Abstract
Women with chronic abnormal uterine bleeding-ovulatory dysfunction (AUB-O) are at increased risk of endometrial neoplasia. We conducted a non-inferiority randomized controlled trial to determine the effectiveness of two cyclic-progestin regimens orally administered 10 d/month for 6 months on endometrial protection and menstruation normalization in women with AUB-O. There were 104 premenopausal women with AUB-O randomized to desogestrel (DSG 150 µg/d, n = 50) or medroxyprogesterone acetate (MPA 10 mg/d, n = 54) group. Both groups were comparable in age (44.8 ± 5.7 vs. 42.5 ± 7.1 years), body mass index (24.8 ± 4.7 vs. 24.9 ± 4.7 kg/m2), and AUB characteristics (100% irregular periods). The primary outcome was endometrial response rate (the proportion of patients having complete pseudodecidualization in endometrial biopsies during treatment cycle-1). The secondary outcome was clinical response rate (the proportion of progestin withdrawal bleeding episodes with acceptable bleeding characteristics during treatment cycle-2 to cycle-6). DSG was not inferior to MPA regarding the endometrial protection (endometrial response rate of 78.0% vs. 70.4%, 95% CI of difference − 9.1–24.4%, non-inferiority limit of − 10%), but it was less effective regarding the menstruation normalization (acceptable bleeding rate of 90.0% vs 96.6%, P = 0.016).
Clinical trial registration: ClinicalTrials.gov (NCT02103764, date of approval 18 Feb 2014).
Similar content being viewed by others
Introduction
Abnormal uterine bleeding (AUB) associated with anovulation without gross uterine abnormality is classified as “abnormal uterine bleeding-ovulatory dysfunction” or AUB-O in the International Federation of Gynecology and Obstetrics (FIGO) classification1. AUB-O is a common gynecologic complaint in women during perimenarcheal or perimenopausal periods, and those with polycystic ovary syndrome or obesity2,3,4,5. Women with AUB-O usually have irregular menstrual periods with or without heavy bleeding. The cases with chronic AUB-O are at increased risk of endometrial neoplasia because of prolonged exposure to unopposed estrogen. Therefore, the treatment of AUB-O aims to normalize menstruation and protect the endometrium from endometrial neoplasia2. Despite the paucity of evidence from randomized controlled trials (RCT), the current treatment options include estrogen-progestin and progestin-only therapy6. The former, in the form of combined hormonal contraceptives, is best for sexually-active women with a need for contraception7. The latter is preferable for women who are in the perimenopausal period, or who are intolerant to or contraindicated to combined hormonal contraceptives.
The commonly used progestin for AUB-O is medroxyprogesterone acetate (MPA, 5–10 mg/d, 10–14 d/month)1. Its strong progestogenic effect is preferable for endometrial protection, but its undesirable glucocorticoid, mineralocorticoid, and androgenic effects may negatively affect glucose and lipid metabolism in long-term users8,9,10,11. A report from a clinical study in 1990 suggested that cyclic progestin (i.e., MPA, norethindrone, or norethisterone), given for 12–14 days each month, was effective management for most women with anovulatory dysfunctional uterine bleeding12. Nowadays, there are a variety of progestins that may be useful for the treatment of AUB-O. Each progestin has its own unique properties, benefits, and disadvantages11. Therefore, prior to adopting a new drug as a treatment option, its efficacy should be proven by an RCT.
Desogestrel (DSG), a third-generation progestin, has high progestogenic activity with low androgenic and little to no glucocorticoid and mineralocorticoid effects13,14. DSG may, therefore, be a better choice for perimenopausal or obese women who are at increased risk for abnormal glucose and lipid metabolism. Information specific to DSG therapy for AUB-O is lacking. This study aimed to evaluate the effectiveness of cyclic DSG compared with cyclic MPA on endometrial protection and menstruation normalization in women with AUB-O. We also assessed the safety of these two progestins on metabolic parameters after 6 months of treatment.
