Abstract
Our aim was to evaluate: (1) the prevalence of coexistence of heart failure (HF) and chronic obstructive pulmonary disease (COPD) in the studied patients; (2) the impact of HF + COPD on exercise performance and contrasting exercise responses in patients with only a diagnosis of HF or COPD; and (3) the relationship between clinical characteristics and measures of cardiorespiratory fitness; (4) verify the occurrence of cardiopulmonary events in the follow-up period of up to 24 months years. The current study included 124 patients (HF: 46, COPD: 53 and HF + COPD: 25) that performed advanced pulmonary function tests, echocardiography, analysis of body composition by bioimpedance and symptom-limited incremental cardiopulmonary exercise testing (CPET) on a cycle ergometer. Key CPET variables were calculated for all patients as previously described. The \({\dot{\text{V}}}\)E/\({\dot{\text{V}}}\)CO2 slope was obtained through linear regression analysis. Additionally, the linear relationship between oxygen uptake and the log transformation of \({\dot{\text{V}}}\)E (OUES) was calculated using the following equation: \({\dot{\text{V}}}\)O2 = a log \({\dot{\text{V}}}\)E + b, with the constant ‘a’ referring to the rate of increase of \({\dot{\text{V}}}\)O2. Circulatory power (CP) was obtained through the product of peak \({\dot{\text{V}}}\)O2 and peak systolic blood pressure and Ventilatory Power (VP) was calculated by dividing peak systolic blood pressure by the \({\dot{\text{V}}}\)E/\({\dot{\text{V}}}\)CO2 slope. After the CPET, all patients were contacted by telephone every 6 months (6, 12, 18, 24) and questioned about exacerbations, hospitalizations for cardiopulmonary causes and death. We found a 20% prevalence of HF + COPD overlap in the studied patients. The COPD and HF + COPD groups were older (HF: 60 ± 8, COPD: 65 ± 7, HF + COPD: 68 ± 7). In relation to cardiac function, as expected, patients with COPD presented preserved ejection fraction (HF: 40 ± 7, COPD: 70 ± 8, HF + COPD: 38 ± 8) while in the HF and HF + COPD demonstrated similar levels of systolic dysfunction. The COPD and HF + COPD patients showed evidence of an obstructive ventilatory disorder confirmed by the value of %FEV1 (HF: 84 ± 20, COPD: 54 ± 21, HF + COPD: 65 ± 25). Patients with HF + COPD demonstrated a lower work rate (WR), peak oxygen uptake (\({\dot{\text{V}}}\)O2), rate pressure product (RPP), CP and VP compared to those only diagnosed with HF and COPD. In addition, significant correlations were observed between lean mass and peak \({\dot{\text{V}}}\)O2 (r: 0.56 p < 0.001), OUES (r: 0.42 p < 0.001), and O2 pulse (r: 0.58 p < 0.001), lung diffusing factor for carbon monoxide (DLCO) and WR (r: 0.51 p < 0.001), DLCO and VP (r: 0.40 p: 0.002), forced expiratory volume in first second (FEV1) and peak \({\dot{\text{V}}}\)O2 (r: 0.52; p < 0.001), and FEV1 and WR (r: 0.62; p < 0.001). There were no significant differences in the occurrence of events and deaths contrasting both groups. The coexistence of HF + COPD induces greater impairment on exercise performance when compared to patients without overlapping diseases, however the overlap of the two diseases did not increase the probability of the occurrence of cardiopulmonary events and deaths when compared to groups with isolated diseases in the period studied. CPET provides important information to guide effective strategies for these patients with the goal of improving exercise performance and functional capacity. Moreover, given our findings related to pulmonary function, body composition and exercise responses, evidenced that the lean mass, FEV1 and DLCO influence important responses to exercise.
Similar content being viewed by others
Introduction
The incidence and prevalence of chronic-degenerative diseases in a progressively elderly population has increased worldwide over the last several decades1. In this context, cardiopulmonary disease (CPD) is increasing and remains the leading cause of death in many countries; in Brazil, CPD is responsible for approximately 20% of all deaths in adults 30 years of age or older2,3.
Heart failure (HF) and chronic obstructive pulmonary disease (COPD) are predominant chronic diseases; the growing prevalence of HF and COPD reflects a combination of aging population, increasing incidence of both diseases in association with diagnostic access4,5,6. The pathophysiology of HF and COPD are well established in the literature7. HF is a syndrome initially characterized by cardiac dysfunction resulting from structural changes or cardiac function, however more advanced stages of the disease leads to compromised lung function and systemic changes, such us pulmonary restrictions, ventilation-perfusion abnormalities, pulmonary congestion and reduced peripheral muscle mass with consequent onset of fatigue7,8,9.
On the other hand, COPD is a common, preventable, and treatable disease characterized by persistent respiratory symptoms and airflow obstruction10. The inflammation resulting from exposure to COPD triggering factors causes a cascade of events that generate pathological abnormalities such as changes in cardiac structure and function and muscle atrophy leading to the onset of dyspnea and inactivity with a consequent reduction in functional capacity11,12,13.
HF and COPD share similar signs and symptoms, and often coexist, leading to a worse prognosis, as well as greater challenges for diagnosis and the establishment of therapeutic interventions. It is estimated that the prevalence of HF in patients with COPD and vice versa is between 10 and 25% in developed countries11,14. The overlap of HF and COPD is associated with increased morbidity, poorer quality of life and greater use of health resources15.
One of the most common and impactful symptoms in patients with either independent or overlapping HF and COPD is decreased exercise capacity; a commonly reported subjective symptom in dyspnea with exertion16,17.
