitro Cellular & Developmental Biology - Plant (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.