Results
Participants
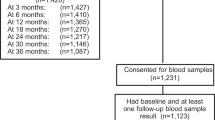
Figure 1 shows flow of study participants. Of 109 eligible patients, 104 cases were enrolled and randomized to the study group (DSG, n = 50) or the control group (MPA, n = 54). According to the exclusion criteria, one case was excluded, and four cases declined to participate. There were two dropouts in the MPA group; one case after treatment cycle-2 because of colon cancer, and the other after cycle-5 because of amenorrhea. Their missing data regarding withdrawal bleeding were imputed from the last observation carried forward. Therefore, there were five imputed data, including four cycles of normal withdrawal bleeding and one cycle of no withdrawal bleeding; the data of which contributed to the clinical outcome of the MPA group.
Table 1 shows demographic and baseline characteristics of the 104 intention-to-treat participants. The DSG and MPA groups were comparable in age (44.8 ± 5.7 vs. 42.5 ± 7.1 years), body mass index (BMI, 24.8 ± 4.7 vs. 24.9 ± 4.7 kg/m2), education > 12 years (64.0% vs. 61.1%), and AUB characteristics (irregular bleeding of 100%). Both groups were comparable in baseline metabolic parameters, including fasting blood glucose, total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels. The non-compliance rate was low in both groups (2.8% in the DSG and 2.6% in the MPA groups).
Efficacy outcomes
Figure 2 and Table 2 show efficacy outcomes. Figure 2 shows various degrees of endometrial response to treatment. The treatment is considered effective if the post-treatment endometrium shows a small inactive gland with pseudodecidual stromal change, as in Fig. 2a. The endometrial response to DSG seemed to be slightly better than that to MPA as determined by the proportion of complete pseudodecidualization (78.0% vs. 70.4%, P = 0.375). The 95% CI of difference (Δ) of this outcome (− 9.1%, 24.4%) indicated the non-inferiority of DSG to MPA as its lower bound lay above the non-inferiority limit of − 10%.
Both DSG and MPA effectively normalized bleeding to an acceptable level (normal or no withdrawal bleeding) for > 90% of cases. However, the clinical response to DSG was not as good as that to MPA, as the proportion of acceptable bleeding was significantly lower in the DSG group than in the MPA group (90.0% vs. 96.6%, P = 0.016). In other words, the DSG group had a significantly higher rate of prolonged bleeding (10.0% vs. 3.4%).
Safety and tolerability
Table 3 shows safety and tolerability data which presents the incidence of adverse events and side effects, and the changes from baseline to month-6 of treatment (δ) for various metabolic parameters. One patient in the MPA group was found to have colon cancer after treatment cycle-2 and was withdrawn from the trial. The most common side effect was drowsiness, which approximately one-third of patients reported in both groups. The incidence of nausea was clinically significantly higher in the DSG group (12.0% vs. 1.9%, P = 0.053). Both DSG and MPA groups were comparable in the δ of every metabolic parameter. Most of the δ had a minus sign indicating favorable changes for total cholesterol, triglycerides, and LDL-C, but an unfavorable change for HDL-C. None of the metabolic parameters had a δ beyond ± 10%; therefore, the δ was not clinically important.
Discussion
Treatment for AUB-O aims to restore normal physiological change of the endometrium to prevent endometrial neoplasia and normalize menstruation2. There is a lack of evidence from randomized controlled trials, especially about the effect of treatment for AUB-O on endometrial histopathology. Most studies showed only intermediate outcomes, such as menstrual cycle regularization15,16,17. Our present study showed that two cyclic-progestin regimens (DSG 150 µg/d or MPA 10 mg/d, 10 d/month) could transform endometrium to complete pseudodecidualization in > 70% of cases in the treatment cycle-1 and could normalize menstruation in > 90% of all treatment cycles.
Obese women with AUB-O are at high risk for developing endometrial neoplasia and may benefit from preventive measures18. Although authorities recommend that endometrial biopsy should be performed in women older than 45 years19,20, obese women may need endometrial biopsy at a younger age18. Our study population was slightly younger than 45 years, but they had BMI in the overweight to obese classification according to the WHO Asia Pacific BMI cut point (≥ 23 kg/M2) 21. The preventive measures for these women include bodyweight reduction by lifestyle modification and progestin therapy18.