In patients with overlap where HF prevails, there is a loss in the delivery or use of oxygen (O2). This is because the stroke volume reduction resulting from cardiac dysfunction is not able to compensate for the high muscle O2 extraction, leading to a low delivery for muscle contraction, causing the anaerobic metabolism to happen earlier, thus increasing lactate rates circulating and thus causing an early sensation of muscle fatigue18. In addition, ventilatory changes also play an important role in exercise limitation: excessive ventilatory stimulation resulting from high cardiac filling pressures increases reflex chemosensitivity, increasing ventilation in relation to demands, moreover, restrictive changes in ventilatory mechanics also occur, increasing thus the feeling of dyspnea on exertion19.
In overlap patients, where COPD has a greater influence on exercise limitation, ventilatory inefficiency is evidenced. The Inflammation, fibrosis, and exudates in the small airways of patients with COPD generate a reduction in FEV1 and progressive airway obstruction during expiration, resulting in lung hyperinflation, reduced inspiratory capacity that influences the increase in functional residual capacity (FRC), resulting in increased dyspnea, which associated with a reduction in the intrinsic contractile properties of the ventilatory muscles and abnormalities in ventilation perfusion, generates ventilatory inefficiency and limited exercise capacity20,21.
A detailed assessment of exercise capacity is relevant in these patient populations from diagnostic, prognostic and therapeutic efficacy perspective. Cardiopulmonary exercise testing (CPET) is the gold-standard approach to assessing exercise capacity and more broadly cardiorespiratory fitness; an evidence-based panel of core CPET measures allows for a more comprehensive evaluation22. Through CPET, ventilatory and gas exchange, as well as heart rate (HR), electrocardiogram, and blood pressures, are measured to provide detailed information on the cardiovascular, pulmonary, and muscular systems as well as detect the primary limitation to exercise in these chronic conditions23.
The coexistence of HF and COPD has important therapeutic and prognostic implications, the knowledge about the prevalence of the concomitance of these diseases is clinically relevant. Moreover, follow-up studies have contributed substantially to the understanding of disease progression, clinical outcomes, mortality, and use of resource in health, as both conditions are quite disabling, generating heightened concern when these conditions coexist.
In the present study, we hypothesized that HF + COPD would further deteriorate cardiorespiratory fitness compared to patients diagnosed with HF and COPD in isolation. We additionally hypothesized that there are relationships between clinical characteristics and CPET measures of cardiorespiratory fitness. The specific aims of this study was to assess: (1) the prevalence of HF and COPD overlap in the studied patients; (2) the impact of the overlapping HF + COPD on exercise capacity and cardiorespiratory fitness and to contrast these measures in patients with either HF or COPD in isolation; and (3) the relationship between clinical characteristics and measures of cardiorespiratory fitness (4) verify the occurrence of exacerbations, hospitalizations and deaths in the follow-up period of up to 24 months.
Methods
Study design and subjects
This longitudinal study was reported following recommendations of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement24. Three-hundred fifteen patients were screened from 3 cardiology and pneumology outpatient clinics of the University Hospital of the Federal University of São Carlos, from 01 June 2017 to 30 December 2019. All patients who attended during this period with the diagnosis of HF with reduced or borderline ejection fraction (EF) and/or COPD were contacted by phone and was asked questions regarding diagnosis, clinical conditions, disease stability, drug optimization, and functional mobility. For all patients, eligibility criteria were: (1) age range of 40–85 years; (2) clinically stable for at least 3 months (i.e., no worsening of symptoms, exacerbation or decompensation); (3) no change in dose or change in medication for at least 3 months; (4) no hospitalizations for any cause for at least 3 months; and (5) absence of any condition that may affect exercise performance (e.g., anemia, neuromuscular disorders, or malignancies). Non-inclusion criteria were: (1) long-term O2 therapy; (2) musculoskeletal disease that would impact exercise performance (e.g., osteoarthritis, osteonecrosis, trauma, etc.); and (3) peripheral arterial disease associated with claudication. Moreover, HF or COPD exacerbation or hospitalization during the study was a criterion for study drop-out. All patients who met the eligibility criteria were invited for an initial assessment and tests to confirm the diagnosis of one (HF or COPD) or both (HF + COPD) diseases being assessed in the current study.
Disease treatment was optimized before study entry and patients underwent CPET only after an agreement had been reached between pneumologists and cardiologists regarding disease stability. As showed in Fig. 1, 124 patients with a confirmed diagnosis of HF and/or COPD were included. The Study followed the resolution no. 466 of the National Health Council (current guideline in Brazil) and The Declaration of Helsinki and was approved by the Ethics and Research Committee of the Federal University of São Carlos. All participants were informed about the objectives, experimental procedures and potential risks involved in this study and gave written informed consent statement prior to participation.
Cardiac and lung function assessments
All patients underwent a transthoracic two-dimensional and Doppler echocardiographic (HD11 XE, Philips, Amsterdam, Netherlands) examination at baseline to confirm the diagnosis, stratify the degree of systolic dysfunction, and obtain the necessary measures for cardiac function in the HF and HF + COPD groups and confirm the absence of reduced ejection fraction in the COPD group. Patients with HF were determined according to left ventricle ejection fraction ≤ 50%25. Advanced pulmonary function assessment (Masterscreen Body, Mijnhardt/Jäger, Würzburg, German) was performed to obtain dynamic and static lung volumes and capacities, such us Forced Expiratory Volume in 1 s (FEV1), Forced Vital Capacity (FVC), Residual Volume (RV), Total Lung Capacity (TLC), Inspiratory Capacity (IC), and Diffusion Capacity Carbon Monoxide (DLCO) pre and post-bronchodilator therapy. The interruption of the use of bronchodilators was requested 12 h before the exam and the GOLD criteria [post-bronchodilator FEV1/ FVC ratio < 0.70] was used to confirm a COPD diagnosis26.