Progestin therapy is available in various forms, including combined hormonal contraceptives, progestin-only contraceptives, and cyclic-progestin therapy. Each form has its own benefits and drawbacks. The progestin therapy results in pseudodecidualization of the endometrium. A case–control study reported that progestins in most combined oral contraceptives were adequate to protect against endometrial cancer22. DSG in the form of progestin-only oral contraceptive might also have this protective effect as it contains a similar dosage of DSG as a combined oral contraceptive does. It is unknown whether cyclic progestin therapy in premenopausal women with AUB-O also retains this protective effect. Evidence from menopausal hormone therapy shows that continuous combined estrogen-progestin regimens reduce the risk of endometrial cancer while cyclic regimens do not alter the risk23. We presume that the risk of endometrial cancer in women with AUB-O treated with cyclic progestin may be the same as the risk in women with regular ovulation.
The equivalent dosages of progestins that are adequate for endometrial transformation were reviewed by Schindler, et al.11 The authors summarized the daily and total doses of progestins. They considered MPA 5–10 mg/d for a total dose of 80 mg and DSG 150 µg/d for a total dose of 2 mg to be equivalent. In the present study, a total dose of 100 mg MPA or 1.5 mg DSG was used within 10 days. Although the total dose of 1.5 mg DSG was less than that suggested by Schindler’s review, this dosage was as effective as 100 mg MPA. The 10-day application of one or the other progestins could not completely transform the endometrium in some patients. Ineffective endometrial transformation may be due to the inadequate cumulative effect of progestin: dosage and duration2. It is suggested that the duration of cyclic progestin therapy should be 12–14 days per cycle to protect the endometrium effectively2,12. However, this recommendation was based on information from the first-generation progestins. Therapy with more potent third-generation progestins may protect the endometrium with a shorter duration of treatment. We found that 10-day DSG seemed to be more effective than 10-day MPA (78% vs. 70% complete pseudodecidualization). However, our study did not have enough power to show the superiority of DSG over MPA in this regard.
Despite the observed comparable effectiveness relating to endometrial response, MPA was superior to DSG for clinical response. The drawback of DSG was the higher rate of bleeding for longer than eight days (11.1% vs. 4.6%). Some participants might have concomitant structural lesions as a confounding cause for prolonged withdrawal bleeding. In the present study, transvaginal ultrasonography could exclude gross structural lesions but not a tiny endometrial polyp which might be detected by saline infusion sonohysterography or hysteroscopy. With the strength of RCT, this confounder should be distributed equally between the groups. Interestingly, the finding that MPA had better bleeding control than DSG was consistent with our previous study that found MPA superior to other progestins regarding menstruation normalization in women with non-atypical endometrial hyperplasia24. Since each progestin has its own unique profiles10,25,26, each may act differently on molecular microenvironment involving hemostasis of endometrium, which may cause different uterine bleeding patterns.
Some progestins have an adverse effect on metabolic profiles, although most of the evidence is derived from studies of menopausal hormone therapy. A review in 2017 regarding progestins and blood lipids found that MPA mitigated the beneficial effects of menopausal hormone therapy on blood lipids27. In our study, we expected the metabolic profile to be better in the DSG group than in the MPA group because DSG has a low androgenic effect and little to no glucocorticoid or mineralocorticoid effect. However, the change in all metabolic parameters from baseline to month-6 was marginal and may not be clinically meaningful. It was possible that cyclical application of these two progestins for 10 d/month did not have an adverse effect on metabolic parameters.
The strength of the present study is its prospective randomized controlled design that can prevent bias. Moreover, we evaluated both endometrial and clinical responses to ensure that the treatment not only normalized menstruation but also protected the endometrium. However, RCT has a limitation in generalizability. We evaluated two cyclic-progestin regimens in overweight premenopausal women with AUB-O and followed up them for 6 months. Application of these regimens to patients with different characteristics in a longer duration of treatment may result in different efficacy and safety outcomes.
In conclusion, according to our definition of non-inferiority, the cyclical administration of DSG (150 µg/d, 10 d/month) is not inferior to MPA (10 mg/d, 10 d/month) for pseudodecidualization of endometrium in women with AUB-O. Both DSG and MPA are effective for menstruation normalization in > 90% of cases, but DSG is less effective than MPA. The 6-month metabolic profile changes are comparable between the DSG and MPA groups, and are not clinically meaningful. Therefore, cyclic DSG can be used as an alternative to MPA for endometrial protection and menstruation normalization in AUB-O.
Methods
This non-inferiority double-blind RCT was conducted at the Gynecologic Endocrinology Unit, Department of Obstetrics and Gynecology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand, from 11 March 2014 to 30 November 2018. This trial was a part of the Siriraj Abnormal Uterine Bleeding (SI-AUB) project, an ongoing project established in 2010. The SI-AUB project comprises many sub-studies, including observational studies and clinical trials. The SI-AUB project and this study were conducted in accordance with the principles set forth in the Declaration of Helsinki.
Participants
Participants were premenopausal women who were newly diagnosed with AUB-O. A diagnosis of AUB-O was made if a patient had unremarkable findings from pelvic ultrasonogram and endometrial histopathology revealed no structural lesions28. Regarding the endometrial histopathology, only patients with proliferative endometrium were recruited. A participant was excluded if she had conditions that might cause AUB (e.g., having coagulation defect or taking certain medications), took hormonal medication within 3 months before enrollment, planned to get pregnant within 6 months, needed hormonal contraception, had contraindications for progestogen administration (e.g., history of breast cancer, severe liver disease, or progestogen allergy), or had diabetes mellitus and/or dyslipidemia with ongoing treatment. All sexually active participants were advised to use barrier contraception. An enrolled participant would be withdrawn from the study if she had a follow-up endometrial histopathology report of endometrial neoplasia or she encountered a serious adverse event. This participant would be counted as a case of treatment failure in the intention-to-treat analysis.
Study procedures
Eligible participants were recruited from the gynecology outpatient department. Routine evaluation for AUB at our clinic includes clinical evaluation and pelvic ultrasonography. Endometrial histopathology is indicated for women at increased risk of endometrial neoplasia, e.g., older than 45 years, irregular menstruation in women with polycystic ovary syndrome, or obesity. Demographic characteristics, medical history, menstrual bleeding pattern, concomitant medications, and findings of general and gynecologic examinations were collected using a structured record form. Transvaginal ultrasonography was performed to assess the structure of pelvic organs. When the ultrasound probe could not be inserted vaginally, transrectal ultrasonography would be performed instead. Endometrial tissue was obtained by an office endometrial biopsy procedure using a flexible instrument. Patients were then scheduled two weeks after the procedure to be enrolled into the present study or another sub-study of the SI-AUB project.
The enrolled participants were scheduled for five visits, including visit-1 at enrollment; visit-2 on day-8, day-9, or day-10 of treatment cycle-1; visit-3 at four weeks; visit-4 at 3 months; and visit-5 at 6 months. Visit-1 was for collecting baseline data, obtaining biochemical blood tests, issuing a menstrual diary card, and providing the cycle-1 medication. Visit-2 was for obtaining endometrial sampling. Visit-3 was for evaluating compliance and side effects, and for providing cycle-2 and cycle-3 medication. Visit-4 was for providing cycle-4 to cycle-6 medication. Visit-5 was for follow-up biochemical blood tests.
All participants were instructed to record data relating to the following in their menstrual diary cards: day of taking trial medication, possible side effects, and vaginal bleeding. One diary card was used to record data from one treatment cycle. The participants had to return the diary card and the leftover medication at the following visit.