Cardiopulmonary exercise testing
All patients underwent a symptom-limited CPET on an electronically braked cycle ergometer (Corival Recumbent, Lode, Groningen, Netherlands) using the Oxycon Mobile System (Mijnhardt/Jäger, Würzburg, German). The patients’ continuous use medications were kept for the test. The exercise protocol started with 5 min of data collection at rest, followed by unloaded cycling for 1 min with a subsequent increment of 5–10 watts each minute (ramp protocol). Patients were instructed to pedal at the cadence of 60 rotation per minute and the work rate (WR) increment was individually selected according to reported exercise tolerance. Breath-by-breath \({\dot{\text{V}}}\)O2 (L/min), \({\dot{\text{V}}}\)CO2 (L/min), and \({\dot{\text{V}}}\)E (L/min) were recorded. The CPET variables were reported as 20-s averaged data. During the rest, exercise test and the recovery phase, the HR (collected with heart rate monitor), 12-lead electrocardiogram (ECG), blood pressure (collected through manual auscultation), and arterial oxygen saturation were monitored. Arterial oxygen saturation was measured non-invasively by pulse oximetry (SpO2, %). Breathlessness and leg effort scores were rated according to the 10-point Borg category ratio scale27. Established criteria to interrupt the test were followed and included angina (score above 2 on a scale of 0–10), life-threatening arrhythmias, electrocardiographic evidence of ischemia, a drop in systolic blood pressure, or arterial oxygen saturation ≤ 84%28. Key CPET variables were calculated for all patients as previously described. The \({\dot{\text{V}}}\)E/\({\dot{\text{V}}}\)CO2 slope was obtained through linear regression analysis29. Additionally, the linear relationship between oxygen uptake and the log transformation of \({\dot{\text{V}}}\)E (OUES) was calculated using the following equation: \({\dot{\text{V}}}\)O2 = a log \({\dot{\text{V}}}\)E + b, with the constant ‘a’ referring to the rate of increase of \({\dot{\text{V}}}\)O230. Circulatory power (CP) was obtained through the product of peak \({\dot{\text{V}}}\)O2 and peak systolic blood pressure and Ventilatory Power (VP) was calculated by dividing peak systolic blood pressure by the \({\dot{\text{V}}}\)E/\({\dot{\text{V}}}\)CO2 slope31,32.
Patients follow-up
All patients were followed for 24 months. The follow-up of patients started after the performance of the CPET and was carried out through telephone contact every 6 months (6, 12, 18, 24 months), where the patient or caregiver (in case the patient was unable to respond) answered a questionnaire with 5 questions regarding occurrence of cardiopulmonary events such as diseases exacerbations, hospitalizations by cardiopulmonary causes [acute myocardial infarction (AMI), stroke, cardiac or pulmonary surgery] and death.
Statistical analysis
A sample calculation was performed (GPower 3.1—University of Kiel, Kiel, Germany) using the peak \({\dot{\text{V}}}\)O2 (HF: 12 ± 3, COPD: 14 ± 3, HF + COPD: 11 ± 3; p: 0, 17; power: 0.80) obtained in pilot studies previously performed in our laboratory with individuals who were diagnosed with HF and COPD. From this sample calculation, 42 subjects, 14 for each group, were needed to reach sufficient statistical power of 0.80. The Shapiro–Wilk test was used to verify the data distribution. Descriptive variables were expressed as mean ± standard deviation, when normal distribution was present, or median and interquartile, when non-normal distribution was not present. Categorical variables are expressed as frequencies and percentages and compared using the chi-square test. A one-way ANOVA was used to compare anthropometric measures, cardiac and pulmonary function measures and CPET measures. A two-way ANOVA was used to compare the exercises responses between groups at different times of exercise. Relationships between measures collected in the current study were assessed by the Pearson Correlation coefficient which correlation strengths will be classified as trivial— < 0.1, small—0.30–0.50, large—0.50–0.70, very large— > 0.70–0.90, nearly perfect— > 0.9033. The analysis of the occurrence of events: number of disease exacerbations, number of hospitalizations for cardiopulmonary causes in the period, onset of AMI, stroke, cardiac or pulmonary surgery and death from cardiopulmonary causes was evaluated by the analysis of Klapan-Meier survival with the groups being compared using the Log-rank test. A p-value < 0.05 was considered as statistically significant for all tests. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) 20.0 (IBM, Armonk, New York) and PRISM 9.0 (GraphPad, San Diego, California).
Results
Clinical and resting characteristics
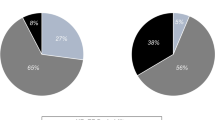
Figure 2 demonstrates that the prevalence of coexisting HF and COPD diagnoses in the studied patients was 20%. Of the 25 patients who were included in the HF + COPD group, only 18 patients (72%) had a previous diagnosis of overlap, while 7 (28%) patients were diagnosed with the overlap after performing the echocardiogram or advanced pulmonary assessment in our study. The 7 patients where a diagnosis of HF or COPD was newly identified were referred for medication optimization and, after 3 months, returned to complete data collection for the current study.
Patient characteristics are reported in Table 1. Significant differences were found for most anthropometric and clinical variables amongst HF, COPD and HF + COPD groups. Most patients in both groups were male while patients in the COPD and HF + COPD groups were older (p < 0.05). In relation to cardiac function, as expected, patients with COPD presented with preserved systolic function, while in the HF and HF + COPD demonstrated similar levels of systolic dysfunction, although differences between HF and HF + COPD groups were found in systolic dysfunction staging (p < 0.05). Differences were found in the Mitral E wave in the HF and COPD groups. As expected, COPD and HF + COPD patients showed evidence of an obstructive ventilatory disorder. The frequency of patients in stage 2 according to the GOLD guidelines was greater in the COPD group. HF patients showed lower static lung volumes when compared to the COPD group (p < 0.05); differences were also found in Residual volume (RV), % predicted TLC and DLCO. In relation to the presence of comorbidities, the HF group was the most affected when contrasted with the COPD group.