Trial medication and treatment allocation
The trial medication was 10 mg MPA or 150 µg DSG administered orally once daily before bedtime for 10 consecutive days per month for 6 months. Each dose of both study medications was encapsulated in a white capsule that was identical in physical appearance. Ten capsules of the same medicines were pre-packed in a zip-locked opaque plastic bag labeled with a serial number according to the randomization code. Patients were allocated to either the control group (MPA) or the study group (DSG) by simple randomization using a random allocation software program29. The randomization codes were individually contained in sealed opaque envelopes sequentially numbered. An independent nurse who had no contact with study participants opened each envelope and dispensed the assigned package of trial medication. The packaging of trial medications and the generation of randomization codes were produced by an independent pharmacist of the Department of Pharmacy. Therefore, the gynecologists and nurses who contacted the patients, the patients themselves, and the gynecologic pathologist were blinded to the group assignments.
Instruction for a missed dose of trial medication included taking the missed dose as soon as the patients remembered or skipping the dose when it was almost the time of the next scheduled dose. In the latter case, the participants would complete the trial medication in > 10 days. The cycles with > 20% skipped doses were counted as non-compliance treatment cycles.
Outcomes
The primary efficacy outcome was the “endometrial response”, which was evaluated from histopathology of endometrial biopsy on medication day-8, day-9, or day-10 of treatment cycle-1. Endometrial biopsy was performed using a disposable, flexible cannula with its own internal suction piston (Endocell®, Wallach Surgical Devices, Trumbull, CT, USA). The endometrial tissue was immediately fixed in formalin and sent to the Department of Pathology for routine tissue processing with hematoxylin and eosin staining. The tissue section was examined under a light microscope by a gynecologic pathologist (M.W.). Endometrial histopathology was categorized into (i) complete pseudodecidualization if the entire endometrial biopsy specimen showed pseudodecidual transformation, (ii) incomplete pseudodecidualization if the specimen showed uneven pseudodecidual transformation, (iii) treatment failure if the specimen showed proliferative endometrium or endometrial neoplasia, and (iv) undetermined if the specimen was inadequate for evaluation. The success rate of endometrial response was the proportion of the cases with complete pseudodecidualization in the intention-to-treat cases. Dropout cases were counted as treatment failure cases.
The secondary efficacy outcome was the “clinical response”, which was evaluated from characteristics of withdrawal bleeding of treatment cycle 2 to cycle 6. The withdrawal bleeding was defined as bleeding that occurred after completion of each course of trial medication and before starting a new one (i.e., between days 11 and 28 of the treatment cycle). The amount of bleeding was categorized according to the participant’s experience30 into (a) light bleeding or spotting (less than normal menstruation), (b) normal bleeding (like normal menstruation), and (c) heavy bleeding (more than normal menstruation). The bleeding pattern was categorized1 into (i) normal bleeding (bleeding lasted up to eight days), (ii) prolonged bleeding (bleeding lasted more than eight days), (iii) breakthrough bleeding (bleeding occurred on the day of taking medication), and (iv) no bleeding (no bleeding during the medication-free days. The success rate of clinical response was the proportion of the treatment cycles with acceptable bleeding [i.e., the cycles with bleeding characteristics (i-a), (i-b), or (iv)] in the intention-to-treat cycles. Missing data were imputed from the last observation carried forward.
Safety of treatment was evaluated from the changes in biochemical blood tests from baseline to month-6, and from side effects that occurred at any time during the study period. Biochemical blood tests were performed at visit-1 and visit-5. A venous blood sample was drawn from an antecubital vein during the 08.00 to 10.00 time period after overnight fasting for 12 h. The blood sample was examined for fasting blood glucose and lipid profile (total cholesterol, triglycerides, HDL-C, and LDL-C). All biochemical assays were performed at the Department of Clinical Pathology (central laboratory certified by IS0 15,189). All assays were performed using automatic analyzers, and all assays had intra-assay and inter-assay coefficients of variation (CV) of < 5%.
Sample size
The sample size was calculated from the primary outcome to determine the non-inferiority of DSG compared with MPA regarding the success rate of endometrial response. A free online sample size calculator was used for comparing two proportions: 2-sample non-inferiority or superiority (HyLown Consulting LLC, GA, USA). Given that the non-inferiority margin was − 10% (δ = − 0.1), the success rate of endometrial response derived from our pilot study in the DSG (θ1) and the MPA (θ2) groups was 80% and 60%, respectively. The required sample size with the equal allocation (r = 1) to achieve 90% power and an α of 0.025 was 47 patients per group. To compensate for 5% dropouts, the final sample size was 50 patients per group.