Metabolic, cardiovascular and ventilatory responses to exercise
Table 2 lists the responses to the CPET and the comparisons between groups. The WR and \({\dot{\text{V}}}\)O2 at peak exercise were significantly lower in the HF + COPD group when compared to the HF group (p < 0.05). Similar ventilatory responses were found between groups; however, the COPD group had higher \({\dot{\text{V}}}\)E/\({\dot{\text{V}}}\)CO2 intercept values compared to the other groups. Ventilatory power (PV) was significantly higher in the COPD group when compared to the HF + COPD group (p < 0.05). However, the COPD group demonstrated a significantly lower O2 pulse compared to the HF group (p < 0.05). The HR recovery was worse in the COPD group when compared to the HF group, and in relation to systolic and diastolic blood pressure at peak exercise, the HF + COPD group presented lower values compared to the other groups (p < 0.05).
The presence of hypoxemia on exertion, assessed by SpO2, did not differ between the HF and HF + COPD groups, but it was significantly reduced in the COPD group (p < 0.05). The subjective perception of exertion (i.e., dyspnea and fatigue in the legs) was higher in the COPD and HF + COPD groups, the main reason for the end of the test being dyspnea.
The HF group showed better cardiorespiratory fitness compared to the other groups when we evaluated different moments of the incremental exercise (Fig. 3). From rest to peak exercise, the increment in \({\dot{\text{V}}}\)O2 and WR were greater in the HF group compared to the COPD and HF + COPD groups.
Relationships between measures of pulmonary function, anthropometric measures, clinical characteristics and exercise responses
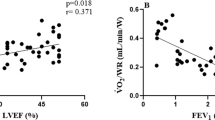
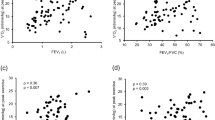
We found associations between components of pulmonary function, anthropometric measures, clinical characteristics and CPET variables (Figs. 4, 5). Combining the three groups, significant correlations were observed between lean mass and peak \({\dot{\text{V}}}\)O2 (r: 0.56 p < 0.001), lean mass and OUES (r: 0.42 p < 0.001), lean mass and peak O2 pulse (r: 0.58 p < 0.001), DLCO and WR (r: 0.51 p < 0.001), and DLCO and VP (r: 0.40 p: 0.002). In addition, we found that FEV1 was correlated with peak \({\dot{\text{V}}}\)O2 (r: 0.52; p < 0.001) and peak WR (r: 0.62; p < 0.001).
Correlation between lung function and CPET responses; used Pearson correlation coefficient. (A) Relationship between diffusion capacity carbon monoxide (DLCO) and work rate; (B) relationship between DLCO and ventilatory power; (C) relationship between forced expiratory volume in the first second (FEV1) and peak oxygen uptake (\({\dot{\text{V}}}\)O2); (D) relationship between FEV1 and peak work rate. Patients that pefromed DLCO: 58 (HF: 28, COPD: 20, HF + COPD: 10).
Occurrence of cardiopulmonary events in the follow-up period
In Table 3, we can see that the occurrence of the events obtained was not different between the groups. The Fig. 6 shows the Kaplan–Meier analysis in both groups. No differences were found between groups in cardiopulmonary outcomes when evaluating disease exacerbations, hospitalizations for cardiopulmonary causes, acute myocardial infarction, stroke, cardiac/pulmonary surgery (Fig. 6A) and survival (Fig. 6B).
Discussion
The main original findings of the present investigation involving patients with HF, COPD and coexisting HF + COPD are as follows: (1) the prevalence of HF + COPD overlap was 20% in the studied patients. (2) Patients with HF + COPD had the greatest impairment with cardiorespiratory fitness, expressed by lower values in key CPET variables; (3) when groups were contrasted at different distinct exercise time points, the HF group has better responses compared to the other two groups, including WR, peak \({\dot{\text{V}}}\)O2; (4) correlations in the overall group suggest that components of pulmonary function and anthropometric characteristics can influence CPET variables; (5) after the follow-up period of both groups, no differences were found in the occurrence of cardiopulmonary events and deaths.
The aging population is a worldwide phenomenon; due to the health issues associated with aging, a higher proportion of the global population are at risk for chronic disease and diagnoses as well as comorbidity34. A coexistance of HF and COPD (overlap syndrome) has been associated with increased morbidity and decreased quality of life as well as a greater use of health resources; the literature indicates a HF + COPD prevalence between 10 and 30%11,35,36. Age, sex and anthropometric characteristics have been shown to influence cardiorespiratory fitness37. In our study, these factors were different between groups, being that the COPD and HF + COPD groups were older than the HF group (p < 0.05), moreover body composition was different between HF and COPD groups. These findings may indicate an advantage for this group, although the groups are in an age range between 60 and 70 years on average. These differences may explain the slightly more favorable exercise response in the HF group.
Left ventricular EF has been an important survival marker in patients with cardiopulmonary diseases. It has been shown that age has an influence on systolic function, and although differences in EF were not found between HF and HF + COPD, the mean age of the overlap group was higher. Shah et al. assessed 18,398 subjects with reduced EF and found that the mean survival of patients aged 65–69 was 4 years38,39. In relation to pulmonary function, no differences were found between the two groups with COPD in spirometric values. Nevertheless, static lung values demonstrated that the COPD group presented with compatible volumes with greater air trapping and a worse DLCO. In general, inflammation and structural changes in the airways resulting from COPD increase expiratory flow limitations and worsens with advancing disease severity. The destruction of the lung parenchyma in association with airflow limitation generates air trapping that results in hyperinflation which associated with a reduced IC leads to reduced ventilation-perfusion and limited gas diffusion compromising the gas exchange40,41. Hyperinflation in association with changes in alveolar ventilation (VA), minute ventilation and reduction of the pulmonary vascular bed leads to hypoventilation and with the progression of the disease there may be an increase in PaCO2 leads to conditions of hypercapnia and hypoxia41,42.
It is already well known that patients with HF and COPD have reduced exercise performance, due to impaired ventilatory function and systemic manifestations that affect the muscular and cardiopulmonary system, increasing the limitation to exertion43,44. CPET allows a rigorous evaluation of the interaction between respiratory deficiencies caused by diseases and reduced exercise capacity in individuals under physiological stress, being possible to verify which is the main limiting factor to physical exercise45. Thus, in the present study, the HF + COPD group had a lower WR when compared to the HF group. The association between advanced age, intrapulmonary conditions, impaired cardiovascular function and loss of muscle strength and endurance in patients with HF + COPD leads to impaired performance during exercise, and intolerance to high workloads46,47. Interestingly, no differences were found in the % of WR predicted achieved at peak exercise between groups. This can be explained by the fact that, due to differences in age, height and weight influence the predicted WR. Thus, although it seems that the individuals performed the same effort in the test, the predicted WR was different between the groups, with each group reaching values of % of the predicted WR, proportionally to the predicted value of the WR.
The V̇O2 is the main marker of aerobic capacity. In our study, we only found differences in absolute values; no differences were found for the relative values. A combination of factors leads to a reduction in peak \({\dot{\text{V}}}\)O2 of patients with HF + COPD: ventilatory abnormalities that generate inefficiency in capitation, changes in the heart pump that lead to impaired delivery, and changes in muscle cell composition that contribute to reduced oxygen utilization48,49. When we contrasted the groups during the entire exercise time, we can see that the HF group had better performance in the exercise, and a combination of factors such us a younger age group, preserved lung function and most individuals in this sample with mild systolic dysfunction (reflected better physiological adjustments to exercise) may have contributed to these individuals tolerating longer exercise time, greater load increments and consequently higher absolute VO2 values.
A comprehensive assessment of several measures obtained from CPET provide for a more comprehensive cardiorespiratory fitness evaluation50. The O2 pulse is a strong predictor of disease severity and adverse events51. Mathematically, the O2 pulse is determined by the product of the stroke volume and arteriovenous oxygen difference, and changes in O2 pulse during exercise suggested alterations in the stroke volume52. In the current study, we observes that the HF group, when compared to the COPD group, have higher O2 pulse values. The RPP was significantly lower in the HF + COPD group when compared with the COPD group, indicating poorer cardiac function, as expected in this comorbidity group. The influence of HF in the group with overlap syndrome can increase myocardial oxygen consumption, leading to exhaustion of coronary blood flow reserve and impaired myocardial perfusion53. Another important variable that reflects central and peripheral components of cardiac work is CP. Our HF + COPD group presented with worse CP values, this can be explained by the fact that the association of the two diseases leads to reduced cardiac function, which during high-intensity exercise can lead to pulmonary congestion, and that, associated with higher pulmonary arterial pressure and pulmonary vascular resistance, increases ventilation–perfusion incompatibility, producing ventilatory inefficiency and contributing to low CP values54.
In recent years, more complex variables derived from CPET have proved to be strong prognostic variables, capable of providing complementary and superior information compared to the isolated use of peak \({\dot{\text{V}}}\)O255. In our study, no differences were found for the \({\dot{\text{V}}}\)E/VCO2 slope, although all groups demonstrated a mean value above the normal threshold (i.e., > 30), thus observing a worse prognosis for these patients. Arena et al. followed 213 cardiac patients and found that \({\dot{\text{V}}}\)E/\({\dot{\text{V}}}\)CO2 slope values ≥ 34 associated with \({\dot{\text{V}}}\)O2 peak ≤ 14 mL kg–1 min−1 were strong predictors of hospitalization and mortality56.
The \({\dot{\text{V}}}\)E/\({\dot{\text{V}}}\)CO2 intercept is a new parameter, and in patients with lung diseases it increases with the severity of disease57. Surprisingly, only the COPD group had higher values of \({\dot{\text{V}}}\)E/\({\dot{\text{V}}}\)CO2 intercept, suggesting an increased dead space on exercise, something that was not seen in our overlap group. Nevertheless, HF + COPD presented reduced VP in comparison with HF group (p < 0.05). The VP has been studied as a prognostic marker in cardiopulmonary diseases58,59. This variable reflects peak cardiac output, alveolar perfusion, peripheral perfusion and the chemo-afferent reflexes of the skeletal muscle, with values below 3.5 mmHg indicating worsening survival32.
Lung volumes at rest influence exercise capacity and directly contribute to ventilatory inefficiency during exercise, The Air trapping does not allow all inhaled gas to be exhaled, generating hyperinflation that reduces the inspiratory capacity, influencing the increase in functional residual capacity (FRC), causing these individuals to breathe more quickly during exercise with a tidal volume (VT) reduced, which associated with low lung compliance and ventilatory muscle weakness, make these patients deplete their ventilatory reserve earlier on exercise60,61. In this context, greater neural activity is needed to increase the muscle contractions necessary to generate adequate ventilation. This increase in the efferent direction of these muscles can contribute to the development of dyspnea, which when combined with a high respiratory effort due to severe obstruction and hyperinflation, generate greater respiratory muscle impairment61.
Pulmonary and systemic cardiocirculatory maladjustments occur in both HF and COPD and in individuals with overlap, these effects are more prominent. Muscle weakness is the most common systemic effect, as it occurs in chronic processes such as what occurs in both diseases62. The increase in circulating inflammatory cytokines, such as tumor necrosis factor α, interleukins 6 and 1β, increase oxidative stress and the increase in reactive oxygen species (ROS) associated with tissue hypoxia, provide protein degradation with a consequent reduction in mass muscle and muscle atrophy affecting directly the ventilatory function, which manifest a pronounced intolerance to exercise63,64. In the current study, the HF + COPD group had worse perception of symptoms, when compared to the HF group; the perception of dyspnea was higher, and when compared to the COPD group, fatigue values were higher.