Statistical analysis
Data were analyzed using SPSS Statistics software version 18.0 (SPSS, Inc., IL, USA). Statistical analyses of the efficacy outcomes were based on the intention-to-treat populations, and those of the safety outcomes were based on the per-protocol population. Data are presented as mean ± standard deviation (SD), median and interquartile range, or number and percent, as appropriate. Shapiro–Wilk test and Q-Q plot were used to examine the normality of continuous data. Unpaired t-test or Mann–Whitney U test was used to compare continuous data. Chi-square test or Fisher’s exact test was used to compare categorical data. Generalized estimating equations model was used to test the difference in the rate of acceptable bleeding from all treatment cycles between DSG and MPA groups. All statistical tests to determine differences between groups were 2-sided, and a P-value of less than 0.05 was considered statistically significant.
Non-inferiority was considered if the lower bound of the 95% confidence interval (CI) for the difference (Δ) in the favorable outcome lay above the non-inferiority limit. Therefore, DSG would be considered non-inferior to MPA if the lower bound for the difference in the endometrial response rate lay above − 10%.
Ethical approval
The protocol for this study was approved by the Siriraj Institutional Review Board (SIRB) of the Faculty of Medicine Siriraj Hospital, Mahidol University (certificate of approval no. Si394/2013, date of approval 8 July 2013). The protocol was also registered with ClinicalTrials.gov (reg. no. NCT02103764, date of approval 18 Feb 2014). The initial participant enrollment was on 11 March 2014. A written informed consent was obtained from all participants before enrollment.
Abbreviations
- AUB-O:
-
Abnormal uterine bleeding-ovulatory dysfunction
- DSG:
-
Desogestrel
- MPA:
-
Medroxyprogesterone acetate
References
Munro, M. G., Critchley, H. O. D. & Fraser, I. S. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int. J. Gynaecol. Obstet. 143, 393–408 (2018).
Fritz, M. A. Abnormal uterine bleeding. In Clinical Gynecologic Endocrinology and Infertility (eds Fritz, M. A. & Speroff, L.) 591–621 (Lippincott Williams & Wilkins, 2011).
Ferenczy, A. Pathophysiology of endometrial bleeding. Maturitas 45, 1–14 (2003).
Horbelt, D. V., Roberts, D. K., Parmley, T. H. & Walker, N. J. Ultrastructure of the microvasculature in human endometrial hyperplasia. Am. J. Obstet. Gynecol. 174, 174–183 (1996).
Abulafia, O. & Sherer, D. M. Angiogenesis of the endometrium. Obstet. Gynecol. 94, 148–153 (1999).
Hickey, M., Higham, J. M. & Fraser, I. Progestogens with or without oestrogen for irregular uterine bleeding associated with anovulation. Cochrane Database Syst. Rev. 9, CD001895 (2012).
Nilsson, L. & Rybo, G. Treatment of menorrhagia. Am. J. Obstet. Gynecol. 110, 713–720 (1971).
Sitruk-Ware, R. Progestins and cardiovascular risk markers. Steroids 65, 651–658 (2000).
Darney, P. D. The androgenicity of progestins. Am. J. Med. 98, 104S-110S (1995).
Stanczyk, F. Z. All progestins are not created equal. Steroids 68, 879–890 (2003).
Schindler, A. E. et al. Classification and pharmacology of progestins. Maturitas 46(Suppl 1), S7–S16 (2003).
Fraser, I. S. Treatment of ovulatory and anovulatory dysfunctional uterine bleeding with oral progestogens. Aust. N Z J. Obstet. Gynaecol. 30, 353–356 (1990).
Stone, S. C. Desogestrel. Clin. Obstet. Gynecol. 38, 821–828 (1995).
Kaplan, B. Desogestrel, norgestimate, and gestodene: The newer progestins. Ann. Pharmacother. 29, 736–742 (1995).