Important correlations between clinical variables, body composition and CPET variables were found in this study. It is important to note that lean mass moderately influenced peak \({\dot{\text{V}}}\)O2, OUES and O2 pulse responses in the patients studied. An adequate interaction between the ventilatory, cardiovascular and muscular systems is a determining factor for appropriate oxygen metabolism during incremental exercise. Cardiopulmonary disease initially leads to a compromise of the pulmonary and cardiovascular systems, however, with disease progression, lean mass alterations occur in these populations affecting muscle performance during exercise. These changes occur due factors such as hypoxia, oxidative stress, disuse, nutritional depletion, systemic inflammation and changes in muscle morphology, fiber type distribution and metabolism65. Other important findings correlate DLCO, and FEV1 with peak \({\dot{\text{V}}}\)O2, WR and VP. Impaired lung function due to air flow limitations, increased intrathoracic pressures, increased intrathoracic blood volume and chronic pulmonary congestion and accumulation of extravascular lung water has a direct effect on the cardiopulmonary response to exercise, leading to increased pulmonary ventilation, ventilatory inefficiency and, consequently, low peak \({\dot{\text{V}}}\)O2 and WR11,66.
Surprisingly, we found no differences between the outcomes assessed in the follow-up of patients in both groups. Although the HF + COPD group has a greater impairment of cardiorespiratory fitness, it is possible to note that the HF and COPD groups also present values of key cardiopulmonary variables for the studied populations compatible with a worse prognosis and high risk of adverse events in the period from 1 to 4 years of follow-up67. Despite the association of comorbidities in individuals with cardiopulmonary diseases increases the risk of clinical events and mortality and in our study the comorbidities were different between groups we believe that the advances in the clinical treatment and the optimized pharmacological therapy favored better control of these diseases, thus, mitigating the risk of events68. Furthermore, the increasing access to cardiopulmonary rehabilitation programs has favored the reduction of adverse events in patients with cardiopulmonary diseases69,70,71,72,73.
Study limitations
This study has some limitations which are inherent to its nature that consider the screening of patients at 3 ambulatory clinics (pneumology and cardiology) diagnosed with at least one of the diseases and aged over 50 years. As the purpose of the present study was to evaluate the coexistence of one condition in the other, it would be expected that some clinical variables would be different. In this context, it was not possible to pair the groups by disease severity, furthermore, the absence of female individuals in the HF + COPD group, the difference of age between groups and the difference in the body composition can influence the CPET response. However, to mitigate this bias, knowing that some variables could be influenced by age and sex, we performed a linear regression analysis to verify the influence on CPET variables that differed. We verified that age and sex had weak but significant influence on WR (R2:0.22 p: 0.000), absolute peak V̇O2 (R2: 0.25 p: 0.000), O2 pulse (R2: 0.20 p: 0.000) and CP (R2: 0.09 p: 0.02).
Implications of the study
The study compared the diagnosis of HF + COPD overlap with the diagnosis of isolated diseases. The comparison of the 3 groups so far is unprecedented in the literature and further strengthened the hypothesis that the coexistence of the HF + COPD has more serious implications for exercise capacity. However, the follow-up of these patients, carried out in the study, was essential to clarify the questions about the occurrence of cardiopulmonary outcomes in the three populations, thus clarifying that in a period of up to 24 months of follow-up, no differences were found in the outcomes studied between groups. This suggests that studies with longer follow-up periods can provide better clarification regarding the severity of the HF + COPD overlap and the functional decline that occurs in the studied population. In addition, conducting a study with a monitoring period of 24 months in this population with overlap of HF + COPD difficult to be carried out, standing out the importance of this study.
Conclusions
In conclusion, the coexistence of HF + COPD induces greater impairment on exercise performance when compared to patients without overlapping diseases, however the overlap of the two diseases did not increase the probability of the occurrence of cardiopulmonary events and deaths when compared to groups with isolated diseases in the period studied. CPET provides important information to guide effective strategies for these patients with the goal of improving exercise performance and functional capacity. Moreover, given our findings related to pulmonary function, body composition and exercise responses, evidenced that the lean mass, FEV1 and DLCO influence important responses to exercise.
References
Vetrano, D. L. et al. Frailty and multimorbidity: A systematic review and meta-analysis. J. Gerontol. A Biol. Sci. Med. Sci. 74(5), 659–666 (2019).
World Health Organization. Cardiovascular Disease. http://www.who.int/mediacentre/factsheets/fs317/en/index.html (Accessed 6 June 2011).
Go, A. S. et al. Heart disease and stroke statistics—2014 update: A report from the American Heart Association. Circulation 129(3), e28–e292 (2014).
Vestbo, J. et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 187(4), 347–365 (2013).
Bleumink, G. S. et al. Quantifying the heart failure epidemic: Prevalence, incidence rate, lifetime risk and prognosis of heart failure—The Rotterdam Study. Eur. Heart J. 18, 1614–1619 (2004).
Liu, L. & Eisen, H. J. Epidemiology of heart failure and scope of the problem. Cardiol. Clin. 32(1), 1–8 (2014).
McMurray, J. J. et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 14, 803–869 (2012).
Abraham, M. R. et al. Angiotensin-converting enzyme genotype modulates pulmonary function and exercise capacity in treated patients with congestive stable heart failure. Circulation 106, 1794–1799 (2002).
Dumitru, L. et al. Disability in COPD and chronic heart failure is the skeletal muscle the final common pathway? Maedica (Buchar). 8(2), 206–213 (2013).
Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Chronic Respir. Dis. 1, 1–164 (2021).
Neder, J. A., Rocha, A., Berton, D. C. & O’Donnell, D. E. Clinical and physiologic implications of negative cardiopulmonary interactions in coexisting chronic obstructive pulmonary disease-heart failure. Clin. Chest Med. 40(2), 421–438 (2019).
Rabe, K. F. et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 176, 532–555 (2007).
Agusti, A. G. N. et al. Systemic effects of chronic obstructive pulmonary disease. Eur. Respir. J. 21, 347–360 (2003).
Dumitru, L. et al. Disability in COPD and chronic heart failure is the skeletal muscle the final common pathway? Maedica (Bucur). 8(2), 206–213 (2013).
Waschki, B. et al. Physical activity is the strongest predictor of all-cause mortality in patient with COPD: A prospective cohort study. Chest 140, 331–342 (2011).
Wagner, P. D. Chronic cardiopulmonary disease and the skeletal muscle. Respir. Care 51, 37–40 (2006).
Neder, J. A. et al. Current challenges in managing comorbid heart failure and COPD. Expert Rev. Cardiovasc. Ther. 16(9), 653–673 (2018).
Agostoni, P. et al. Cardiopulmonary interaction in heart failure. Pulm. Pharmacol. Ther. 20, 130–134 (2007).
O’Donnell, D. E. et al. Exertional dyspnoea in COPD: The clinical utility of cardiopulmonary exercise testing. Eur. Respir. Rev. 25, 333 (2016).
Hogg, J. C. et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N. Engl. J. Med. 350, 2645–2653 (2004).
Rodriguez-Roisin, R. et al. Ventilation-perfusion imbalance and chronic obstructive pulmonary disease staging severity. J. Appl. Physiol. 106, 1902–1908 (2009).
Mozaffarian, D. et al. Heart disease and stroke statistics 2016 update: A report from the American Heart Association. Circulation 133, e38–e360 (2016).
Nelson, N. & Asplund, C. A. Exercise testing: Who, when, and why? PM R 8(3 Suppl), S16–S23 (2016).
Von Elm, E. et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 61(4), 344–349 (2008).
Lang, R. M. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 28(1), 1–39 (2015).
Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Chronic Respir. Dis. 1, 1–141 (2020).
Borg, G. A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 14(5), 377–381 (1982).
Balady, G. J. et al. Clinician’s Guide to cardiopulmonary exercise testing in adults: A scientific statement from the American Heart Association. Circulation 122(2), 191–225 (2010).
Arena, R., Myers, J., Aslam, S. S., Varughese, E. B. & Peberdy, M. A. Technical considerations related to the minute ventilation/carbon dioxide output slope in patients with heart failure. Chest 124(2), 720–727 (2003).
Baba, R. et al. Oxygen uptake efficiency slope: A new index of cardiorespiratory functional reserve derived from the relation between oxygen uptake and minute ventilation during incremental exercise. J. Am. Coll. Cardiol. 28(6), 1567–1572 (1996).
Cohen-Solal, A. et al. A non-invasively determined surrogate of cardiac power ('circulatory power’) at peak exercise is a powerful prognostic factor in chronic heart failure. Eur. Heart J. 23(10), 806–814 (2002).
Forman, D. E. et al. Ventilatory power: A novel index that enhances prognostic assessment of patients with heart failure. Circ. Heart Fail. 5(5), 621–626 (2012).
Hinkle, D. E., Wiersma, W. & Jurs, S. G. Applied Statistics for the Behavioral Sciences 5th edn. (Houghton Mifflin, 2003).
Gijsen, R. et al. Causes and consequences of comorbidity: A review. J. Clin. Epidemiol. 54(7), 661–674 (2001).
Macchia, A. et al. Unrecognised ventricular dysfunction in COPD. Eur. Respir. J. 39(1), 51–58 (2012).
de Miguel, D. J., Chancafe Morgan, J. & Jiménez, G. R. The association between COPD and heart failure risk: A review. Int. J. Chron. Obstruct. Pulm. Dis. 8, 305–312 (2013).
Neder, J. A. et al. Prediction of metabolic and cardiopulmonary responses to maximum cycle ergometry: A randomised study. Eur. Respir. J. 14(6), 1304–1313 (1999).
Lakatta, E. G. & Levy, D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: The aging heart in health: Links to heart disease. Circulation 107(2), 346–354 (2003).
Shah, K. S. et al. Heart failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J. Am. Coll. Cardiol. 70(20), 2476–2486 (2017).
McDonough, J. E. et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N. Engl. J. Med. 365(17), 1567–1575 (2011).
O’Donnell, D. E., D’Arsigny, C., Fitzpatrick, M. & Webb, K. A. Exercise hypercapnia in advanced chronic obstructive pulmonary disease: The role of lung hyperinflation. Am. J. Respir. Crit. Care Med. 166(5), 663–668 (2002).
Rodríguez-Roisin, R. et al. Ventilation-perfusion imbalance and chronic obstructive pulmonary disease staging severity. J. Appl. Physiol. 106(6), 1902–1908 (2009).
Güder, G. & Rutten, F. H. Comorbidity of heart failure and chronic obstructive pulmonary disease: More than coincidence. Curr. Heart Fail. Rep. 11(3), 337–346 (2014).
Hawkins, N. M., Virani, S. & Ceconi, C. Heart failure and chronic obstructive pulmonary disease: The challenges facing physicians and health services. Eur. Heart J. 34(36), 2795–2803 (2013).
Ingle, L. Theoretical rationale and practical recommendations for cardiopulmonary exercise testing in patients with chronic heart failure. Heart Fail. Rev. 12(1), 12–22 (2007).
Steinborn, W. & Anker, S. Cardiac cachexia: Pathophysiology and clinical implications. Basic Appl. Myol. 13, 191–201 (2003).