Trivedi, N., Chauhan, N. & Vaidya, V. Effectiveness and safety of dydrogesterone in regularization of menstrual cycle: A post-marketing study. Gynecol. Endocrinol. 32, 667–671 (2016).
Podzolkova, N., Tatarchuk, T., Doshchanova, A., Eshimbetova, G. & Pexman-Fieth, C. Dydrogesterone treatment for menstrual-cycle regularization in routine clinical practice: A multicenter observational study. Gynecol. Endocrinol. 32, 246–249 (2016).
Wang, L., Guan, H. Y., Xia, H. X., Chen, X. Y. & Zhang, W. Dydrogesterone treatment for menstrual-cycle regularization in abnormal uterine bleeding - ovulation dysfunction patients. World J. Clin. Cases 8, 3259–3266 (2020).
Seif, M. W., Diamond, K. & Nickkho-Amiry, M. Obesity and menstrual disorders. Best Pract. Res. Clin. Obstet. Gynaecol. 29, 516–527 (2015).
National Institute for Health Care and Excellence. Heavy menstrual bleeding, (London, 2007).
Committee on Practice, B.-G. Practice bulletin no. 128: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet. Gynecol. 120, 197–206 (2012).
World Health Organization. Regional Office for the Western Pacific. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment, (Health Communications Australia, 2000).
Maxwell, G. L. et al. Progestin and estrogen potency of combination oral contraceptives and endometrial cancer risk. Gynecol. Oncol. 103, 535–540 (2006).
Beral, V., Bull, D., Reeves, G. & Million Women Study, C. Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet 365, 1543–1551 (2005).
Rattanachaiyanont, M. et al. Clinical and pathological responses of progestin therapy for non-atypical endometrial hyperplasia: A prospective study. J. Obstet. Gynaecol. Res. 31, 98–106 (2005).
Di Carlo, C. et al. Bleeding patterns during continuous estradiol with different sequential progestogens therapy. Menopause 12, 520–525 (2005).
Roland, M., Clyman, M. J., Decker, A. & Ober, W. B. Classification of endometrial response to synthetic progestogen-estrogen compounds. Fertil. Steril. 15, 143–163 (1964).
Jiang, Y. & Tian, W. The effects of progesterones on blood lipids in hormone replacement therapy. Lipids Health Dis. 16, 219 (2017).
Committee on Practice, B.-G. Practice bulletin no. 136: Management of abnormal uterine bleeding associated with ovulatory dysfunction. Obstet. Gynecol. 122, 176–185 (2013).
Saghaei, M. Random allocation software for parallel group randomized trials. BMC Med. Res. Methodol. 4, 26 (2004).
Fraser, I. S. et al. Improving the objective quality of large-scale clinical trials for women with heavy menstrual bleeding: Experience from 2 multi-center, randomized trials. Reprod. Sci. 20, 745–754 (2013).
Acknowledgements
We would like to thank Dr. Chulaluk Komoltri, DrPh, Office for Research and Development, Faculty of Medicine Siriraj Hospital, Mahidol University for comments on research methodology and statistical analysis, and Dr. Kevin P. Jones, Ph.D., Khon Kaen University for English language editing. We also thank Ms. Chongdee Dangrat, M.Sc. and all Gynecologic Endocrinology Unit staff members, Department of Obstetrics and Gynecology, for coordinating the project and taking care of the study participants, respectively.
Funding
The study was supported by the Research and Development Fund of the Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand.
Author information
Authors and Affiliations
Contributions
N.S. and M.R. contributed to the research initiation and study design, analysis and interpretation of data, and writing the manuscript. M.W. contributed to histopathology examination and interpretation of data. T.W., S.I., P.T., K.T. and S.A. contributed to data collection, interpretation of data, and drafting the manuscript. All authors have approved the submitted version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Soontrapa, N., Rattanachaiyanont, M., Warnnissorn, M. et al. The effectiveness of desogestrel for endometrial protection in women with abnormal uterine bleeding-ovulatory dysfunction: a non-inferiority randomized controlled trial. Sci Rep 12, 1662 (2022). https://doi.org/10.1038/s41598-022-05578-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-05578-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.