Agustí, A. G. et al. Systemic effects of chronic obstructive pulmonary disease. Eur. Respir J. 21(2), 347–360 (2003).
Agostoni, P., Cattadori, G., Bussotti, M. & Apostolo, A. Cardiopulmonary interaction in heart failure. Pulm. Pharmacol. Ther. 20(2), 130–134. https://doi.org/10.1016/j.pupt.2006.03.001 (2007).
Arena, R. & Sietsema, K. E. Cardiopulmonary exercise testing in the clinical evaluation of patients with heart and lung disease. Circulation 123(6), 668–680 (2011).
Guazzi, M. et al. 2016 focused update: Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur. Heart J. 39(14), 1144–1161. https://doi.org/10.1093/eurheartj/ehw180 (2018).
Bhambhani, Y., Norris, S. & Bell, G. Prediction of stroke volume from oxygen pulse measurements in untrained and trained men. Can J Appl Physiol. 19(1), 49–59 (1994).
Mahler, D. A., Parker, H. W. & Andresen, D. C. Physiologic changes in rowing performance associated with training in collegiate women rowers. Int. J. Sports Med. 6(4), 229–233 (1985).
Dini, F. L. et al. Coronary flow reserve in idiopathic dilated cardiomyopathy: Relation with left ventricular wall stress, natriuretic peptides, and endothelial dysfunction. J. Am. Soc. Echocardiogr. 22(4), 354–360 (2009).
Witte, K. K. & Clark, A. L. Why does chronic heart failure cause breathlessness and fatigue? Prog. Cardiovasc. Dis. 49, 366–384 (2007).
Arena, R., Myers, J. & Guazzi, M. Cardiopulmonary exercise testing is a core assessment for patients with heart failure. Congest Heart Fail. 17(3), 115–119 (2011).
Arena, R., Myers, J., Aslam, S. S., Varughese, E. B. & Peberdy, M. A. Peak VO2 and VE/VCO2 slope in patients with heart failure: A prognostic comparison. Am. Heart J. 147(2), 354–360 (2004).
Apostolo, A. et al. Impact of chronic obstructive pulmonary disease on exercise ventilatory efficiency in heart failure. Int. J. Cardiol. 189, 134–140 (2015).
Goulart, C. D. L. et al. The value of cardiopulmonary exercise testing in determining severity in patients with both systolic heart failure and COPD. Sci. Rep. 10(1), 4309 (2020).
Dos Santos, P. B. et al. Eccentric left ventricular hypertrophy and left and right cardiac function in chronic heart failure with or without coexisting COPD: Impact on exercise performance. Int. J. Chron. Obstruct. Pulm. Dis. 16, 203–214 (2021).
Arbex, F. F. et al. Exercise ventilation in COPD: Influence of Systolic Heart Failure. COPD 13, 1–8 (2016).
Cherniack, R. M. & Snidal, D. P. The effect of obstruction to breathing on the ventilatory response to CO2. J. Clin. Investig. 35(11), 1286–1290 (1956).
de Oca, M. et al. Peripheral muscle composition and health status in patients with COPD. Respir. Med. 100(10), 1800–1806 (2006).
Agusti, A. G. et al. Skeletal muscle apoptosis and weight loss in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 166, 485–489 (2002).
Barreiro, E. et al. Oxidative stress and respiratory muscle dysfunction in severe chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 171, 1116–1124 (2005).
Gosker, H. R., Wouters, E. F., van der Vusse, G. J. & Schols, A. M. Skeletal muscle dysfunction in chronic obstructive pulmonary disease and chronic heart failure: Underlying mechanisms and therapy perspectives. Am. J. Clin. Nutr. 71(5), 1033–1047 (2000).
Alencar, M. C. et al. Does exercise ventilatory inefficiency predict poor outcome in heart failure patients with COPD? J. Cardiopulm. Rehabil. Prev. 36(6), 454–459 (2016).
Guazzi, M. et al. 2016 focused update: Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation https://doi.org/10.1161/CIR.0000000000000406 (2016).
World Health Organization. Innovative Care for Chronic Conditions: Building Blocks for Action (WHO, 2012).
Anderson, L. et al. Exercise-based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta-analysis. J. Am. Coll. Cardiol. 67(1), 1–12 (2016).
Taylor, R. S. et al. Exercise-based rehabilitation for heart failure. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD003331.pub4 (2014).
De Ferrari, G. M. et al. Physical inactivity is a risk factor for primary ventricular fibrillatton. JACC 73(16), 2117–2118 (2019).
Saint-Maurice, P. F. et al. Association of leisure-time physical activity across the adult life course with all-cause and cause-specific mortality. JAMA Netw. Open 2(3), e190355 (2019).
Stamatakis, E. et al. Sitting time, physical activity, and risk os mortality in adults. JAAC 73(16), 2062–2072 (2019).
Acknowledgements
We would like to thank the Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP) and the Coordenação de Apoio a Pessoal do Ensino Superior (CAPES) for providing financial support, allowing this work to be carried out. We extend our gratitude to the University Hospital of the Federal University of São Carlos for supporting patient recruitment for this study. Borghi-Silva A is an Established Investigator (level IB) of the Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil. We are also grateful to all physical therapy team for technical support. More importantly, however, we are indebted to the patients for their effort and enthusiastic cooperation throughout the study.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dos Santos, P.B., Simões, R.P., Goulart, C.L. et al. Responses to incremental exercise and the impact of the coexistence of HF and COPD on exercise capacity: a follow-up study. Sci Rep 12, 1592 (2022). https://doi.org/10.1038/s41598-022-05503-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-05503-5
This article is cited by
-
Chronic Obstructive Pulmonary Disease in Heart Failure: Challenges in Diagnosis and Treatment for HFpEF and HFrEF
Current Heart Failure Reports (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.